Abstract
The aim of the present study was to evaluate mitotic abnormality and micronuclei inducing potentials of colchicine and the leaf aqueous extracts of Clerodendrum viscosum (LAECV) in Allium cepa root apical meristem cells. A. cepa roots were exposed to LAECV (1–8 mg ml–1) and colchicine (0.4 mg ml–1) for 4 h and allowed to recover in water for 16 h. Root tips (treated and recovery samples) were fixed, stained and squashed; the mitotic abnormalities and micronuclei frequency were analyzed under light microscope. The microscopic study revealed both colchicine and LAECV treatment could induce different types of mitotic abnormalities including c-metaphase, vagrant chromosomes, sticky chromosomes, anaphase bridges and increased frequency of micronuclei, along with a reduction in mitotic index in onion root apical meristem cells. Thus cyto-genotoxic effects of colchicine and LAECV are explored and compared here. Data indicate colchicine and LAECV treatment show similarity in cyto-genotoxic potentials. In conclusion, as both the substances have therapeutic potentials and also with cyto-genotoxic actions; therefore, extreme caution should be exercised in prescribing them in medication.
1. Introduction
Colchicine, N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide, is a toxic natural product and secondary metabolite, originally extracted from a plant, Colchicum autumnale (commonly known as autumn crocus, meadow saffron or naked lady). In De Materia Medica, Colchicum extract was first illustrated as a treatment for gout by Pedanius Dioscorides in the first century AD. The drug colchicine is now often used to prevent gout attacks (Van et al. Citation2014). It can relieve a gout attack between 12 and 24 h by reducing inflammation in the joints. This drug acts as an anti-inflammatory to ease pain from gout outbreaks by inhibiting neutrophil motility and action, leading to a net anti-inflammatory effect, although it is unable to lower uric acid levels in the body. As an anti-inflammatory agent it is also used for long-term treatment of Behcet’s disease (Cocco et al. Citation2010), and useful for the treatment of chondritis (Puéchal et al. Citation2014), constipation-predominant irritable bowel syndrome in women (Verne et al. Citation2003), severe or persistent aphthous stomatitis (Porter and Scully Citation2005), and pericarditis (Alabed et al. Citation2014). Moreover, colchicine functions effectively as a mitotic poison or spindle poison by binding to tubulin and inhibiting microtubule polymerization which is essential to mitosis. Each molecule of colchicine binds to one of tubulin by replacement of a methyl group, thus preventing its polymerization (Salmon et al. Citation1984). In cytogenetic study the mitosis inhibiting function of colchicine has been of great use in the observation of the metaphase chromosomes of cells under light microscopes, and also for karyotype studies as well as polyploidy induction in plants. Colchicine treatment to apical meristem leads to growth inhibition, root swelling, chromosome condensation and increase in frequency of metaphase with haphazardly arranged chromosomes and also reduces metaphase to anaphase transition frequency (Ray et al. Citation2013). As a result of colchicine treatment, when microtubules fail to connect to one or more kinetochores, components of the checkpoint continue to generate signals that delay metaphase to anaphase transition. Disruption of the spindle with the drug treatment would be expected to generate a strong signal that greatly prolongs metaphase (Salmon et al. Citation1984). In the present study cyto-genotoxic effects of colchicine are studied and compared with the leaf aqueous extract of Clerodendrum viscosum Vent., a traditionally used medicinal plant.
C. viscosum, a perennial shrub (Family: Lamiaceae, common name: Ghetu), is widely used in many indigenous systems of healthcare (Prajapati et al. Citation2002; Khatry et al. Citation2006). The leaf aqueous extract of C. viscosum (LAECV) contains tannins, terpenoids, triterpenoids, glycosides, carbohydrates, saponins and alkaloids (Ray et al. Citation2012). The aerial parts yield a number of sterols, namely clerosterol and its 22E-dehydro derivative, 24β–ethylcholesta-5,22E,25-trien-3β–ol as major sterols in addition to cholesterol, campestral, 24a-stigmasterol, sitosterol, 24β–stigmasterol (Prajapati et al. Citation2002) and a fixed oil consisting of oleic acid, linolenic acid, lingnoceric acid and stearic acid (Sajem and Gosai Citation2006). It is commonly prescribed in all types of worm infection, stomach pain, skin disease and malarial fever (Bhattacharya Citation2004). C. viscosum leaves are mixed with vegetables for the treatment of high blood pressure and asthma (Sajem and Gosai Citation2006) and the leaf juice is prescribed in headache, stomach trouble and benign tumors (Bhattacharya Citation2004) and also for the management of cancer by some tribal communities (Panda and Das Citation1999). Moreover, the leaf methanolic extract of C. viscosum could reduce blood sugar level in Wistar diabetic rats (Das et al. Citation2011) and also antinociceptive, anti-inflammatory and neuropharmacological activities (Khatry et al. Citation2006). Since ancient times many plant products have been traditionally used in the treatment of a variety of ailments due to their high remedial properties. But, unfortunately some secondary metabolites used for therapeutic purposes have been found likely to have teratogenic and mutagenic effects (Ping et al. Citation2012). Recent studies have discovered that plants used for therapeutic purposes might cause cyto-genotoxic effects on life forms when used long-term or in case of overdosage (Celik and Aslantürk Citation2010; Kayraldiz et al. Citation2010; Özmen Citation2010; Ping et al. Citation2012). Furthermore, recent reports indicate that many plants include cyto-genotoxic materials (Schimmer et al. Citation1994; Celik and Aslantürk Citation2007, 2010, Chaudhuri and Ray Citation2014). However, the potential toxicities of most medical plants are yet to be defined by the expert groups (Soetan and Aiyelaagbe Citation2009). Therefore, assessment of the cyto-genotoxic effects of these plants has significant implications and is essential for the evaluation of undesirable effects on living organisms.
