Abstract
Genotoxic effects of a heavy metal, i.e. lead (Pb), on cyto-morphological parameters of chilli were investigated. Seeds were treated with five different concentrations (20, 40, 60, 80, 100 ppm) of this heavy metal. The higher concentrations of lead nitrate significantly reduced plant height, pollen fertility and yield and caused variations in the plant at the seedling and mature stages. In addition, lead affected the pairing of homologous chromosomes and spindle formation: as the concentration of lead increases chromosomal abnormalities also significantly increase but chiasma frequency reduced. On the basis of these results, it was concluded that lower concentrations of metal did not significantly affect the cyto-morphology of Capsicum, while higher concentrations of lead nitrate were found to be more mutagenic and genotoxic.
Introduction
Heavy metal pollution results from natural sources including volcanic eruptions and weathering of rocks, and anthropogenic sources including mining. These activities have significantly increased in the past few decades as a result of the burning of fossil fuels, industrial activities, automobile emissions, use of metal-enriched materials, mining, wastewater irrigation, sewage sludge, pesticide usage, etc. The continuous production and release of chemicals into the environment emphasize the necessity to assess their genotoxicity (Elena et al. Citation2013). Various workers have determined a relationship between heavy metals in natural and industrial environments and an increased frequency of chromosomal mutations and cancerous processes in organisms (Brüning et al. Citation1999). Analysis of the genotoxic potential of a substance through the investigation of the induction of chromosome alterations represents an effective method for biomonitoring studies and for the analysis of the extent of pollution (Harden Citation2001).
Capsicum annuum L. belongs to family Solanaceae, 2n=24 and is used as a pungent flavour in food, a natural plant colour, a pharmaceutical ingredient and as a spray for riot control and self-defence. It is a rich source of vitamins C (ascorbic acid), A and E. It also has various medicinal activities, e.g. for treating cancer, mutagenesis, neurogenic inflammation, high cholestrol, obesity and antioxidant (Morre and Morre Citation2003; Szolcsanyi Citation2004; Kempaiah et al. Citation2005; Sim and Sil Citation2008).
The purpose of the present investigation was to evaluate the influence of Pb concentrations on somatic as well as gametic cells of chilli, since the most pronounced effect of heavy metals on plant development is growth inhibition, which is inseparably connected with cell division.
Material and methods
Certified Capsicum annuum L. seeds were obtained from the Indian Agricultural Research Institute (IARI), New Delhi, India. Fresh, healthy, and uniform Capsicum seeds were pre-soaked in distilled water for 24 h and then treated with five different concentrations (20, 40, 60, 80 and 100 ppm) of lead nitrate (PbNO3)2 solution (pH 4) prepared in phosphate buffer for 24 h. One set of seeds was soaked in distilled water to act as the control. The treated sets of seeds were washed in running tap water to remove the residual metal. Each set of seeds was sown in a pot along with the control to raise the M1 generation. Control seeds germinated after 10 days, whilst lead-treated seeds showed a delay in germination time. For meiotic studies, young flower buds from randomly selected plants at each mutagen dosage level, as well as from the control, were fixed in freshly prepared Carnoy’s fixative (absolute alcohol, chloroform, and acetic acid 6:3:1) for 24 h and preserved in 70% alcohol. Anthers from the collected flower buds were compacted in 1.0% propionocarmine and made permanent through an NBA-GAA series for pollen mother cells (PMCs) and pollen grain analysis.
Statistical analysis
A total of 15 replicates for each treatment were conducted. Statistical analysis of data was done with SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Analysis of variance (ANOVA) was performed on the data to determine the least significant difference (LSD) between treatments; means were set at 5% and 1% significance levels (p < 0.05, 0.01). Photographs were taken from both temporary and permanent slides.
