Abstract
Heracleum sosnowskyi is one of the most aggressive alien species in Europe. However, no information is available on its genotoxic effects. The goal of this study was to investigate cytological aberrations and genotoxic effects of H. sosnowskyi juice using the well-established Allium test. Root tip cells were treated with varying concentrations (1, 5, 10, 50, 100 and 300 ml l–1) of solution in water of H. sosnowskyi juice. The same slides with root tips were used for mitotic abnormalities, chromosome aberrations and micronuclei scoring. Mitotic and phase indexes were also analyzed. No genotoxic effects were found at a concentration of 1 ml l–1. Mitotic abnormalities were detected at concentrations of 10, 50, 100, and 300 ml l–1. Chromosomal abnormalities and micronuclei were detected at concentrations of 50, 100, and 300 ml l–1. Significant decrease in mitotic index was found at 5, 10, 50, 100, and 300 ml l–1 concentrations of H. sosnowskyi juice. Lagging chromosomes were the most common abnormalities. The data demonstrated that H. sosnowskyi juice exhibits both clastogenic and aneugenic activity. However, aneugenic activity is more pronounced.
1. Introduction
Currently Sosnowsky’s hogweed (Heracleum sosnowskyi Manden, family Apiaceae), together with giant hogweed, Heracleum mantegazzianum (H. mantegazzianum Manden), and Persian hogweed (H. persicum Manden), is an active invasive species in Europe. The alien range of H. sosnowskyi covers the Baltic States, European part of Russia, Belarus and Ukraine, sporadically occurring in Poland, Hungary, Germany and Denmark. However, detailed distribution data could not be found (Nielsen et al. Citation2005). H. sosnowskyi is included in the lists of the most aggressive alien species in all the Baltic States. It is also included in the European and Mediterranean Plant Protection Organization’s (EPPO) list of invasive alien plants. Giant hogweed has a serious negative impact on biodiversity, destroying natural ecosystems, causing significant economic damage and posing a danger to human health. It is important to know that H. sosnowskyi belongs to a group of toxic plants, dangerous both to humans and animals (Jakubowicz et al. Citation2012). However, only during the last few years has the community been informed about the negative impacts caused by H. sosnowskyi (Rzymski et al. Citation2015). Universal ways to eliminate them have not been developed, so these species require detailed study (Rzymski et al. Citation2015).
There is a well-known phototoxic effect of juice from H. sosnowskyi, H. mantegazzianum, and H. persicum. These plants produce a clear watery sap, which contains photosensitizing compounds furanocoumarins. When it is in contact with skin, and in the presence of ultraviolet radiation, these compounds are activated and cause burns. Furanocoumarins present in the sap of hogweeds can cause cancer (Nielsen et al. Citation2005). Sap extracts are also considered to have allelopathic activity (Jakubska-Busse et al. Citation2013).
The juice of different kinds of hogweed can have other negative properties. Experiments on brine shrimp (Artemia salina L.) revealed that the extracts of H. persicum and H. sphondylium have a strong toxic effect without photoactivation, and cause the death of aquatic organisms (Mosham et al. Citation2009). Another study found that the extract of H. sibiricum Manden. has a mutagenic effect in culture of mammalian lymphocytes (Bogucka-Kocka et al. Citation2008). Allelopathic activity for several Heracleum species (H. sosnowskyi, H. asperum, H. dissectum, H. lescovii, and H. hirtum) was reported (Mishyna et al. Citation2015). Despite the seriousness of the problem and the need for detailed toxicological and genotoxicological study of different plants species (especially invasive ones), these data remain lacking (Modallal et al. Citation2008; Abderrahman and Shbailat Citation2014; Karaismailoglu Citation2014; Falistocc Citation2016; Arshad et al. Citation2016). Therefore, extensive studies are required to understand the effect of H. sosnowskyi on human health and environment. There have been no genotoxicological studies regarding H. sosnowskyi.