Allium cepa roots were originally used by Levan as a standard plant model to study genotoxicity (Fiskesjö Citation1985). Plants are useful for studying the cyto-genotoxic effects of substances on living organisms (Singh et al. Citation2008) with chromosomal aberrations and micronuclei tests, some of which are usually Allium cepa (Fiskesjö Citation1985), Helianthus annuus (Kaymak Citation2005) and Vicia faba (Khadra et al. Citation2012), along with some lichens such as Pseudevernia furfuracea (Yildiz et al. Citation2011, Cansaran-Duman et al. Citation2012). A. cepa is one of the most frequently used plants in genotoxicity assays (Grant Citation1994).
In our earlier study, root growth inhibitory effect of LAECV was observed in wheat and onion root apical meristems (Ray et al. Citation2012). LAECV treatment of wheat and onion root tip cells resulted in increased percentage of metaphase and interphase cells while the overall anaphase-telophase frequency and mitotic index decreased, indicating that LAECV has an anti-proliferative effect, delays cell cycle kinetics, and arrests metaphase (Ray et al. Citation2013). There are reports on the correlation between the root growth retardation and the suppression of cell division and chromosomal aberrations (Fiskesjö Citation1985; Siddiqui Citation2007).We have also shown the effects of LAECV on the cell cycle kinetics of mouse bone marrow cells. Moreover, both colchicine and LAECV could induce a typical DNA laddering effect in wheat and onion root apical meristem and mouse bone marrow cells which correlate with their apoptosis inducing anticancer therapeutic potential (Ray et al. Citation2012). However, cyto-genotoxic potentials of LAECV and colchicine are not well studied in root apical meristem cells. Therefore, the novel aspect of the present study was to determine colchicine and LAECV induced cyto-genotoxic stress in terms of micronuclei frequency and mitotic abnormalities in root apical meristem.
2. Materials and methods
2.1. Chemicals
Orcein, colchicine, glacial acetic acid and methanol were obtained from BDH Chemicals Ltd, Poole, UK. Ethidium bromide was obtained from Sigma Chemical Company (St Louis, MO, USA). Other analytical grade chemicals were obtained from reputed manufacturers.
2.2. Plant products collection, authentication and extract preparation
Fresh leaves of C. viscosum were collected from the Burdwan University Tarabag residential complex, West Bengal, India. The voucher specimen (no. BUTBSR011) is maintained for future reference in the Department of Zoology, The University of Burdwan. It was taxonomically identified by Taxonomist, Professor Ambarish Mukherjee, Department of Botany, The University of Burdwan.
Freshly collected C. viscosum leaves were washed in running tap water, shade dried and pulverized using an electric grinder (Philips Mixer Grinder HL1605, Kolkata, India) and leaf powder was stored in air tight container for future use. Fifty grams of dried, pulverized, plant material were extracted using 500 ml of distilled water for 6 h at slow heat (50°C) in water bath and at the end the filtrate was concentrated in a water bath at 60°C for about 4 h to make the final volume one-fifth of the original volume (Parekh et al. Citation2005). The extract was then stored at –20°C for further use.
2.3. Experimental plants
Onion (Allium cepa) root apical meristems (48 h aged and 2–3 cm long) were used as plant model for determining for cyto-genotoxic potentials of colchicine and LAECV.