Results
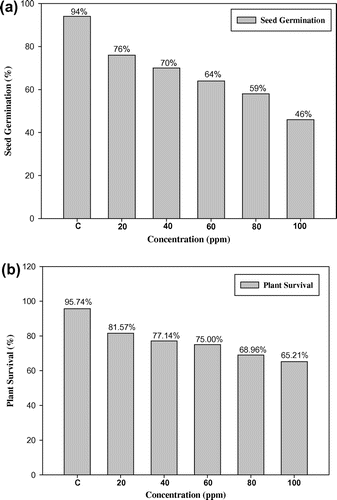
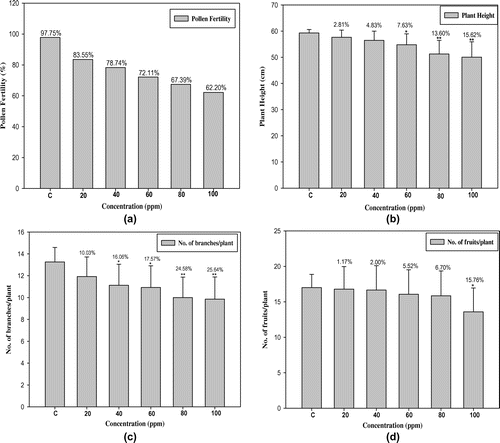
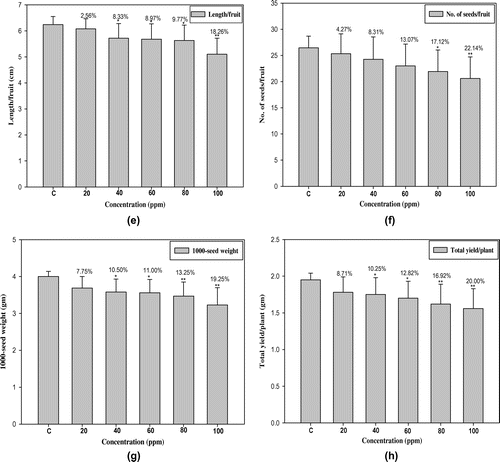
The data recorded for cyto-morphological parameters in Capsicum annuum L. are presented in Figures and Table . The maximum germination and plant survival was recorded in the control group (94.00% and 95.74%) and decreased in treated populations, ranging from 76.00 to 46.00% and 81.57–65.21% in 20 to 100 ppm lead nitrate, respectively (Figure (a) and 1(b)). Dose-dependent decreases in plant height, number of branches per plant, number of fruits per plant, length of fruit, number of seeds per fruit, 1000-seed weight (g) and total yield per plant (g) were also observed, with significant values at 5% and 1% levels (Figure (b–h)). The higher concentration of Pb (100 ppm) shows the maximum reduction percentage up to 15.62% in plant height, 25.64% in number of branches per plant, 15.78% in number of fruits per plant, 18.26% in length of fruit, 22.14% in number of seeds per fruit, 19.25% in1000-seed weight and 20.00% in total yield per plant when compared to control plants (Figure (b–h)). There were dose dependent increases in meiotic anomalies in pollen mother cells of Capsicum annuum L. exposed to different concentrations of Pb(NO3)2 (Table ). Different abnormalities observed in the treated sets were; multivalents, stickiness, precocious separation, laggards, bridges, fragments, micronuclei and disturbed polarity (Figure (a–f)). The overall percentage of abnormal PMCs (pollen mother cells) increased from 3.23 to 22.11% when the lead nitrate increased from 20 to 100 ppm. The maximum aberrations were found with 80 and 100 ppm of lead nitrate (Table ). Laggards, bridge and disturbed polarity were absent in the lower concentration (20 ppm) of lead nitrate, but their frequencies increased from 40 to 100 ppm, respectively. The frequency of precocious separation, multivalents, fragments and micronuclei also increased significantly with increasing concentrations of heavy metal (Table ). The pollen fertility showed a very low percentage of sterile pollen grains in control sets. Pollen fertility was found to be significantly correlated with meiotic abnormalities: as the meiotic abnormalities increased along with dose of lead treatment, the percentage of fertile pollen grains decreased (Figure (a)).
Figure 2. Effect of lead on (a) pollen fertility in Capsicum annuum L.; (b–h) morphological parameters in Capsicum annuum L. Values are the mean ± SD (n=5). Mean value significantly different between the treatments and control: *p<0.05 and **p<0.01.
Figure 3. Chromosomal abnormalities (a) diakinesis; (b) stray bivalent at metaphase II; (c) unequal separation at anaphase I; (d) bridge at anaphase I; (e) laggards with fragment at anaphase I; (f) micronuclei at telophase II (scale bar=10 μm).
