Abstract
Phenylboronic acids and their conjugates as well as methods for their preparation and use have been the subject of several US patents. A novel phenylboronic acid derivative, 2-(bromoacetamido) phenylboronic acid (2-BAPBA), which may be useful in treatment of the human or animal diseases, has recently been prepared. Questions that need to be answered include: whether 2-BAPBA has any potential cytogenotoxic effects; and if so, whether they are commensurate with a reasonable risk/benefit ratio for its intended use. In this study, a well-established in vivo assessment bioassay, the Allium cepa test, was used to examine the effect of a dimethyl sulfoxide (DMSO) solution of 2-BAPBA at doses equivalent to 1/4, 1/2, 1 EC50 (half maximal effective concentration) and 2 EC50 (2.23, 4.45, 8.90, or 17.8 mg l–1, respectively) for 24 or 48 h. The EC50 of 2-BAPBA was 8.9 mg l–1. The compound was found to be cytotoxic, causing statistically significant reductions in root growth and mitotic index in a dose- and time-dependent manner. Furthermore, 2-BAPBA induced significant increases in the percentage of aberrant cells (% Abc) at all concentrations tested. It induced chromosome and nuclear aberrations, as well as morphological alterations in shape and size of cells, spindle disturbance and polar deviation in root tip meristem cells of Allium cepa. Under the present experimental conditions, 2-BAPBA is cytotoxic and genotoxic in onion root tips. The described work is the first of its type. To reach more definitive conclusions about this subject, further studies should be performed with different test systems.
Introduction
Continuous efforts are being made to identify new pharmaceutically acceptable and potentially useful anti-cancer, anti-inflammatory, and anti-microbial agents. Because of their unique structural features, numerous boronic acid compounds and their ester derivatives have been reported for the treatment of various diseases, including cancer (Hall et al. Citation2012; Wang et al. Citation2013; Dunetz et al. Citation2016). Epidemiological, in vitro, and animal studies revealed a possible role for boric acid (BA), the most abundant physiological form of boron in the plasma, as a chemotherapeutic agent (Gallardo-Williams et al. Citation2004; Barranco and Eckhert Citation2006). In a nude mouse model of prostate cancer, BA decreases tumor size, insulin-like growth factor 1 (IGF-1) serum levels and prostate specific antigen (PSA) levels and proteolytic activity (Gallardo-Williams et al. Citation2004). Boric acid at 1 mM significantly decreased migration and proliferation of the prostate cancer cell line DU-145 in vitro (Barranco and Eckhert Citation2006). Bortezomib (Velcade®, PS-341) is a boronic acid-based thrombin inhibitor that was the first FDA-approved boronic acid in 2008 as a single agent for the treatment of multiple myeloma (Al-Zoubi Citation2011). Phenylboronic acid (PBA) was a more potent inhibitor than BA of key signaling networks involved in prostate and breast cancer cell migration (Bradke et al. Citation2008; McAuley et al. Citation2011), suggesting that these compounds target actomyosin-based contractility of metastatic and proliferative properties of cancer cells. Furthermore, AN-2690 is a fluoroarene boroxole-based antifungal agent developed specifically for the treatment of onychomycosis, a common disorder of nails caused by diverse spectrum of fungi ranging from dermatophytes to nondermatophyte molds and yeasts (Gupta and Simpson Citation2014). In addition, 3-amino-PBA (APBA) modified graphene oxide (GO) has been applied for the electrochemical monitoring of glycated hemoglobin (HbA1c) level in blood and controlled insulin release (Zhang et al. Citation2013; Pundir and Chawla Citation2014). Tests using boronic acid compounds, mainly 3-APBA, with disks of cefotetan or cefoxitin were found to be successful for detecting AmpC enzymes in organisms that classically did not produce these enzymes (Doi and Paterson Citation2007). More recently, Ji et al. (Citation2016) conjugated PBA onto low molecular weight polyethylenimine to generate a nanovector which facilitates cancer-targeted RNA delivery. These compounds and other boronic acids have initiated significant interest in boron based small molecules in drug discovery.