2.4. Onion root sprouting, treatment and preparation of mitotic phases from root apical meristem cells
Mitotic abnormalities and micronuclei frequency were analyzed to evaluate colchicine and LAECV induced cyto-genotoxic effects on onion root-tip cells. Similar sized onion bulbs were selected; the outer scales of the bulbs were removed without damaging the primordia of the root, and they were allowed to sprout roots in test tubes containing distilled water in the culture room in darkness at 25–27°C. After 48 h the onion roots (2–3 cm root length) were used for the experiments. Onion roots were treated with the different concentrations, 1–8 mg ml–1 of LAECV and a single concentration of colchicine, 0.4 mg ml–1, for 4 h. At the end of 4 h exposure, all the roots were removed from LAECV and colchicine and instantly 50% roots were fixed and processed for squash preparation following the standard procedure (Chaudhuri and Ray Citation2015). The remaining 50% of the roots were allowed to grow further for another 16 h in distilled water and then root tips were fixed and subsequently processed. The control group, which had not received any treatment, were maintained in distilled water simultaneously with the treatment groups. The treated and untreated root tips were fixed in aceto-methanol (three parts methanol: one part glacial acetic acid) for 24 h and then hydrolyzed for 10 min in 1 N HCl at 60°C, stained with 2% aceto-orcein and finally squashed in 45% acetic acid (Sharma and Sharma Citation1999; Ray et al. Citation2013). The well-spread areas of squashed roots were focused under the bright field light microscope for observation and scoring cellular abnormality.
2.5. Scoring and statistical analysis
In onion root tip cells, the slides were randomly coded and for each set of experiments at least five slides were studied under bright field light microscope with a 40× objective lens. The mitotic index, mitotic abnormalities and micronucleus frequencies were analyzed on the basis of the nucleus and chromosomal characteristics. The pattern of cell cycle kinetics was determined by scoring of the mitotic index (MI), MI% = number of metaphases/total number of cells scored × 100. The statistical significance of the difference between the control and treated groups for the different types of mitotic abnormalities, MI and mitotic phase frequencies were analyzed using a 2 × 2 contingency χ2-test.
3. Results
3.1. Effect of LAECV and colchicine on mitotic abnormality
Mitotic abnormalities were monitored in all phases of mitosis and the percentages of abnormalities (c-metaphase, vagrant chromosome, stickiness, anaphase bridges, and micronucleus) caused by the LAECV and colchicine treatments are presented in Table and Figure and are described in the following sections 3.2–3.7.
Table 1. Effects of LAECV and colchicine on the frequency of mitotic abnormalities and micronuclei induction in A. cepa root apical meristem cells.
Figure 1. Photomicrographs of onion root apical meristem cells. (A–D) Normal mitotic phases: (A) prophase, (B) metaphase, (C) anaphase, and (D) telophase. (E–I) Colchicine induced abnormalities: (E) C-metaphase, (F) sticky chromosome, (G) polar deviation, (G, H) anaphase bridge, and (I) micronucleus (arrow). (J–P) LAECV induced abnormalities: (J) sticky chromosomes, (K) c-metaphase, (L) anaphase bridge, (M) polar deviation, (N) vagrant chromosome (arrow), and (O, P) micronucleus (arrows). Photomicrographs (400×) were further magnified (2×) using Microsoft Office Power Point.
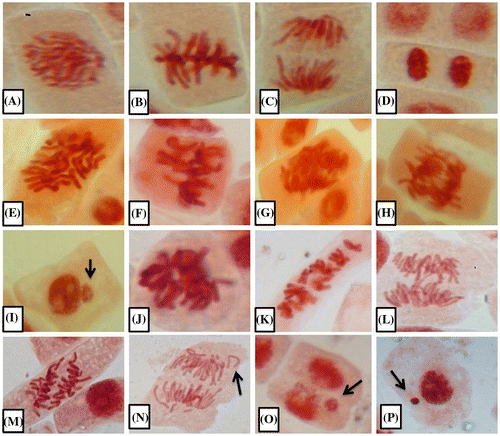
3.2. Aberrant cell percentage
Aberrant cell frequency increased in LAECV (4 mg ml–1 for 4 h) treated root apical meristem cells and the highest aberrant cell percentage scored as 3.81 and colchicine (0.4 mg ml–1 treatment for 4 h) treatment also show almost similar (3.35%) trends of aberrant cell inducing potentials. In 16 h recovery samples, the LAECV and colchicine induced aberrant cell frequency decreased slightly compared though concentration dependent aberrant cell percentage inducing pattern maintained (Table ).
3.3. C-metaphase frequency
LAECV treatment for 4 h induced C-metaphase in onion root apical meristem cells and the C-metaphase frequency recorded as 7.7, 8.3, 12.0 and 10.8% respectively for concentrations 1, 2, 3 and 4 mg ml–1. Colchicine treatment (0.4 mg ml–1 for 4 h) could induce 9.8% C-metaphase. In the 16 h recovery samples, the C-metaphase frequency decreased as compared to 4 h treated samples, excepting 8 mg ml–1 of LAECV treatment where the increased C-metaphase frequency recorded (15.4%) (Table ).