Plant bioassays are important tests in the detection of genotoxic and toxic agents and contamination in the environment (Frescura et al. Citation2013; Dixit et al. Citation2013; Adamakis et al. Citation2013). Allium cepa L. has been used to evaluate DNA damage, such as chromosome aberrations, micronuclei and disturbances in the mitotic cycle (Bakare et al. Citation2012; Achary et al. Citation2013). Due to its sensitivity, the A. cepa test was the first of nine plant assay systems evaluated by the Gene-Tox Program of the US Environmental Protection Agency (Grant Citation1994). The Allium test is now frequently used for environmental monitoring (Leme and Marine-Morels Citation2009; Olorunfemi et al. Citation2012; Olorunfemi et al., Citation2015), laboratory studies (Pesnya and Romanovsky Citation2013; Pesnya Citation2013; Kibrik et al. Citation2013; Prokhorova et al. Citation2013; Ślusarczyk et al. Citation2014; Karaismailoglu Citation2016), nanomaterials (Kumari et al. Citation2009; Klancnik et al. Citation2011; Panda et al. Citation2011; Sobieh et al. Citation2016) and is also proposed for assessing plant extracts (Kuhn et al. Citation2015).
The aim of this study was to investigate the genotoxic effects of H. sosnowskyi juice.
2. Materials and methods
2.1. H. sosnowskyi juice preparation
Stems and leaves of H. sosnowskyi were used. Plants were collected from abandoned farms (Nekrasovskiy district of the Yaroslavl region, village Burmakino 57°26′00″ N, 40°18′00″ E). Fresh stems and leaves were homogenized and filtered to obtain juice. The juice was diluted with distilled water (pH = 6.7) for different concentrations. The following concentrations were used: 300, 100, 50, 10, 5, and 1 ml l–1 (Celik and Aslanturk Citation2009). All preparations were immediately used in the Allium cepa test.
2.2. Allium test and treatment
The Allium test was used to analyze genotoxic activity (Constantin and Owens Citation1982; Fiskesjo Citation1985).
The onion bulbs (Allium cepa L., 2n = 16) were of the Stuttgarten-Risen variety, average weight 25 g. Bulbs of A. cepa were placed in small glass jars with their basal ends dipped in distilled water and germinated at room temperature (24 ± 3°C). When the newly emerged roots were 0.50 cm in length they were used in the test. Roots of A. cepa were treated with a series of concentrations of H. sosnowskyi juice, i.e. 300, 100, 50, 10, 5, and 1 ml l–1 for 96 h in the absence of sunlight and direct light. Control groups were treated with deionized distilled water. After the treatment the root-tips were placed in a solution of ethanol (96%) and glacial acetic acid (3:1) for 48 h then washed with distilled water and dyed using aceto-orcein for 1 h. The squash technique was applied for the study of the mitotic index (MI) and phase indexes, mitotic and chromosomal aberrations and micronuclei. Five replicates (bulbs) were performed for each group and scoring was given from the three roots of each replicate (15 slides for each concentration) (Barbério et al. Citation2011). The MI was calculated for each treatment as a number of dividing cells per 700 cells and also scored the proportions of mitotic phases.
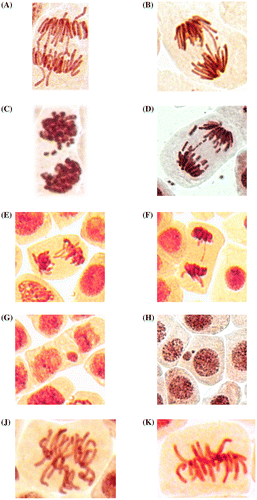
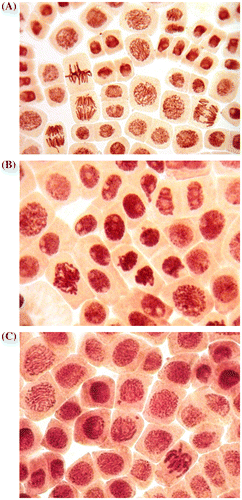
Chromosomal aberrations (chromatid (single) and chromosome (double) bridges and fragments) were scored in 100 ana-telophases per slide. Mitotic abnormalities (lagging chromosomes and polyploidy) were scored in 1000 mitotic cells per slide. Micronuclei frequency was expressed as the number of interphase cells with micronuclei per 3000 for every slide. All examinations were done under a light microscope at 400 magnifications. The most frequent abnormalities (Figures (B) and (C) and ) and normal cells (Figure (A)) are shown in photomicrographs. The results regarding MI and phase indexes and the frequency of mitotic and chromosomal abnormalities in root-tip cells of A. cepa are summarized in Tables , and .