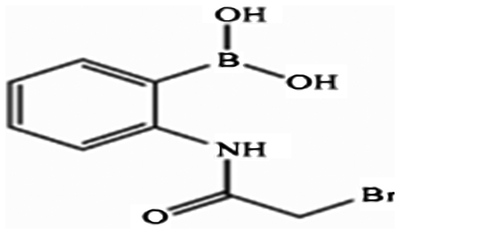
Phenylboronic acid reagents and boronic compound complexing reagents, their conjugates and bioconjugates as well as methods for their preparation and use have been the subject of several US patents. Recently, a novel phenylboronic acid derivative, 2-(bromoacetamido) phenylboronic acid (2-BAPBA; Figure ), which possesses anti-proliferative (e.g. anti-cancer) activity and is accordingly useful in methods of treatment of the human or animal body, has been prepared by Al-Qaoud et al. (Citation2014) (patent number US 8877980 B2).
Most short-term mutagenicity studies (National Toxicology Program Citation1987; Arslan et al. Citation2008; Kahraman et al. Citation2013) indicated that, in mammalian systems, most tested boronic acids have very low or no toxicity compared to other boron organic compounds because the eventual end product in the breakdown of boronic acid-containing compounds is generally boric acid, which has low toxicity to animals and humans. Similarly, in Drosophila, no significant genotoxic effect was detected at all boron concentrations by the somatic mutation and recombination test (SMART) (Sarıkayaa et al. Citation2016). In fact boron was able to abolish the genotoxic effects induced by the ethyl methane sulfonate (Sarıkayaa et al. Citation2016). However, contradictory results have been observed in bacteria, where a number of boronic acids have been tested using Salmonella typhimurium strains TA1535, TA1537, TA98 and TA100 and Escherichia coli strain WP2uvrA(pKM101) (O’Donovan et al. Citation2011). The results with some compounds proved mutagenicity in both TA100 and WP2uvrA (pKM101). 3-Aminophenylboronic acid was reported to be toxic in Swiss albino mice (Qureshi et al. Citation2001) and boronic acid can be toxic for rainbow trout and cause histopathological damage in fish tissue (Ceyhunc and Atamanalp Citation2016).
Despite the recognition of some potential boronic acid-based therapeutics by the FDA (Wang et al. Citation2013; Dunetz et al. Citation2016) medical applications of boron-containing drugs have not achieved success in clinical trials and are still limited due to the lack of a toxicological profile or the conflicting findings (Cambre and Sumerlin Citation2011; Lee et al. Citation2015). The present work was designed to examine the pros and cons of 2-BAPBA by a well-established in vivo assessment bioassay, the Allium cepa Chromosome aberrations (CA) (Fiskesjö Citation1993). The toxicity or adverse effects, if any, associated with this novel drug should be commensurate with a reasonable risk/benefit ratio for its intended use. The compound has been reported to have cytotoxic effects on Chinese hamster ovary cell line as determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Al-Qaoud et al. Citation2014). To the best of our knowledge, the toxicological properties of this substance have not yet been thoroughly investigated.
Materials and methods
Chemicals
2-BAPBA was a generous gift from The Jordan Company for Antibody Production (MONOJ), Al-Jubaiha, Amman, Jordan. Methyl methane sulfonate (MMS; CAS number 67–27-3) and dimethyl sulfoxide (DMSO; CAS number 67–68-5) were purchased from Sigma Aldrich, St. Louis, MO, USA. Colcemid (CAS number 477–30-5) was obtained from Life Technologies/Gibco, Carlsbad, CA, USA. All other chemicals were of analytical grade.
Preparation of 2-BAPBA
Solutions of 2-BAPBA (3.125, 6.25, 12.5, 25, 50 and 100 mg l–1) were prepared by gradually dissolving it to the maximum solubility in 100% DMSO (1 mg ml–1). DMSO at concentrations of 0.3125, 0.625, 1.25, 2.5, 5, and 10% correspond to the different BAPBA concentrations, respectively. DMSO (0.31%) served as negative control, while methyl methane sulfonate (MMS, 10 mg l–1) was used as positive control.