3.4. Chromosomal stickiness
LAECV induced increased percentage of chromosomal stickiness in treated root apical meristem cells and the percentage of sticky chromosome containing cells increased to 7.7, 10.0, 13.3 and 20.3 respectively at concentrations 1, 2, 3 and 4 mg ml–1 while colchicine 0.4 mg ml–1 treatment for 4 h could induce 6.9% cells with sticky chromosome. The 16 h recovery samples also show increased percentage of cells with sticky chromosomes, and with increasing concentrations of LAECV the sticky chromosome cell frequency decreased, compared to 4 h treated samples, except in the case of colchicine treatment where it increased to 10.6% (Table ).
3.5. Anaphase bridge
LAECV treatment induced increased the percentage of anaphase bridges in onion root apical meristem cells; at concentrations of 1, 2, 3 and 4 mg ml–1 respectively the cells containing anaphase bridges increased to 4.6, 3.3, 5.3 and 9.45%; meanwhile, colchicine 0.4 mg ml–1 treatment for 4 h could induce 8.8% cells with anaphase bridges. Also in the 16 h recovery samples, increasing concentrations of LAECV led to increased frequency of cells with anaphase bridges, whereas colchicine treatment decreased the frequency from 8.8% to 5.3% (Table ).
3.6. Vagrant chromosomes
The percentage of root apical meristem cells containing vagrant chromosomes increased to 6.1, 10.0, 12.0 and 13.5% respectively at concentrations of 1, 2, 3 and 4 mg ml–1 LAECV. Colchicine treatment at 0.4 mg ml–1 for 4 h could induce 8.8% cells with vagrant chromosomes. The 16 h recovery samples also show an increased percentage of cells with vagrant chromosomes with the increasing concentrations of LAECV treatment. The vagrant chromosome bearing cell frequency decreased as compared to 4 h treated samples. In colchicine treated samples, both at 4 h treatment and 16 h recovery samples, vagrant chromosome frequency was maintained at 8.8% (Table ).
3.7. Micronucleus
The outcomes of the micronucleus analysis of A. cepa root tips exposed to colchicine and LAECV are given in Table and Figure . Micronucleus frequency increased in the LAECV treated onion root apical meristem cells to 0.38% at a concentration of 4 mg ml–1 with 4 h treatment, while 0.4 mg ml–1 colchicine treatment could induce 0.57% cells with micronuclei. There was also increased percentage of micronuclei for the 16 h recovery samples with increasing concentrations of LAECV, compared to 4 h treated samples. At 8 mg ml–1 of LAECV treatment MN frequency decreased to 0.26% while at a concentration of 4 mg ml–1 could induce the highest MN frequency (3.85%).
3.8. Effect of LAECV and colchicine on mitotic index and mitotic phase frequency
Data indicate mitotic index percentage in root apical meristem cells varies with the growing period. In the case of untreated root apical meristem cells, mitotic index percentage was recorded as 12.13 ± 0.96 at 4 h (52 h of culture set) and 16.12 ± 0.23% at 20 h (68 h). Dose-dependent mitotic index reduced in LAECV treated (1–4 mg ml–1) samples at 4 h and also in 16 h recovery samples. Four-hour colchicine (0.4 mg ml–1) treatment reduced mitotic index 20.3% while LAECV (4 mg ml–1) could reduce mitotic index 42.0%. This mitotic index reducing tendency was also maintained in the post treated recovery samples. Both colchicine and LAECV treatment could significantly decrease the mitotic phase frequency, except for the metaphase frequency which instead increased with the increasing concentrations of LAECV. Similar patterns of mitotic phase frequency modulating effects of colchicine and LAECV were also maintained in 16 h recovery samples (Table ).
Table 2. Effects of LAECV and colchicine on the mitotic index and mitotic phase frequency in A. cepa root apical meristem cells.