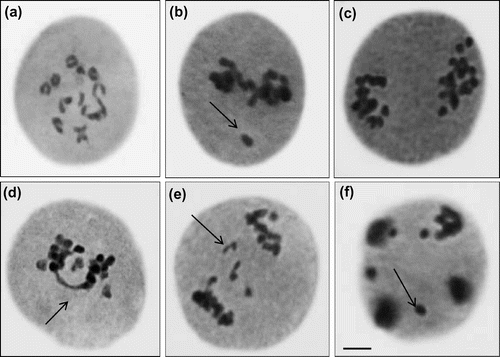
Table 1. Different chromosomal abnormalities induced by Pb(NO3)2 in Capsicum annuum L.
The results of the present study indicate that lead nitrate has a considerable inhibitory effect on morphological parameters but these parameters increased at lower concentration. Chromosomal aberrations in Capsicum were dose dependent; these effects were highest at greater concentrations of lead (Figure and Table ).
Discussion
Seed germination was inhibited parallel to the increasing concentrations of mutagens and antiparallel to the germination percentage. Reduction in seed germination of treated populations has been explained due to delay or inhibition in physiological and biological processes necessary for seed germination, which include hormonal imbalance and inhibition of meiotic process (Ananthaswamy et al. Citation1971). Heavy metal (Pb) decreased germination percentage, germination index, root/shoot length, tolerance index and dry mass of root and shoot (Kabir et al. Citation2008; Farooqi et al. Citation2009). Reduction in survival occurred due to various factors, such as cytogenetic damage and physiological disturbances (Girija et al. Citation2013), and chromosomal damage leading to mitotic arrest (Khursheed et al. Citation2008). Morphological analysis of M1 plants showed that plant height was reduced in the treated population as compared to control. The reduction can be attributed to chromosomal damage. High concentrations of heavy metals suppressed the growth of vegetative organs (Dimitrova Citation1993). Dimitrova (Citation1993) also reported that increased levels of heavy metals in the soil decreased not only the growth of vegetative organs but also the rate of cell division. The data concerning yield parameters showed a significant decrease in yield. Laxmi et al. (Citation1983) also recorded a significant decrease in yield as compared to control. Similar results were reported in soybean (Pavadai and Dhanavel Citation2004), cotton (Sundaravadivelu et al. Citation2006) and in broad bean (Bhat et al. Citation2006).
Mutagen-induced chromosomal variations have been extensively investigated to understand the damaging effects of heavy metals (Pb) on important biological systems like chilli. Cytological analysis revealed that the level of chromosomal aberration gradually increased along with increasing concentrations of lead nitrate (PbNO3)2, conforming to the observations of earlier studies (Dimitrova Citation1993; Kumar and Tripathi Citation2007). The spectrum of chromosomal abnormalities induced by heavy metal was broad and included a comparatively higher proportion of stickiness. Stickiness has been reported to be a result of partial dissociation of nucleoproteins and alteration in the pattern of cyto-chemically balanced reactions (Jayabalan and Rao Citation1987). Stray chromosomes at metaphase-I seem to be caused by spindle dysfunction and clumping of chromosomes (Bhat et al. Citation2007). Kumar and Rai (Citation2007) observed that precocious movements are possibly due to the effect of chemicals in breaking the protein moiety of the nucleoprotein backbone. Precocious separation was observed by several workers in different plants (Srivastava and Kapoor Citation2008; Aslam et al. Citation2012). Presence of laggards may be attributed to the inability of multivalents to separate properly (Ganai et al. Citation2005). Laggards may arise by breakage or faulty spindle, resulting in imbalanced daughter nuclei and micronuclei (Singh and Chaudhary Citation2005). Bridges observed seem to be due to non-separation of chiasma due to stickiness. Kumar and Gupta (Citation2009) reported that gene mutation, or the direct action of a mutagen on the target protein responsible for chiasmata terminalization during diakinesis at meiosis-I, causes some structural defects in the protein which lead to their improper functioning, thus resulting in bridges. Micronuclei are true mutagenic feature, which may lead to a loss of genetic material and have been regarded as an indication of the mutagenicity of their inducers (Ruan et al. Citation1992). Disturbed polarity at anaphase and telophase stages could be due to spindle disturbance. Disturbed polarity was also reported in maize (Kumar and Rai Citation2007) and Trigonella (Srivastava and Kapoor Citation2008).
