Abstract
Twenty ISSR markers were used to amplify the genome of Brassica campestris L. ssp. chinensis Makino (Pak-choi). A total of 173 scorable alleles have been detected among 68 B. campestris genotypes, and the polymorphic alleles was 84.21%. Average diversity index (I) was 0.4284, and the mean number of the per locus of effective alleles was 1.4796. Moreover, the mean value of the Nei’s gene diversity (He) was 0.2883. The results revealed a higher genetic diversity in B. campestris from China than that from Japan and Thailand. The dendrogram divided the 68 B. campestris accessions into four groups. The dendrogram clustered the accessions of similar morphology together, and it indicated that the samples from the same geographical region were much closer than those from different regions. Meanwhile, the dendrogram also revealed that male sterile lines and their maintainer lines clustered together.
Introduction
Brassica campestris L. ssp. chinensis Makino (Pak-choi) is an allogamous crop with obvious heterosis originating from China, chromosomal group AA, 2n = 2x = 20, of Cruciferae (Zhang et al. Citation2010; Qiu et al. Citation2012). Additionally, it is one of the most economically important leafy vegetables in large cities of the middle and lower Yangtze River region of China (Shan et al. Citation2009; Liu et al. Citation2014). The cultivated forms of Pak-choi consist of morphologically distinct groups, distinguished by the appearance of their edible or useful parts. The major representatives of this diversified crop include common Pak-choi (var. communis Tsen et Lee), Wutacai (var. rosularis Tsen et Lee), Caitai (var. cai tai Hort) and Taicai (var. tai cai Hort) (Yu et al. Citation2010). Various phenotypes with wide morphological variation are found in China, Thailand, and Japan. Some morphological features is peculiar to a certain geographical region (Yu et al. Citation2010).
The assessment of genetic diversity is important in designing crop improvement programs. In particular, molecular markers can help to identify the abundance of genetic diversity in the material available to breeders, since understanding the genetic relationships among germplasm helps to select appropriate parental plants for crossing, and it also helps to decide breeding strategies (Ibitoye and Akin-Idowu Citation2010). In addition, molecular markers can provide a rapid method to screen parental germplasm for genetic variation, to develop genetic linkage maps, and to tag genes controlling important traits (Ibitoye and Akin-Idowu). In order to accelerate the breeding process of Pak-choi, marker-assisted selection (MAS) must be combined with conventional breeding. Different types of genetic molecular marker system have been used in biodiversity analyses, including inter-simple sequence repeat (ISSR), simple sequence repeats (SSR), sequence characterized amplified region (SCAR), random amplification of polymorphic DNA (RAPD), and amplified fragment length polymorphisms (AFLP). Some previous studies on the genetic diversity of germplasm resources of Brassica campestris L. ssp. chinensis Makino were conducted only with RAPD (Han et al. Citation2008) and SSR markers (Wang et al. Citation2008; Song et al. Citation2015). RAPD markers have limitations including poor reproducibility, a small amount of information provided by each marker, testing easily influenced by reaction conditions, and low reliability of results. SSR are preferred in breeding programs due to their multi-allelic nature, co-dominant inheritance, relative abundance, and extensive genome coverage (Ijaz and Khan Citation2009). However, the development of SSR markers is high cost and complicated. In contrast, ISSR markers are based on arbitrary primer sequences, with the advantages of low economic cost, low processing time, and high polymorphism (Moysés et al. Citation2010). ISSR-PCR is a technique that can overcome most of the limitations of RAPD and SSR (Meyer et al. Citation1993; Gupta et al. Citation1994; Wu et al. Citation1994; Zietkiewicz et al. Citation1994). It has been used by the research community in various fields of plant improvement (Godwin et al. Citation1997; Sharma et al. Citation2014), e.g. in Jerusalem artichoke (Helianthus tuberosus L.) (Wangsomnuk et al. Citation2011), micro-propagated guava (Psidium guajava) plants (Liu and Yang Citation2012), as well as Cassia occidentalis L. plantlets (Naz et al. Citation2016). Many Brassica species have been investigated with ISSR markers, including cauliflower (Brassica oleracea var. botrytis L.) (Bornet et al. Citation2002), Brassica rapa var. chinensis (Shen et al. Citation2016), winter oilseed rape (Brassica napus L.) (Lenka et al. Citation2014), and Brassica juncea (L.) Czern & Coss (Verma et al. Citation2016).