EC50 determination
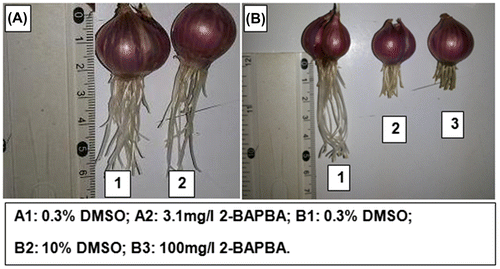
To determine the EC50 of 2-BAPBA, a total of 42 commercial equal-sized Allium cepa onion bulbs of 2–3 g per concentration were used (Figure ). They were carefully unscaled and the old roots were scraped away from the primordial without harming the root ring. The bulbs were placed on top of test tubes containing distilled water at room temperature (RT; ~ 21 ± 4°C), in the dark for 24 h.
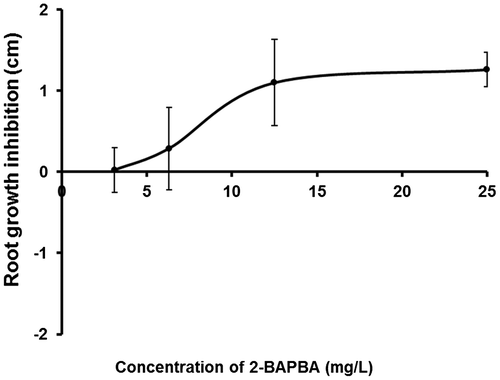
The bulbs were divided into 14 sets, three bulbs in each, with uniform root growth and were chosen for the experiment and treated with different concentrations of 2-BAPBA (3.125, 6.25, 12.5, 25, 50 and 100 mg l–1), or DMSO (0.3125, 0.625, 1.25, 2.5, 5, and 10%) as solvent concentrations for the different 2-BAPBA concentrations, respectively. Tap water was used as sham control and 10 mg ml–1 MMS as positive control. After 96 h exposure, the length of the whole root bundle from control and experimental sets was measured as described by Fiskesjö (Citation1993). Inhibition of root growth resulted from each concentration of 2-BAPBA was calculated after subtracting the effect of DMSO. The highest two concentrations of 2-BAPBA (50 and 100 m ml–1) were considered toxic. To calculate EC50 value, concentration–response (inhibition of root growth) curve (Figure ) was graphed using Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). The EC50 of the curve was calculated by nonlinear regression analysis and interpolation according to the method described previously (Alexander et al. Citation1999) using the following formula:(1)
Figure 3. Concentration–response curve of 2-BAPBA effect on root growth inhibition. Vertical bars shown the standard deviation.
Where y = A – B; A, and B are the actually recorded responses selected on either side of the 50% of the maximum response and are from an approximately straight line; x = D – C; and C and D are the actually recorded concentrations which caused responses A and B, respectively.
Allium cepa test
To perform this test, the method reported before (Khalil et al. Citation2009) was adapted. A cepa bulbs were placed in vials and rooted in tap water until the roots reached 1.5–2.0 cm. Three rooted bulbs per concentration were placed for 24 or 48 h in vials each containing 8 ml of the test chemical dissolved in 0.5% DMSO at concentrations equivalent to 1/4 EC50, 1/2 EC50, EC50, or 2× EC50, i.e. 2.23, 4.45, 8.90, or 17.8 mg l–1, respectively. DMSO (0.31%) was used as solvent control while MMS (10 mg l–1) and tap water served as positive and sham control, respectively.
Before termination of the experiment, the roots of bulbs were treated with 0.05% colcemid for 2.5 h. Then, they were cut and fixed in 3:1 (methanol: glacial acetic acid). The roots were subsequently hydrolyzed in a hydrochloric acid solution (1 N) for 6 min at 60°C, rinsed in distilled water and stained with 2% aceto-carmine stain. Root tips were squashed in 45% acetic acid and examined microscopically. Under blind codes, 500 cells were screened in randomly picked three zones per slide. Two slides were examined per bulb in each group which included three onions, totaling 3000 cells/experiment. The following parameters were observed: (a) Mitotic index (MI); (b) the frequency of cells with CA (bridges, fragments, sticky chromosomes, polar deviation, pulverization and others); and (c) the frequency of micronucleus (MN) formation.