Kumar and Gupta (Citation2008) treated the Capsicum seeds with heavy metal and reported a gradual decrease in pollen fertility. The pollen fertility was significantly correlated with meiotic irregularities; as the meiotic abnormalities increased along with the increasing dose of heavy metal (mercury and cadmium) (Rai and Kumar Citation2010; Choudhary et al. Citation2012). Similarly decrease in pollen fertility was reported earlier in Cichorium intybus L. (Jafri et al. Citation2011), Trigonella foenum-graecum (Choudhary et al. Citation2012) and Capsicum annuum (Gulfishan et al. Citation2012; Aslam et al. Citationin press).
Conclusion
The present investigation clearly demonstrates that lead (Pb) stress had a significant effect on the cyto-morphological characters of Capsicum annuum L. The observed chromosomal aberrations represent the direct effect of pollution on the genetic material and morphological variations were positively correlated with chromosomal aberrations. It was observed that plants can tolerate lead toxicity at low concentration without losing viability; but at higher concentration lead toxicity was genotoxic and mutagenic.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The University Grants Commission (UGC) New Delhi, India provided financial assistance.
Acknowledgements
The authors are grateful to Chairmen, Department of Botany, Aligarh Muslim University, Aligarh for providing the necessary facilities required for the completion of this study.
References
- Ananthaswamy HM, Vakil UK, Sreenivasan A. 1971. Biochemical and physiological changes in gamma-irradiated wheat during germination. Radiat Bot. 11(1):1–12.10.1016/S0033-7560(71)91257-9
- Aslam R, Choudhary S, Ansari MYK, Alka Haneef I, Bhat TM. 2012. Assessment of genetic variability induced by MMS and 6-AP in a medicinal herb Cichorium intybus L. Biosci Int. 1(2):30–39.
- Aslam R, Bhat TM, Choudhary S, Ansari MYK, Shahwar D. in press. Estimation of genetic variability, mutagenic effectiveness and efficiency in M2 flower mutant lines of Capsicum annuum L. treated with caffeine and their analysis through RAPD markers. J King Saud Univ – Sci.
- Bhat TA, Khan AH, Parveen S. 2006. Effect of gamma rays on certain cytomorphological parameters in two varieties of Vicia faba L. Adv Plant Sci. 19(1):227–232.
- Bhat TA, Parveen S, Khan AH. 2007. Meiotic studies in two varieties of Vicia faba L. (Fabaceae) after EMS treatment. Asian J Plant Sci. 6(1):51–55.
- Brüning T, Chronz C, Thier R, et al. 1999. Occurrence of urinary tract tumors in miners highly exposed to dinitrotoluene. Int J Occup Env Med. 41(3):144–149.10.1097/00043764-199903000-00003
- Choudhary S, Ansari MYK, Khan Z, Gupta H. 2012. Cytotoxic action of lead nitrate on cytomorphology of Trigonella foenum-graecum L. Turkish J Biol. 36(3):267–273.
- Dimitrova I. 1993. Studies of grass plant under conditions of industrial pollution with heavy metal and sulphur dioxide [ author’s summary of PhD]. Bulgaria: Plovdiv University “P. Hilendarski”.
- Elena CT, Daniela NG, Iulia Csilla IB, Gabriela VV. 2013. Zinc-induced genotoxic effects in root meristems of barley seedlings. Not Bot Hort Agrobot Cluj, [ZDB]. 41(1):150–156.
- Farooqi ZR, Iqbal ZM, Kabir M, Nad Shafiq M. 2009. Toxic effect of lead and cadmium on germination and seedling growth of Albizia lebbeck (L.) Benth. Pakistan J Bot. 41(1):27–33.
- Ganai FA, Khan AH, Bhat TA, Parveen S, Wani NA. 2005. Cytogenetic effects of methyl methane sulphonate (MMS) on two varieties of chickpea (Cicer arietinum L.). J Cytol Genet. 6(2):97–102.
- Girija M, Gnanamurthy S, Dhanavel D. 2013. Genetic diversity analysis of cowpea mutant (Vigna unguiculata (L.) Walp) as revealed by RAPD marker. Int J Adv Res. 1(4):139–147.