In this study, ISSR markers have been used to assess the genetic diversity of Pak-choi with the aims of offering useful information for efficient utilization and conservation of B. campestris germplasm resources.
Materials and methods
Plant materials
The seeds of B. campestris (68 accessions) were provided by the WuHan Vegetable Research Institute, collected from different places in China, Japan, and Thailand (Table ).
Table 1. The accessions of Brassica campestris L. ssp. chinensis Makino from different countries investigated in this study.
DNA extraction
Genomic DNA was extracted from young fresh leaves of five plants for each cultivar (1 g), using the modified CTAB protocol described by Tang et al. (Citation2007).
ISSR analysis
ISSR analysis was performed according to the protocol described by Fernández et al. (Citation2002). A total of 20 primers were selected from a set of designed ISSR primers by University of British Columbia (Table ).
Table 2. Analysis of genetic variations generated by ISSR marker of Brassica campestris L. ssp. chinensis.
ISSR reactions were performed in a final volume of 20 μl containing 10 ng of template DNA, 2.0 μl (10 pM) primer DNA, 0.4 μl each dNTP with 2.5 mM, 0.1 μl Taq DNA polymerase (MBI) with 5 U μl–1, 2 μl of 10 × buffer (100 mM Tris-HCl, pH8.8 at 25°C, 500 mM KCl, 0.8% Nonidet P40) and 2.5 μl of 1.5 mM MgCl2. PCR amplification was performed on an Eppendorf Mastercycler gradient (Thermo Hybaid, Waltham, MA, USA) with the program of 28 cycles: 94°C for 30 s, 50°C for 45 s and 72°C for 1 min followed by a final extension at 72°C for 10 min. The PCR amplified products were separated and verified using 1% agarose gel.
Data analysis
Each ISSR marker was referred to as a locus. The amplified fragments were scored as either present (1) or absent (0), each of which was viewed as an allele. Thus, one matrix of different ISSR phenotypes was formed. The matrix was then used for the statistical analyses described below, and the genetic variation was analyzed using the Popgene program (version 1.32). The gene diversity index for each locus and population was calculated using the formula: He = 1 − , in which Pi was the ith allele frequency (Nei Citation1973). The distribution of variabilities between and within populations were also calculated for each ISSR locus using the methodology of Nei (Citation1973). The total gene diversity (HT) was partitioned into within-population diversity (HS) and between-population diversity (DST) components, where: HT = HS + DST. Subsequently, the genetic diversity between populations relative to the total population diversity was expressed as: GST = DST/HT. The NTSYS-pc software ver.2.11 was used to calculate Nei’s similarity coefficients for all pairs of individuals. Similarity matrices were used to construct the unweighted pair group with arithmetic average (UPGMA) dendrograms using the NTSYS-pc ver.2.11 software.
Results
Allelic variation of ISSR markers
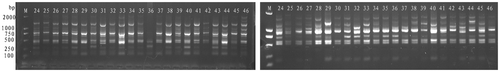
A total of 30 ISSR markers were used, but only 20 ISSR markers were found to effectively and reproducibly amplify polymorphic fragments for all B. campestris accessions surveyed. A total of 173 scorable alleles were detected in the 68 genotypes (Figure ) ranging from 200 bp to 2000 bp, with 173 alleles (83.82%) being polymorphic (Table ). The number of scorable fragments amplified by each ISSR primer varied from six to 12, with an average of 8.65. Of all the primers used, primer 851 gave rise to the most reproducible bands (as many as 12) and primers 823, 846 and 844 produced the least bands (only 6). Average diversity index (I) was 0.4284 ranging from 0.2362 (primer 851) to 0.6781 (primer 826), and the effective number (Ne) of alleles in each locus ranged from 1.2564 to 1.9453 with the average value being 1.4796. The mean value of the Nei’s (Citation1973) gene diversity (He) was 0.2883 with the range from 0.1495 to 0.4851 for ISSR.
Genetic diversity of the 68 genotypes was shown in Table . The percentage of polymorphic loci in B. campestris from China was highest (82.66%), while the percentage of that from Thailand was the lowest (17.60%). In this study, the total gene diversity (Ht) was 0.2501 and the mean value of within the group (Hs) was 0.1955. The genetic differentiation coefficient was 0.2182 among Pak-choi from different locations. Therefore, for the present data, 21.82% of the variation (on average) happened between groups and 78.18% of the variation (on average) occurred within groups, which indicated that the degree of diversity was much greater within a group than between groups in this study (Table ). Meanwhile, low gene flow (Nm, 1.7919) existed among different groups, which maintained the diversity of genetic variation among different groups.