Statistical analysis
All experiments were done in triplicate, and the results were presented as mean ± standard deviation. Data were analyzed using Minitab v.14 (Minitab inc., State College, PA, USA). For the determination of the significance among the means, an independent t-test was conducted (p < 0.01).
Results
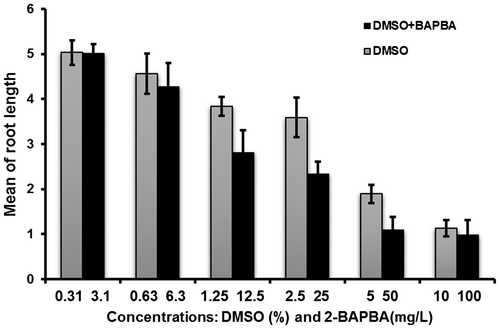
The results from the four-day A. cepa root growth inhibition test of 2-BAPBA and the corresponding controls are shown in Table and Figure . The inhibition of root growth by DMSO was concentration-dependent and with a clear inverse relationship. The inhibition of root growth progressively increased and reached a maximum at 10% DMSO, where the mean of root length was 1.13 ± 0.18 cm. The 2-BAPBA-treated roots showed a similar trend with means of root length of 5.01 ± 0.21 cm and 0.97 ± 0.34 cm at 3.1 and 100 mg l–1, respectively. Maximum inhibition was observed at 25 mg l–1 of 2-BAPBA and the EC50 was 8.9 mg l–1.
Table 1. The inhibition effects of various concentration of 2-(bromoacetamido) phenylboronic acid (2-BAPBA) and dimethyl sulfoxide (DMSO) on root growth of A. cepa.
Figure 4. Effect of different 2-BAPBA concentrations on A. cepa root length compared with concurrent DMSO concentrations.
Exposure of roots inhibited the MI in a concentration-dependent manner when compared to the mitotic index of about 50% in the control group (Table , Figure ). The lowest MI value of 18.2 ± 1.00 was recorded for 2.23 mg l–1 2-BAPBA for 48 h. There were highly significant reductions (p < 0.01) in the MI values between treated groups and control group.
Table 2. Types of chromosome aberrations and % aberrant cells induced by 2-BAPBA in A. cepa at different times and concentrations.
Figure 5. Effect of 2-BAPBA at different times and concentrations on the mitotic index and chromosomal aberrations detected by percentage of aberrant cells of treated roots in A. cepa. Vertical bars show standard deviations.
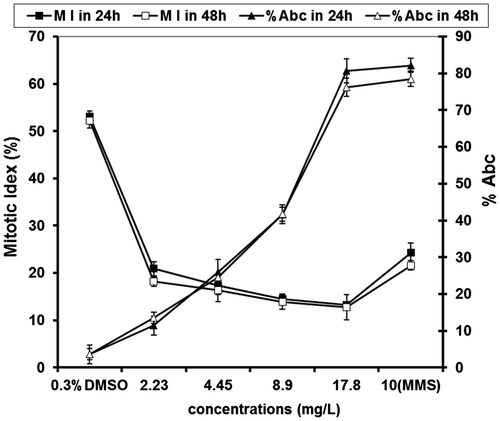
The results regarding the type and frequency of abnormalities in root meristematic cells of A. cepa following treatment with different concentrations of 2-BAPBA are given in Table and Figure . Various types of clastogenic and physiological CA were observed at all stages of cell division. Clastogenic aberrations include chromosome bridge(s) and chromosomal break(s), whereas physiological aberrations include c-mitosis, vagrant(s), stickiness, and laggard(s). In the positive control and at high concentration of 2-BAPBA (17.8 mg l–1), sticky chromosomes and micronuclei followed by c-mitosis and fragments, respectively, were the most common chromosome aberrations observed. In DMSO root tip samples, percentage of aberrations cells was low; 2.8 ± 2.00 compared to highest concentration of BAPBA, which scored 62.7 ± 2.52% and 76.2 ± 2.52% at 24 and 48 h, respectively. These increases were dose- and time-dependent.