4. Discussion
Colchicine, a toxic secondary metabolite, is used as a drug for the treatment of gout and also as an anti-inflammatory agent for long-term treatment of Behcet’s disease. It works as a spindle poison by binding to tubulin, and inhibits microtubule polymerization (Salmon et al. Citation1984; Cocco et al. Citation2010; Van et al. Citation2014). C. viscosum is a widely used medicinal plant in India (Prajapati et al. Citation2002; Bhattacharya Citation2004; Sajem and Gosai Citation2006). Here, cyto-genotoxic effects of colchicine are studied and compared with the aqueous leaf extract of C. viscosum (LAECV). The cyto-genotoxic effects of colchicine and LAECV were evaluated by analyzing mitotic abnormalitities like c-metaphases, anaphase bridges, chromosomal stickiness, vagrant chromosomes, micronuclei, mitotic index and mitotic phase frequency in A. cepa root apical meristem cells, which are commonly used as a plant model in toxicity tests that contribute to better understanding of the harmful effects of ethno-phyto-medicines on living organisms. The used concentrations of LAECV and colchicine caused a significant increase in the frequency of defects in the mitotic phases of A. cepa root apical meristem cells. Chromosomal aberrations can be considered as an effective test to investigate the cyto-genotoxic effects of the applied substances (Carita and Marin-Morales Citation2008). Stickiness of chromosomes may be due to increased chromosome condensation and contraction or nucleoproteins dissolution and DNA depolymerization (Merykutty and Stephen Citation1980; Ford and Correll Citation1992; Babich, Segall and Fox Citation1997). Similarly, anaphase bridges are probably formed during breakage and fusion of chromatids and chromosomes, suggesting that colchicine and the constituents of LAECV may have a clastogenic effect (Permjit and Grover Citation1985; Burke Citation2000). Stickiness and anaphase bridges have irreversible and toxic consequences and may be associated with cell death (Fiskesjö and Levan Citation1993). The formation of spindles was inhibited with colchicine and LAECV applications (Badr Citation1983; Ray et al. Citation2013). The onset of c-metaphase is related to spindle poison and shows a turbogenic influence (Shahin and El-Amoodi Citation1991; Özmen Citation2010).Vagrant chromosomes occur due to the inhibition or failure of spindle fiber formation and these reversible mitotic abnormalities are established as low genotoxic influences (Fiskesjö Citation1985, Citation1993). Colchicine treatment of apical meristems leads to growth inhibition and swelling, chromosome condensation, increased frequency of metaphase with haphazardly arranged condensed chromosomes and reduced rate of transition from metaphase to anaphase (Ray et al. Citation2013). The LAECV induced root growth retardation effect was analyzed earlier using green gram seedlings, and compared with colchicine (0.025 to 1 mg ml–1) induced root growth retardation and for cell cycle kinetics. Moreover, metaphase arresting activity of LAECV and colchicine was analyzed using wheat and onion root apical meristem cells (Ray et al. Citation2013).
Here, the frequency of apical meristem cells with micronuclei increased with the increasing concentrations of LAECV and the colchicine treatment as compared to the untreated control. In the present investigation, data indicate there is a positive correlation between the increasing mitotic abnormality percentage and the micronucleus frequency in almost all of the treated LAECV concentrations when compared with the untreated control. Thus micronuclei analysis plays an important role in testing the possible genotoxic potentials of a substance and its quantitative assessment is used as indicator for the chromosomal abnormalities (Gebel et al. Citation1997).
The % MI reducing effects of the various concentrations of LAECV on the A. cepa root tip cells are given in Table . A MI reduction below 50% possesses sublethal influence and is referred to as the cytotoxic limit value (Sharma Citation1983; Panda and Sahu Citation1985). In this study, the highest sublethal influence (46.92%) was determined in the 8 mg ml–1 LAECV concentration. With colchicine (0.4 mg ml–1) treatment for 4 h, followed by a 16 h recovery phase, the mitotic index reduced 40.38% in comparison to the negative control. These findings are well matched with the outcomes obtained by previous studies (Merykutty and Stephen Citation1980; Schulze and Kirschner Citation1986; Çelik and Aslantürk Citation2006; Akinboro and Bakare Citation2007; Celik and Aslantürk Citation2009; Al-Zubairi et al. Citation2010; Uckan and Sak Citation2010; Kayraldız et al. Citation2010, Ping et al. Citation2012, Ilbas et al. Citation2012; Ray et al. Citation2013). This reduction in MI% may be due to a variety of reasons, e.g.cell cycle arrest or delay, slow progression of cells from the S phase to the M phase, the inhibition of DNA synthesis; preventing the cells from entering in mitosis or metaphase arrest due to spindle disruption and it may be attributed as the antiproliferative and apoptotic death of cells which are interpreted as direct toxic effects of LAECV. The mitotic index is considered as an indicator of cell cycle progression, and determines the frequencies of the cells in the various mitotic phases (Ping et al. Citation2012; Ray et al. Citation2013). MI observations in plant test systems are excellent monitoring tests for detecting environmental chemicals that cause risk to genetic material during mitosis and meiosis. Studies using the A. cepa assay have shown correlation with in vivo cytogenetic studies in mammalian systems (Chauhan et al. Citation1999; Vicentini et al. Citation2001; Ray et al. Citation2013).The effects of the different concentrations of the LAECV on the mitotic phase frequencies of the root cells of A. cepa are given in Table . The different mitotic phase frequencies were compared with the untreated control and the differences were found to be statistically significant (p < 0.05). In all the treated samples, metaphase cell frequency increased and the other mitotic phase frequencies decreased, which explains anti proliferative and metaphase arresting activities of colchicine and LAECV (Ray et al. Citation2013). The medicinal properties of plants have been claimed to lie in their chemical ingredients which can produce specific action on the human physiology (Sato et al. Citation1994; Nepka et al. Citation1999; Phan et al. Citation2001; Shoeb Citation2006; Liby et al. Citation2007; Ovesna et al. Citation2007). Anti-neoplastic and anticancer agents exert their effect through cell cycle progression machinery (Li et al. Citation2002). These results indicate that LAECV may contain bio-active compound(s) that interact with the mitotic apparatus. Previous study indicated the presence of bioactive compounds including carbohydrate, glycosides, terpenoids, saponins, tannins, triterpenoids and trace amounts of alkaloids in LAECV (Haque et al. Citation2010; Ray et al. Citation2012).