Table 1. Data on mitotic and phase indexes (mean ± SD) in the meristematic cells of A. cepa roots treated with solutions of H. sosnowskyi juice.
Table 2. Data on the number of mitotic disturbances (mean ± SD) in the meristematic cells of A. cepa roots treated by H. sosnowskyi juice.
Table 3. Data on the number of chromosomal aberrations and micronuclei (mean ± SD) in the meristematic cells of A. cepa roots treated with solutions of H. sosnowskyi juice.
Figure 1. (A) Control meristematic cells of A. cepa; (B) destruction of interphase chromatin (nuclear budding) and formation of micronuclei in root meristem of A. cepa after incubation in solution of 100 ml l–1 H. sosnowskyi juice; (C) micronuclei at the center in root meristem of A. cepa after incubation in solution of 100 ml l–1 H. sosnowskyi juice.
2.3. Statistical analysis
Statistical calculations were done using Statistica 8.0 (Dell Software, https://software.dell.com/products/statistica/). The differences in the mitotic index and phases indexes between treated and control groups were tested applying the non-parametric Mann–Whitney test. Frequencies of chromosome aberrations, micronuclei and mitotic abnormalities were statistically analyzed by Student’s t-test and ANOVA. The level of significance was accepted at p ≤ 0.05 (*).
3. Results and discussion
3.1. Mitotic and phase indexes
H. sosnowskyi juice at concentrations of 5–3000 ml l–1 significantly decreased the number of dividing cells in A. cepa root meristems. The most pronounced effect was registered at 300 ml l–1 concentration (Table ). It should be noted that the antiproliferative activity of other medicinal plants using the A. cepa test has already been observed in many studies (Leme Marine-Morels Citation2009; Frescura et al. Citation2012; Frescura et al. Citation2013; Kuhn et al. Citation2015). Additionally, phase indexes were affected at 10–300 ml l–1 concentrations of H. sosnowskyi juice. The prophase index is decreased, while metaphase index is increased. Anaphase and telophase index are increased at a 300 ml l–1 concentration of H. sosnowskyi juice (Table ). According to Prokhorova et al. (Citation2008), a decreasing frequency of prophases indicates that the percentage of cells entering into the first phase of mitosis is decreasing. This confirms the strong antiproliferative effect of H. sosnowskyi juice. The situation, when the frequency of metaphases increased can be associated with action of H. sosnowskyi juice on the achromatic spindle (Figure (J)). In this case chromosomal segregation cannot occur, which may result in the appearance of lagging chromosomes and genomic mutations (polyploidy, aneuploidy, etc.) (Prokhorova et al. Citation2008). For polyploidy occurrence, several mechanisms were proposed (Zimmet and Ravid Citation2000; Beyaz et al. Citation2013).
H. sosnowskyi juice at 1 ml l–1 concentration did not cause statistically significant changes in MI and phase indexes (Table ).
Our results indicate that H. sosnowskyi juice disturbs the proportions of mitotic phases, which may result in mitotic abnormalities.
3.2. Mitotic abnormalities
In the control root tip cells were registered lagging chromosomes with a frequency of 0.43 ± 0.17% (spontaneous level). H. sosnowskyi juice caused a significant increase in the frequency of lagging chromosomes at concentrations of 10, 50, 100 and 300 ml l–1 (Table , Figure (E) and (F)). The most pronounced aneugenic effect was registered at a 300 ml l–1 concentration of H. sosnowskyi juice (Table ).
3.3. Chromosomal abnormalities and micronuclei
Spontaneous frequencies of chromosomal aberrations (bridges and fragments) were 0.60 ± 0.16 and for micronuclei 0.012 ± 0.006 (Table ).
Treatments with 1, 5 and 10 ml l–1 concentrations of H. sosnowskyi juice did not increase the frequencies of chromosomal abnormalities or micronuclei over the control values.