Table 3. Genetic polymorphism of different geographical distribution revealed by ISSR markers of Brassica campestris L. ssp. chinensis.
The calculation of the genetic similarity coefficient and the conversion from the genetic similarity to genetic distance between different B. campestris were calculated using NTSYS-pc ver.2.11 software (http://www.xdowns.com/soft/23/bioinformatic/2016/Soft_156403.html). The genetic distance varied from 0.0417 for the accessions of “Xiashang” between “Xiadi” to 0.5098 for the accessions of “Huangxinwu” between “Beijing510”. This revealed that the difference of genetic distance among 68 Pak-choi accessions involved in this study was significant, and the genetic relationship between various accessions was also different, inferring that these B. campestris accessions used in this study had abundant genetic diversity.
The smaller the genetic distance is, the closer the relationship between the two given accessions is. Therefore, the value of the genetic distance between two accessions of B. campestris can be used to measure their genetic differential. Generally, it is easy to gain strong heterosis by selecting accessions with large genetic differential as parent plants for crossbreeding. In this study, values of genetic distance between these 68 B. campestris accessions were listed in Table (values lower than 0.1) and Table (the top 10 largest values). Thus, our results offered theoretical reference for B. campestris breeding.
Table 4. Genetic distance value under 0.1 between different accessions of Pak-choi.
Table 5. The 10 largest genetic distance values of 10 male sterile lines with a given maintainer line (Fertility) involved in this study.
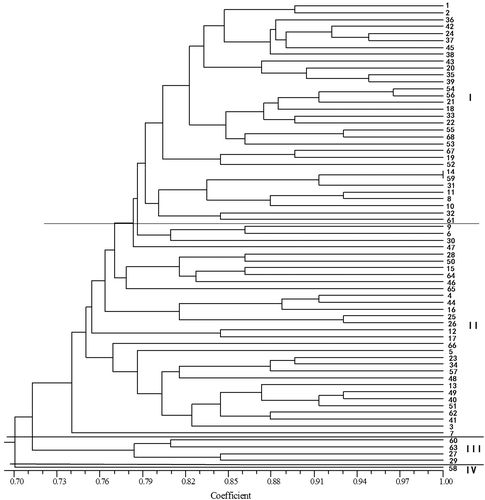
A similarity matrix was used to construct the UPGMA dendrogram by the NTSYS-pc (ver. 2.11) software (Figure ). The dendrogram separated the 68 B. campestris accessions into four groups. Cluster I included 32 accessions from different geographical origins representing the wide, smooth oval leaves, and could be further divided into two subclusters, representing thick and light-green petiole and waist type group, and the semi-round blade and white petiole group and waist group. Thirty-one were grouped into cluster II, representing round petioles, loose plant-type, and could be divided into two subclusters, representing dwarf planet, blistering, white petiole and dark green leaves, and the semi-round blade, light green leaves and green petiole. Cluster III included four accessions and these accessions were very similar to Chinese seeding cabbage; all accessions in this cluster had smooth, dark green leaves, as well as the long, thin leaves, round petioles, loose plant type and fast growth, which showed the close relationship between these type accessions with Chinese cabbage. “Qingjiangbai” was clustered into Cluster IV.
Discussion
In our study, 20 ISSR primers were selected to detect the genetic diversity of 68 B. campestris accessions, and all these primers could amplify polymorphic bands. The Shannon average polymorphic index (I) was 0.4477, ranging from 0.2362 to 0.6781. Nei’s (Citation1973) genetic polymorphic index was 0.3006, varying from 0.1495 to 0.4851 for different loci. The polymorphism of locus was 83.82% with variation ranging from 60% to 100%, which was higher than the values detected by SRAPs (52.09%; Han et al. Citation2007), RAPDs (70.73%; Chuang et al. Citation2004), and SSRs (73.68%; Wang et al. Citation2008). These data show that the 68 accessions used in this study have high genetic diversity and polymorphism. The gene flow of all these accessions is 1.7919. This finding is consistent with that reported by Han et al. (Citation2007). The reason might be the different Pak-choi populations from China, Japan and Thailand grown in different geographical environments, including different altitude, temperature, rainfall, light intensity and UV intensity. It is the different geographical environments in which different populations grow that lead to the distinct morphological characteristics. The latter in turn leads to the genetic differentiation of different B. campestris populations.