5. Conclusion
Although colchicine and LAECV have therapeutic potential, they showed similar cyto-genotoxic effects, namely increased mitotic abnormality and micronucleus frequency. Therefore, indiscriminate use of colchicine drugs and LAECV in traditional medicine should be avoided, and they should be recommended for medication with caution.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors gratefully acknowledge the financial support of UGC MRP [University Grant Commission Major Research Project] [F.No.42-563/2013 (SR) dt. 22.3.13], UGC-DRS [Departmental Research Support] and infrastructural supports of the Department of Zoology (DST [Department of Science & Technology]-FIST [Fund for Improvement of S&T Infrastructure in Universities and Higher Educational Institutions] and UGC-DRS Sponsored Department), The University of Burdwan, Burdwan, West Bengal, India.
References
- Akinboro A, Bakare AA. 2007. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants. J Ethnopharmacol. 112(3):470–475.10.1016/j.jep.2007.04.014
- Alabed S, Cabello JB, Irving GJ, Qintar M, Burls A. 2014. Colchicine for pericarditis. Cochrane Database Syst Rev. doi:http://dx.doi.org/10.1002/14651858.CD010652.pub2.
- Al-Zubairi AS, Abdul AB, Yousif M, Abdelwahab SI, Elhassan MM, Mohan S. 2010. In vivo and in vitro genotoxic effects of Zerumbone. Caryologia. 63:11–17.10.1080/00087114.2010.10589704
- Babich H, Segall MA, Fox KD. 1997. The Allium test— a simple, eukaryote genotoxicity assay. Am Biol Teacher. 59(9):580–583.10.2307/4450386
- Badr A. 1983. Mitodepressive and chromotoxic activities of two herbicides in A. cepa. Cytologia. 48:451–457.10.1508/cytologia.48.451
- Bhattacharya S. 2004. Chironjib Bonoushodhi. Vol. III. Kolkata, India: Ananda Publisher. p. 38–43.
- Burke DJ. 2000. Complexity in the spindle checkpoint. Curr Opin Genet Dev. 10:26–31.10.1016/S0959-437X(99)00040-4
- Cansaran-Duman D, Aras S, Atakol O, Atasoy I. 2012. Accumulation of trace elements and the.assessment of the genotoxicity in the Lichen Pseudevernia furfuracea transplanted to a polluted site in Ankara. Ekoloji. 85:1–14.10.5053/ekoloji
- Carita R, Marin-Morales MA. 2008. Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere. 72:722–725.10.1016/j.chemosphere.2008.03.056
- Çelik TA, Aslantürk ÖS. 2006. Anti-mitotic and anti-genotoxic effects of Plantago lanceolata aqueous extract on Allium cepa root tip meristem cells. Biologia. 61(6):693–697.
- Çelik TA, Aslantürk ÖS. 2007. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia. 62(3):292–296.
- Çelik TA, Aslantürk ÖS. 2009. Investigation of cytotoxic and genotoxic effects of Ecballium elaterium juice based on Allium test. Methods Find Exp Clin Pharmacol. 31(9):591–596.
- Çelik TA, Aslantürk ÖS. 2010. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J Biomed Biotechnol. 2010:1–8.
- Chaudhuri A, Ray S. 2014. Evaluation of phytotoxic and cytogenotoxic potentials of leaf aqueous extract of Ampelocissus latifolia (Roxb.) planch. in relation to its total polyphenol content. Int J Pharm Bio Sci. 5(4):225–235.
- Chaudhuri A, Ray S. 2015. Antiproliferative activity of phytochemicals present in aerial parts aqueous extract of Ampelocissus latifolia (Roxb.) planch. on apical meristem cells. Int J Pharm Bio Sci. 6(2):99–108.
- Chauhan LKS, Saxena PM, Gupta SK. 1999. Cytogenetics effects of cypermethrin and fenvalerate on the root meristem cells of a. cepa. Environ Exp Bot. 42:181–189.10.1016/S0098-8472(99)00033-7
- Cocco G, Chu DCC, Pandolfi S. 2010. Colchicine in clinical medicine. A guide for internists. Euro J Intern Med. 21:503–508.
- Das S, Bhattacharya S, Prasanna A, Suresh Kumar RB, Pramanik G, Haldar PK. 2011. Preclinical evaluation of antihyperglycemic activity of Clerodendron infortunatum leaf against streptozotocin-induced diabetic rats. Diabetes Ther. 2(2):1–9.