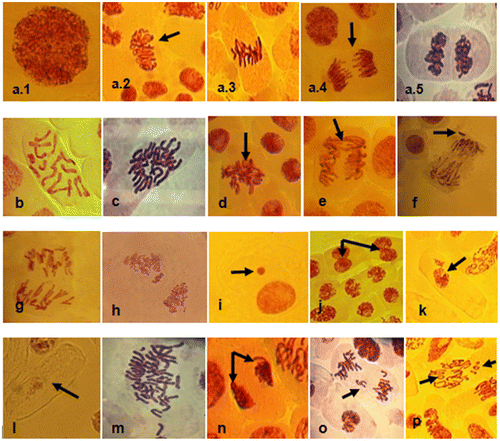
Figure 6. Photomicrographs of squash preparations of root tips of Allium cepa stained with aceto-carmine showing different types of chromosomal aberrations observed in meristematic cells exposed to 2-BAPBA. (a) Normal stages of cell division: (a.1) interphase; (a.2) prophase; (a.3) metaphase; (a.4) anaphase; (a.5) telophase. (b) Normal cell (2n = 16). (c) C-mitosis (2n = 32). (d) Sticky chromosomes. (e) Anaphase bridge. (f) Vagrant chromosome. (g) Multipolar anaphase. (h) Pulverized chromosomes. (i) Micronucleated cell. (j) Binucleated cells. (k) Damaged nucleus. (l) Ghost cell. (m) Polyploid cell. (n) Polar deviation. (o) Anaphase lagging chromosome. (p) Ring chromosomes. Magnification 400×.
Discussion
The Allium cepa roots test is an important in vivo test: the roots grow in direct contact with a substance of interest, enabling possible damage to human DNA to be predicted. The test has many advantages as genotoxicity screening assay, one being that root cells of A. cepa possess a mixed function oxidase system which is capable of activating promutagens or genotoxic chemicals (Namita and Sonia Citation2013). Furthermore, the effects of specified concentrations of a test chemical on MI and CA are used as parameters of cytotoxicity and genotoxicity, respectively (Nefic et al. Citation2013).
The study described here represents the first attempt to investigate the biological activity of 2-BAPBA in A. cepa. Since 2-BAPBA has only recently been synthesized and patented (Ji et al. Citation2016), there are no published data on cytotoxicity and genotoxicity of this compound for comparison. The toxicity of chemicals is commonly expressed in terms of the dosage which gives 50% effect (EC50) to the response compared to the control; the effect can be either an increase or a decrease in response; this was shown to be a useful parameter for choosing the test concentrations for genotoxicity tests (Seth et al. Citation2007). The EC50 of 2-BAPBA was 8.9 mg l–1. A decrease in root growth over 45% is used as an indicator of toxicity. At this level, a substance has sublethal effects on plants (Fiskesjö Citation1993). The variation in root elongation is very useful as a parameter for assessing the cytotoxicity. This inhibition is generally related to cell elongation during the differentiation, apical meristematic activity (Fusconi et al. Citation2007; Mustafa and Arikan Citation2008) and/or the inhibition of protein synthesis (Seth et al. Citation2007).
MI is a quantitative estimation of the mitotic activities in an organism or a particular organ. This study observed a reduction of MI in meristematic cells of A. cepa root tips with increasing concentrations of 2-BAPBA, as well as inhibition of the root growth and the appearance of stunted roots. This could be interpreted as cytotoxic effect and cellular death (Ping et al. Citation2012) induced by 2-BAPBA. The reduction of the MI might be explained as being due to: the effect on microtubule configuration; the obstruction of the onset of prophase; the blocking of one or more mitotic phases; the slowing of the rate of cell progression through mitosis (Christopher and Kapoor Citation1988); the inhibition of certain cell cycle specific proteins which inhibit DNA polymerase and other enzymes (Hidalgo et al. Citation1989); or a blocking in the G2 phase of the cell cycle (Sudhakar et al. Citation2001; Türkoğlu Citation2012). This is explained by the anti-mitotic effect hence, i.e. due to impaired nucleoprotein synthesis and reduced level of ATP to provide energy to nuclear protein synthesis in the cell cycle, spindle elongation, microtubule dynamics and chromosome movement (Sudhakar et al. Citation2001; Türkoğlu Citation2012).