- Gulfishan M, Khan AH, Jafri IF, Bhat TA. 2012. Assessment of mutagenicity induced by MMS and DES in Capsicum annuum L. Saudi J Biol Sci. 19(2):251–255.10.1016/j.sjbs.2012.01.008
- Harden RM. 2001. The learning environment and the curriculum. Med Teach. 23:335–336.10.1080/01421590120063321
- Jafri IF, Khan AH, Gulfishan M. 2011. Genotoxic effects of 5-bromouracil on cytomorphological characters of Cichorium intybus L. Afr J Biotechnol. 10(52):10595–10599.
- Jayabalan N, Rao GR. 1987. Gamma radiation induced cytological abnormalities in Lycopersicon esculentum Mill. Var Pusa Ruby Cytologia. 52(1):1–4.10.1508/cytologia.52.1
- Kabir M, Iqbal MZ, Shafigh M, Faroogi ZR. 2008. Reduction in germination and seedling growth of Thespesia populnea L. caused by lead and cadmium treatments. Pakistan J Bot. 40(6):2419–2426.
- Kempaiah RK, Manjunatha H, Srinivasan K. 2005. Protective effect of dietary capsaicin on induced oxidation of low-density lipoprotein in rats. Mol Cell Biochem. 275(1–2):7–13.10.1007/s11010-005-7643-3
- Khursheed T, Ansari MYK, Shahab D. 2008. Studies on the effect of caffeine on seed germination, seedling survival and pollen fertility in Helianthus annuus L. Var Mod Adv Plant Sci. 20 (II): 311-312.
- Kumar G, Gupta P. 2008. Lead induced differential genotoxicity in Capsicum annumm L. Curr Biotica. 3(2):261–273.
- Kumar G, Gupta P. 2009. Induced karyomorphological variations in three phenodeviants of Capsicum annuum L. Turkish J Biol. 33(2):123–128.
- Kumar G, Rai P. 2007. EMS induced karyomorphoological variations in maize (Zea mays L.). Turkish J Biol. 33(4):187–195.
- Kumar G, Tripathi R. 2007. Anomalous nucleolar and chromosomal organization in induced phenodeviants of grass pea. Cytologia. 72(3):345–350.10.1508/cytologia.72.345
- Laxmi V, Gupta MN, Datta SK. 1983. Investigation on an induced green seed coat mutant of Trigonella foenum-graecum L. Cytologia. 48(2):373–378.10.1508/cytologia.48.373
- Morré DJ, Morré DM. 2003. Synergistic Capsicum-tea mixtures with anticancer activity. J Pharm Pharmacol. 55(7):987–994.10.1211/0022357021521
- Pavadai P, Dhanavel D. 2004. Effect of EMS, DES and colchicines treatment in soybean. Crop Res. 28(1–3):118–120.
- Rai PK, Kumar G. 2010. The genotoxic potential of two heavy metals in inbred lines of maize (Zea mays L.). Turkish J Bot. 34(1):39–46.
- Ruan C, Lian Y, Lium J. 1992. Application of micronucleus test in Vicia faba root tips in the rapid detection of mutagenic environmental pollutants. Chinese J Environ Sci. 4:56–58.
- Sim KH, Sil HY. 2008. Antioxidant activities of red pepper pericarp and seed extracts. Int J Food Sci Technol. 43(10):1813–1823.10.1111/ifs.2008.43.issue-10
- Singh AK, Chaudhary BR. 2005. γ- rays induced chromosome anomalies in two morphologically distinct varieties of Capsicum annuum L. Nucleus. 48(3):80–84.
- Srivastava A, Kapoor K. 2008. Seed yield is not impaired by chromosome stickiness in sodium azide treated Trigonella foenum-graecum. Cytologia. 73(2):115–121.10.1508/cytologia.73.115
- Sundaravadivelu K, Ranjithselvi P, Reddy VRK. 2006. Induced genetic variability in cotton (Gossypium hirsutum L.) for yield and its components. Crop Res. 32(3): 442–446.
- Szolcsányi J. 2004. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 38(6):377–384.10.1016/j.npep.2004.07.005