- Fiskesjö G. 1985. The Allium tests as a standard in environmental monitoring. Hereditas. 102:99–112.
- Fiskesjö G. 1993. A 2-3 day plant test for toxicity assessment by measuring the mean root growth of onion (Allium cepa L.). Environ Toxicol Water Qual. 8(4):461–470.
- Fiskesjö G, Levan A. 1993. Evaluation of the first ten MEIC chemicals in the Allium-test. Alta. 2:139–149.
- Ford JH, Correll AT. 1992. Chromosome errors at mitotic anaphase. Genome. 35:702–705.10.1139/g92-107
- Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H. 1997. In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch Toxicol. 71:193–197.10.1007/s002040050375
- Grant WF. 1994. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 310:175–185.10.1016/0027-5107(94)90112-0
- Haque MZ, Rouf MA, Jalil MA, Islam BM, Islam MR. 2010. Screening of phytochemical and biological potential of Clerodendron viscosum leaves extracts. Bangladesh J Sci Ind Res. 45:381–386.
- Ilbas A, Gönen U, Yılmaz S, Dadandı MY. 2012. Cytotoxicity of Aloe vera gel extracts on Allium cepa root tip cells. Turk J Bot. 36:263–268.
- Kaymak F. 2005. Cytogenetic effects of maleic hydrazide on Helianthus annuus L. Pak J Biol Sci. 8(1):104–108.
- Kayraldız A, Yavuz Kocaman A, Rencüzogullari E, Istifli ES, Ila HB, Topaktas M, Daglioglu YK. 2010. The genotoxic and antigenotoxic effects of Aloe vera leaf extract in vivo and in vitro. Turk J Biol. 34:235–246.
- Khadra A, Pinelli E, Lacroix MZ, Bousquet-Melou A, Hamdi H, Merlina G, Guiresse M, Hafidi M. 2012. Assessment of the genotoxicity of quinolone and fluoroquinolones contaminated soil with the Vicia faba micronucleus test. Ecotoxicol Environ Saf. 76(2):187–192.10.1016/j.ecoenv.2011.10.012
- Khatry N, Kundu J, Bachar SC, Uddin MN, Kundu JK. 2006. Studies on antinociceptive, antiinflammatory and diuretic activities of methanol extract of the aerial parts of Clerodendron viscosum Vent. Dhaka Univ J Pharm Sci. 5:63–66.
- Li Y, Shan F, Wu JM, Wu GS, Ding J, Xiao D. 2002. Novel antitumor artemisinin derivatives targeting G1 phase of the cell cycle. Bioorg Med Chem Lett. 11:5–8.
- Liby KT, Yore MM, Sporn MB. 2007. Triterpenoids and retinoid as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 7:357–369.10.1038/nrc2129
- Merykutty VC, Stephen J. 1980. Adriamycin induced genetic toxicity as demonstrated by Allium cepa test. Cytologia. 45:769–777.10.1508/cytologia.45.769
- Nepka CH, Asprodini E, Kouretas D. 1999. Tannins, xenobiotic metabolism and cancer chemoprevention in experimental animals. Eur J Drug Metab Ph. 24:183–189.10.1007/BF03190367
- Ovesna Z, Vachalkova A, Horvathova K, Tothova D. 2007. Pentacyclic triterpenoic acids: new chemoprotective compounds. Minireview Neoplasma. 51:327–333.
- Özmen A. 2010. Cytotoxicity of Hibiscus rosa-sinensis flower extract. Caryologia. 63(2):157–161.
- Panda PC, Das P. 1999. Medicinal plant-lore of the tribals of Baliguda sub-division, Phulbani district. Orissa J Econ Taxon Bot. 23(2):515–521.
- Panda BB, Sahu UK. 1985. Induction of abnormal spindle function and cytokinesis inhibition in mitoticcells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 167:147–155.
- Parekh J, Nair R, Chanda S. 2005. Preliminary screening of some folkloric plants from Western India for potential antimicrobial activity. Ind J Pharmacol. 37:408–409.
- Permjit K, Grover IS. 1985. Cytological effects of some organophosphorus pesticides. meiotic effects. Cytologia. 50:199–211.
- Phan TT, Wang L, See P, Grayer RJ, Chan SY, Lee ST. 2001. Phenolic compounds of Chromolaena odorata protect cultured skin cells from oxidative damage: implication for cutaneous wound healing. Biol Pharm Bull. 24:1373–1379.10.1248/bpb.24.1373
- Ping KY, Darah I, Yusuf UK, Yeng C, Sasidharan S. 2012. Genotoxicity of Euphorbia hirta: an Allium cepa assay. Molecules. 17:7782–7791.10.3390/molecules17077782
- Porter S, Scully C. 2005. Aphthous Ulcers (recurrent). Clin Evid. 13:1687–1694.