Chromosome aberrations are changes in chromosome structure resulting from a break or exchange of chromosomal material. Analysis of CA not only allowed estimation of genotoxic effects of chemicals in cells, but also enabled evaluation of their clastogenic and aneugenic actions (Fiskesjö Citation1993; Leme and Marin-Morales Citation2009; Akinboro et al. Citation2011). When the total aberrations observed are considered, the percentage of Abc decreases in 2-BAPBA treatment groups compared with positive control group.
The occurrence of the different CA types, in all phases of the cell cycle, enables a better investigation of the clastogenic, aneugenic and tubergenic effects of 2-BAPBA. Most CA observed in the meristem cells of A. cepa are lethal, but there are many corresponding aberrations that are viable and can cause genetic effects (Akinboro et al. Citation2011). Breaks may occur and subsequent inhibition of repair mechanisms may lead to base mismatch, mutation and CA such as fragmented chromosomes and DNA breaks (Türkoğlu Citation2012). Anaphase chromosome fragments and bridges are useful for obtaining information on clastogenic activity, whereas vagrant chromosomes and c-metaphases increase the risk for aneuploidy (Leme and Marin-Morales Citation2009; Nefic et al. Citation2013). The presence of fragmented nuclei and polynucleated cells can indicate a cell death process and this may lead to aneuploidy and then to cell death (Nefic et al. Citation2013). The presence of dicentric chromosomes and unequally exchanged chromatids undergoing translocation has been reported to be responsible for chromosomal bridges at anaphase. Stickiness of chromosomes, which is the most frequent aberration showed in this study, was interpreted as a result of depolymerization of DNA, partial dissolution of nucleoproteins, breakage and exchanges of the basic folded fiber units of chromatids and the stripping of the protein covering of DNA in chromosomes (Mercykutty and Stephen Citation1980). However, stickiness may be due to defective functioning of one or two types of specific nonhistone proteins involving chromosome organization that makes the chromatids connected by means of subchromatid bridges (Türkoğlu Citation2012). It may be also occurring through immediate reactions with DNA during its inhibition periods, causing DNA–DNA or DNA–protein cross linking. Sticky chromosomes indicated a highly toxic, irreversible effect, probably leading to cell death (Fiskesjö Citation1993). A toxicant may affect DNA and consequently affect telomeres which protect chromatids and chromosomes from sticking together. Thus, chromosomes aggregate together in a sticky mass as recently reported (Abdel-Rahman et al. Citation2015).
The induction of micronuclei in root meristem cells of A. cepa is the manifestation of chromosome breakage (clastogenic agent) or whole chromosomes (aneugenic agent) that were not incorporated to the main nucleus during the cell division cycle (Leme and Marin-Morales Citation2009; Fenech et al. Citation2011). Inhibition of cytokinesis following telophase is responsible for binucleated cell formation (Fenech et al. Citation2011). Nuclear lesions are genotoxic analogs of MN that may also be the result of the action of a genotoxic agent (Osman Citation2014). Such abnormal cell division would result in genetic imbalance of the cells, and may also be involved in carcinogenesis. The chromosome abnormalities could be due to the blockage of DNA synthesis or inhibition of spindle formation. The test chemical may even not allow the initiation of their biosynthesis (Akinboro et al. Citation2011).
In conclusion, under the present experimental conditions, 2-BAPBA is cytotoxic and genotoxic in onion root tips. Due to the diversity of the genetic endpoints, it is clear that the potential genotoxicity and/or mutagenicity of a compound cannot be assessed by a single assay system. The results are preliminary and further experiments are needed to support them. Therefore, to obtain more information and to reach more definitive conclusions about this subject, further research should be performed with different test systems. Some of these activities are being done in our laboratory.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Abdel-Rahman HHM, Abdel Migid HM, Attia SA, Rizkalla AA. 2015. Cytogenetical, biochemical and chemical analytical studies for assessment of the water quality of Nile water using three bioassays. Middle East J Appl Sci. 5(5):112–124.