The UPGMA cluster analysis revealed that accessions with similar morphological characteristics were clustered together. This result agreed with that from another study on the genetic diversity and marker-trait associations in a collection of Pak-choi accessions (Yu et al. Citation2010). Male sterile lines and their maintainer lines were also clustered together, such as “Zaoshenghuajing” and its male sterile line “ZaoshenghuajingA”, and “Jinyinfeng” and its male sterile line “JinyinfengA”. However, samples in our study were from three countries (China, Japan, and Thailand), and most from China. In the dendrogram, samples from Japan and Thailand respectively are assembled together, but they were mixed with the samples from China in a bigger group. Therefore, they are not separated from each other by their geographical origins in a strict sense. It is likely that China is the center of origin for cultivated B. campestris samples (Dong et al. Citation2001) and there is abundant genetic diversity in B. campestris from China, while narrow genetic diversity in B. campestris collected from Japan and Thailand.
In conclusion, the genetic relationship of various Pak-choi accessions revealed by ISSR markers is in accord with their morphologic traits. Moreover, the high polymorphism detected by ISSR markers suggests strong heterosis between the crossing combinations of various accessions of B. campestris. Therefore, this research can provide a reliable molecular guide for heterosis breeding of B. campestris.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Fund of National Commodity Vegetable Technology System [CARS-25-G-30]; the Natural Science Foundation of Hubei Province of China [2013CFA102].
References
- Bornet B, Muller C, Paulus F, Branchard M. 2002. Highly informative nature of inter simple sequence repeat (ISSR) sequences amplified using tri- and tetra-nucleotide primers from DNA of cauliflower (Brassica oleracea var. botrytis L.). Genome. 45(5):890–898.10.1139/g02-061
- Chuang HY, Tsao SJ, Lin JN, Shu CK, Liou TD, Chung MC, Yang YW. 2004. Genetic diversity and relationship of non-heading Chinese cabbage in Taiwan. Bot Bull Acad Sin. 45:331–337.
- Dong YS, Zhuang LM, Zhao LM, Sun H, He MY. 2001. The genetic diversity of annual wild soybeans grown in China. Theor Appl Genet. 103:98–103.10.1007/s001220000522
- Fernández ME, Figueiras AM, Benito C. 2002. The use of ISSR and RAPD markers for detecting DNA polymorphism, genotype identification and genetic diversity among barley cultivars with known origin. Theor Appl Genet. 104:845–851.
- Godwin ID, Aitken EAB, Smith LW. 1997. Application of inter-simple sequence repeat (ISSR) markers to plant genetics. Electrophoresis. 18(9):1524–1528.10.1002/(ISSN)1522-2683
- Gupta MYS, Chyi JRS, Owen JL. 1994. Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theor Appl Genet. 89:998–1006.
- Han JM, Hou XL, Xu HM, Shi GJ, Geng JF, Deng XH. 2007. Genetic differentiation of non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino) Germplasm based on SRAP markers. Cro J 33(11):1862–1868.
- Han JM, Hou Xl, Xu HM, Shi GJ, Wang JJ. 2008. RAPD analysis of genetic diversity of non- heading Chinese cabbage (Brassica campestris L. ssp. chinensis Makino) germplasm. J Nanjing Agric Univ. 31(3):31–36.
- Ibitoye DO, Akin-Idowu PE. 2010. Marker-assisted-selection (MAS): a fast track to increase genetic gain in horticultural crop breeding. Afr J Biotechnol. 9(52):8889–8895.
- Ijaz S, Khan IA. 2009. Molecular characterization of wheat germplasm using microsatellite markers. Genet Mol Res. 8(3):809–815.10.4238/vol8-2
- Lenka H, Eva J, Andrea R, Miroslav K, Vratislav K, Vladislav Č. 2014. Genetic diversity assessment in winter oilseed rape (Brassica napus L.) collection using AFLP, ISSR and SSR markers. Czech J GenetPlant Breed. 50(3):216–225.
- Liu T, Dai W, Sun F, Yang X, Xiong A, Hou X. 2014. Cloning and characterization of the nitrate transporter gene branrt2.1 in non-heading Chinese cabbage. Acta Physiol Plant. 36(4):815–823.10.1007/s11738-013-1460-1
- Liu XM, Yang GC. 2012. Assessment of clonal fidelity of micro-propagated guava (Psidium guajava) plants by ISSR markers. Aust J Crop Sci. 6(2):291–295.