- Prajapati ND, Purohit SS, Sharma AK, Kumar TA. 2002. Hand book of medicinal plants: a complete source book, section II. Jodhpur, India: Agrobios (India) Publishers. p. 154.
- Puéchal X, Terrier B, Mouthon L, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C. 2014. Relapsing polychondritis. Joint Bone Spine. 81(2):118–124.
- Ray S, Kundu LM, Goswami S, Chakrabarti CS. 2012. Antiproliferative and apoptosis inducing activity of allelochemicals present in leaf aqueous extract of traditionally used antitumor medicinal plant, Clerodendrum viscosum vent. IJPRD. 4(6):33–34.
- Ray S, Kundu LM, Goswami S, Roy GC, Chatterjee S, Dutta S, Chaudhuri A, Chakrabarti CS. 2013. Metaphase arrest and delay in cell cycle kinetics of root apical meristems and mouse bone marrow cells treated with leaf aqueous extracts of Clerodendrum viscosum Vent. Cell Prolif. 46:109–117.10.1111/cpr.12011
- Sajem AL, Gosai K. 2006. Traditional use of medicinal plants by the Jaintia Tribes in North Cachar Hills district of Assam, Northeast India. J Ethnobiol Ethnomed. 2:33. doi:http://dx.doi.org/10.1186/1746-4269-2-33.
- Salmon ED, Mickseel M, Hays T. 1984. Rapid rate of tubulin dissociation from microtubule in the mitotic spindle in vivo measured by blocking polymerization with colchicine. J Cell Biol. 99:1066–1075.10.1083/jcb.99.3.1066
- Sato KM, Mochizuki I, Saiki YC, Yoo K, Samukawa I, Azuma I. 1994. Inhibition of tumor angiogenesis and metastasis by a saponins of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 17:635–639.10.1248/bpb.17.635
- Schimmer O, Kruger A, Paulini H, Haefele F. 1994. An evaluation of 55 commercial plant extracts in the Ames mutagenicity test. Pharmazie. 49(6):448–451.
- Schulze E, Kirschner M0. 1986. Microtubule dynamics in interphase cells. J Cell Biol. 102(3):1020–1031.10.1083/jcb.102.3.1020
- Shahin SA, El-Amoodi KHH. 1991. Induction of numerical chromosomal aberrations during DNA synthesis using the fungicides nimrod and rumigan-4 in root tips of Vicia faba L. Mutat Res. 261:169–176.10.1016/0165-1218(91)90064-S
- Sharma CBSR. 1983. Plant meristems as monitors of genetic toxicity of environmental chemicals. Current Science India. 52:1000–1002.
- Sharma AK, Sharma A. 1999. Plant chromosomes: analysis, manipulation and engineering. Amsterdam, Netherlands: Harwood Academic Publishers.
- Shoeb M. 2006. Anticancer agents from medicinal plants. Bangladesh J Pharmacol. 1:35–41.
- Siddiqui ZS. 2007. Allelopathic effects of black pepper leaching on Vigna mungo (L.). Hepper Acta Physiol Plant. 29:303–308.10.1007/s11738-007-0039-0
- Singh P, Srivastava AK, Singh AK. 2008. Cell cycle stagespecific application of cypermethrin and carbendazim to assess the genotoxicity in somatic cells of Hordeum vulgare L. Bull Environ Contam Toxicol. 81:258–261.10.1007/s00128-008-9469-7
- Soetan KO, Aiyelaagbe OO. 2009. The need for bioactivity-safety evaluation and conservation of medicinal plants: a review. J Med Plants Res. 3:324–328.
- Uckan F, Sak O. 2010. Cytotoxic effect of cypermethrin on Pimpla turionellae (Hymenoptera: Ichneumonidae) Larval Hemocytes. Ekoloji. 75:20–26.10.5053/ekoloji
- Van Echteld I, Wechalekar MD, Schlesinger N, Buchbinder R, Aletaha D. 2014. Colchicine for acute gout. Cochrane Database Syst Rev. 8.
- Verne GN, Davis RH, Robinson ME, Gordon JM, Eaker EY, Sninksy CA. 2003. Treatment of chronic constipation with colchicine: randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 98(5):1112–1116.
- Vicentini VEP, Camparoto ML, Teixeira RO, Mantovani MS. 2001. Averrhoa carambola L, Syzygium cumini (L.) Skeels and Cissus sicyoides L.: medicinal herbal tea effects on vegetal and test systems. Acta Scientiarum. 23:593–598.
- Yildiz A, Aksoy A, Akbulut G, Demirezen D, Islek C, Altuner EM, Duman F. 2011. Correlation between chlorophyll degradation and the amount of heavy metals found in Pseudevernia furfuracea in Kayseri (Turkey). Ekoloji. 78:82–88.10.5053/ekoloji