- Akinboro A, Mohamed KB, Asmawi MZ, Sulaiman SF, Sofiman OA. 2011. Antioxidants in aqueous extract of Myristica fragrans (Houtt.) suppress mitosis and cyclophosphamide-induced chromosomal aberrations in Allium cepa L. cells. J Zhejiang Univ Sci B. 12(11):915–922. 10.5897/AJB
- Alexander B, Browse DJ, Reading SJ, Benjamin IS. 1999. A simple and accurate mathematical method for calculation of the EC50. J Pharmacol Toxicol Methods. 41:55–58. 10.1016/S1056-8719(98)00038-0
- Al-Qaoud KM, Shihab PA, Abu-Qatouseh LF, Lowe CR, Rawashdeh AM, Alkhayyat YA, Ratrout SS, Naser SM 2014. Phenylboronic acid. Patent N0. : US 8,877,980 B2. 2014.
- Al-Zoubi RM. 2011. Mild, green and catalytic: Ortho-iodoarylboronic acids for direct amide bond formation at room temperature [ Ph. D. Thesis]. University of Alberta: Edmonton, Alberta.
- Arslan M, Topaktas M, Rencuzogullari E. 2008. The effects of boric acid on sister chromatid exchanges and chromosome aberrations in cultured human lymphocytes. Cytotechnology. 56:91–96. 10.1007/s10616-007-9094-z
- Barranco WT, Eckhert CD. 2006. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer. 94:884–890. 10.1038/sj.bjc.6603009
- Bradke TM, Hall C, Carper SW, Plopper GE. 2008. Phenylboronic acid selectively inhibits human prostate and breast cancer cell migration and decreases viability. Cell Adh Migr. 2:153–160. 10.4161/cam.2.3.6484
- Cambre NJ, Sumerlin SB. 2011. Biomedical applications of boronic acid polymers. Polymer. 52:4631–4643. 10.1016/j.polymer.2011.07.057
- Ceyhunc B, Atamanalp M. 2016. The effects of acute boric acid treatment on gill, kidney and muscle tissues in juvenile rainbow trout. J Appl Anim Res. 44:297–302.
- Christopher HB, Kapoor MB. 1988. The cytogenetic effects of sodium salicylate on the root meristem cells of Allium sativa L. Cytologia. 54:203–209.
- Doi Y, Paterson DL 2007. Detection of plasmid-mediated class C β-lactamases. Int J Infect Dis. 11: 191–197.
- Dunetz JR, Magano J, Weisenburger GA. 2016. Large-Scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org Process Res Dev. 20:140–177. 10.1021/op500305s
- Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmond DA, Tucker JD, Thomas P. 2011. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 26:125–132. 10.1093/mutage/geq052
- Fiskesjö G. 1993. The Allium test in waste-water monitoring. Environ Toxicol Water Qual. 8:291–298. 10.1002/(ISSN)1098-2256
- Fusconi A, Gallo C, Camusso W. 2007. Effects of cadmium on root apical meristem of Pisumsativum L: cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat Res. 632:9–19. 10.1016/j.mrgentox.2007.03.012
- Gallardo-Williams MT, Chapin RE, King PE, Moser GJ, Goldsworthy TL, Morrison JP, Maronpot RR. 2004. Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol. 32:73–78. 10.1080/01926230490260899
- Gupta AK, Simpson FC. 2014. Investigational drugs for onychomycosis. Expert Opin Investig Drugs. 23:97–106. 10.1517/13543784.2013.840289
- Hall DG, Lee JCH, Ding J. 2012. Catalytic enantioselective transformations of borylated substrates: preparation and synthetic applications of chiral alkylboronates. Pure Appl Chem. 84:2263–2277.
- Hidalgo A, Gonzales JA, Navas P, Garcia-Herdugo G. 1989. Abnormal mitosis and growth inhibition in Allium cepa roots induced by propham and chlorpropham. Cytobios. 57:7–17.
- Ji M, Li P, Sheng N, Liu L, Pan H, Wang C, Cai L, Ma Y. 2016. Sialic acid-targeted nanovectors with phenylboronic acid-grafted polyethylenimine robustly enhance siRNA-based cancer therapy. ACS Appl. Mater. Interfaces. 8:9565–9576. 10.1021/acsami.5b11866
- Kahraman E, Gürhan ID, Korkmaz M. 2013. Investigation of possible genotoxic and cytotoxic effects of differential boron compounds in CCL 62 (Hela contaminant) human amniotic epithelial cell line. Med Sci. 2:454–468.