- Meyer W, Mitchell TG, Freedman EZ, Vilgays R. 1993. Hybridization probes for conventional DNA fingerprinting used as single primers in the polymerase chain reaction to distinguish strains of Cryptococcus neoformans. J Clin Microbiol. 31:2274–2280.
- Moysés CB, Daniel-Silva MF, Lopes CE, De Almeida-Toledo LF. 2010. Cytotype-specific ISSR profiles and karyotypes in the Neotropical genus Eigenmannia (Teleostei: Gymnotiformes). Genet. 138(2):179–189.10.1007/s10709-009-9407-6
- Naz R, Anis M, Alatar AA. 2016. ISSR marker-based detection of genomic stability in Cassia occidentalis L. plantlets derived from somatic embryogenesis. Eng. Life Sci. 16(1):17–24.
- Nei M. 1973. Analysis of diversity in subdivided populations. Proc Natl Acad Sci USA. 70:3321–3323.10.1073/pnas.70.12.3321
- Qiu Z, Zhang L, Hu Y, He S, Li L. 2012. The acetylation level of rDNA in Brassica campestris. J Plant Biol. 55(4):298–302.10.1007/s12374-011-0317-7
- Shan XZ, Li Y, Gao SY, Ma CY. 2009. Molecular Genetic Map of Brassica campestris ssp. chinensis. Acta Botanica Boreali-Occidentalia Sinica. 29(6):1116–1121.
- Sharma P, Sharma V, Kumar V. 2014. Genetic diversity analysis of cluster bean [Cyamopsis tetragonoloba (L.) Taub] genotypes using RAPD and ISSR markers. J Agr Sci Tech. 16:433–443.
- Shen XL, Zhang YM, Xue JY, Li MM, Lin YB, Sun XQ, Hang YY.2016. Analysis of genetic diversity of Brassica rapa var. chinensis using ISSR markers and development of SCAR marker specific for Fragrant Bok Choy, a product of geographic indication. Gente Mol Res. 15(2):1–11.
- Song X, Ge T, Li Y, Hou X. 2015. Genome-wide identification of SSR and SNP markers from the non-heading Chinese cabbage for comparative genomic analyses. Bmc Genomics. 16(1):1–18.
- Tang YJ, Ashcroft JM, Chen D, Min G, Kim CH, Murkhejee B, Larabell C, Keasling JD, Chen FF. 2007. Charge-associated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 7(3):754–760.
- Verma V, Thakur AK, Singh BK, Chauhan DK, Singh KH, Chauhan JS. 2016. Assessment of genetic fidelity of in vitro regenerated plants of Brassica juncea (L.) Czern&Cross. Using RAPD and ISSR markers. Indian J Biotech. 15:120–123.
- Wang XY, Yu SC, Zhang FL, Yu YJ, Zhao XY, Zhang DS. 2008. SSR fingerprinting and genetic distinctness of Pak-choi (Brassica rapa L. ssp. chinensis Makino). Acta Agric Boreali-Sim. 23(5):97-103.
- Wangsomnuk PP, Khampa S, Jogloy S, Srivong T, Patanothai A, Fu YB. 2011. Assessing genetic structure and relatedness of Jerusalem Artichoke (Helianthus tuberosus L.) Germplasm with RAPD, ISSR and SRAP markers. Amer J Plant Sci. 2:753–764.10.4236/ajps.2011.26090
- Wu K, Jones R, Dannaeberger L, Scolnik PA. 1994. Detection of microsatellite polymorphisms without cloning. Nucleic Acids Research. 22:3257–3258.10.1093/nar/22.15.3257
- Yu SC, Zhang FL, Wang XY, Zhao XY, Zhang DS, Yu YJ, Xu JB. 2010. Genetic diversity and marker-trait associations in a collection of Pak-choi (Brassica rapa L. ssp. chinensis Makino) Accessions. Genes Genomics. 32:419–428.
- Zhang C, Qi L, Hou X, Shi G, Zhang J. 2010. Differential gene expression analysis of a new Ogura CMS line and its maintainer in non-heading Chinese cabbage by cDNA-AFLP. Acta Physiol Plant. 32(4):781–787.10.1007/s11738-010-0463-4
- Zietkiewicz E, Rafalski A, Labuda D. 1994. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerease chain reaction amplification. Genomics. 20:176–183.10.1006/geno.1994.1151