- Khalil A, Maslat A, Hafez A, Mizyed S, Ashram M. 2009. Genotoxicity of different tert-butyl calyx [4] crowns. Zeitschrift für Naturforschung C. 63:167–175.
- Lee DY, Choe K, Jeong YJ, Yoo J, Lee SM, Park JH, Kim P, Kim YC. 2015. Establishment of a controlled insulin delivery system using a glucose-responsive double-layered nanogel. RSC Adv. 5:14482–14491. 10.1039/C4RA16656F
- Leme DM, Marin-Morales MA. 2009. Allium cepa test in environmental monitoring: a review on its application. Mutat Res. 682:71–81. 10.1016/j.mrrev.2009.06.002
- McAuley EM, Bradke TA, Plopper GE. 2011. Phenylboronic acid is a more potent inhibitor than boric acid of key signaling networks involved in cancer cell migration. Cell Adh Migr. 5:382–386. 10.4161/cam.5.5.18162
- Mercykutty VC, Stephen J. 1980. Adriamycin induced genetic toxicity as demonstrated by the Allium test. Cytologia. 45:769–777. 10.1508/cytologia.45.769
- Mustafa Y, Arikan ES. 2008. Genotoxicity testing of quizalofop-P-ethyl herbicide using the Allium cepa anaphase-telophase chromosome aberration assay. Caryologia. 61:45–52. 10.1080/00087114.2008.10589608
- Namita K, Sonia S. 2013. Allium cepa root chromosomal aberration assay: a review. Indian J Pharm Biol Res. 1:105–119.
- National Toxicology Program. 1987. Toxicology and carcinogenesis studies of boric acid. Natl Toxicol Program Tech Rep Ser. 324:1–126.
- Nefic H, Musanovic J, Metovic A, Kurteshi K. 2013. Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by alprazolam. Med Arh. 67:388–392.10.5455/medarh
- O’Donovan MR, Mee CD, Fenner S, Teasdale A, Phillips DH. 2011. Boronic acids—a novel class of bacterial mutagen. Mutat Res. 724:1–6. 10.1016/j.mrgentox.2011.05.006
- Osman AGM. 2014. Genotoxicity tests and their contributions in aquatic environmental research. J Environ Prot. 05:1391–1399. 10.4236/jep.2014.514132
- Ping KY, Darah I, Yusuf UK, Yeng C, Sasidharan S. 2012. Genotoxicity of Euphorbia hirta: an Allium cepa assay. Molecules. 17:7782–7791. 10.3390/molecules17077782
- Pundir CS, Chawla S. 2014. Determination of glycated hemoglobin with special emphasis on biosensing methods. Anal Biochem. 444:47–56. 10.1016/j.ab.2013.09.023
- Qureshi S, Al-Shabanah OA, Al-Harbi MM, Al-Bekairi AM, Raza M. 2001. Boric acid enhances in vivo Ehrlich ascites carcinoma cell proliferation in Swiss albino mice. Toxicology. 165:1–11. 10.1016/S0300-483X(01)00396-1
- Sarıkayaa R, Erciyasb K, Karac MI, Sezer U, Erciyas AF, Ay S. 2016. Evaluation of genotoxic and antigenotoxic effects of boron by the somatic mutation and recombination test (SMART) on Drosophila. Drug Chem Toxicol. 12:1–7.
- Seth CS, Chaturvedi PK, Misra V. 2007. Toxic effect of arsenate and cadmium alone and in combination on giant duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ Toxicol. 22:539–549. 10.1002/(ISSN)1522-7278
- Sudhakar R, Gowda N, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 66:235–239. 10.1508/cytologia.66.235
- Türkoğlu, Ș. 2012. Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pest Biochem Physiol. 103:224–23025.
- Wang X, Xia N, Liu L. 2013. Boronic acid-based approach for separation and immobilization of glycoproteins and its application in sensing. Int J Mol Sci. 14:20890–20912. 10.3390/ijms141020890
- Zhang C, Losego MD, Braun PV. 2013. Hydrogel-based glucose sensors: effects of phenylboronic acid chemical structure on response. Chem Mater. 25:3239–3250. 10.1021/cm401738p