Significant increase in the frequency of chromosomal aberrations was observed only after exposure to 50 ml l–1 of H. sosnowskyi juice. Bridges (Figure (A) and (B)) and fragments (Figure (C) and (D)) were registered in root meristems of A. cepa at this concentration. Additionally, a significant increase in frequency of micronuclei (Figure (G) and (H)) was registered (Table ). Micronuclei may arise mostly from acentric fragments or lagging chromosomes (Fenech Citation2000). Fragments (Figure (C) and (D)) can be derived from chromosomal breakages caused by clastogenic effect or they may alternatively derive from chromosome aberrations, such as chromosomal bridges, which break up and originate acentric fragments (Fiskesjo Citation1993; Leme and Marin-Morales, Citation2008). Micronuclei may additionally be generated through nuclear budding in interphase (Lindberg et al. Citation2007).
The vast majority of chromosomal abnormalities constitute lagging chromosomes (15.56 ± 1.70%), indicating that the achromatic spindle is damaged or centromeric regions of chromosomes are damaged.
This is the first genotoxicological study on H. sosnowskyi showing that H. sosnowskyi juice has strong genotoxic activity and can induce mitotic abnormalities, chromosomal aberrations and micronuclei in plant cells. Additionally, nuclear budding in interphase and formation of micronuclei was registered (Figure (B) and (C)). This study shows that H. sosnowskyi exhibits genotoxic effects in vivo even without photoactivation.
Limited information about the chemical components of Sosnowski’s hogweed is available. It was believed that the most biologically active as well as hazardous components of hogweeds from different locations are various coumarins (Jakubska-Busse et al. Citation2013). Coumarins, as mentioned in earlier research include isoimperatorin, bergapten, psoralen, pangelin, angelicin, umbelliferone, isobergapten, isopimpinellin, marmezin, osthole, (+)-oxypeucedanin, pimpinellin, sphondin, and methoxalen (Abyshev and Denisenko Citation1973; Malikov and Saidkhodzahaev Citation2004). Malikov and Saidkhodzahaev (Citation2004) described coumarins common to both H. sosnowskyi and H. mantegazzianum species: bergapten, methoxalen, angelicin, isobergapten, isopimpinellin, osthole, pimpinellin, sphondin and umbelliferone. Other early investigation of chemical composition of H. sosnowskyi showed 15 coumarins (3 oxicoumarins and 12 furanocoumarins) including psoralen, xanthotoxin, bergapten, and angelicin (Satsyperova Citation1984). A recent study showed that the five main furanocoumarins of H. sosnowskyi are xanthotoxin (1.15%), bergapten (1.04%), umbelliferone (0.83%), angelicin (0.63%) and sphondin (0.35%) (Yourlova et al. Citation2013). Genotoxic activity of xanthotoxin, bergapten and angelicin have been shown in early studies (Venturini et al. Citation1980; Schimmer Citation1981). On the other hand, antigenotoxic activity of umbeliferone has been shown (Rezaee et al. Citation2014). It is possible that the cytological aberrations and genotoxic effects of H. sosnowskyi juice observed in the present study is synergetic, i.e. the result of the combined activity of the all chemical compounds.
Several Heracleum species were already evaluated for their cytotoxic and anticancer activities. The apoptotic activity of H. sibiricum against human acute myeloblastic leukemia, human acute T cell leukemia, human eosinophilic leukemia, human Caucasian promyelocytic leukemia, human T cell leukemia lymphoblast, human T cell leukemia, human myeloma, human Caucasian normal B cell, and human T cell was studied (Bogucka-Kocka et al. Citation2008). Cytotoxic activities of four Heracleum species on Hela, LS180, and Raji cell lines were investigated. H. pastinacifolium and H. transcaucasicum exhibited strong activities. H. rechingeri showed activity only on LS180, while H. persicum did not show cytotoxicity (Firuzi et al. Citation2010). It was reported that the leaves of H. sprengelianum have strong toxicity against liver cancer cells and less toxicity toward normal cells (Sathak et al. Citation2014).
However, there have not been any epidemiologic or intervention studies on the genus Heracleum, and information about safety is not sufficient. All of the cytotoxicity studies on Heracleum species are in vitro. There is a lack of in vivo investigations on their cytotoxic and anticancer effects. Advanced studies are necessary to explore the mechanism of their cytotoxic effects on different models and especially on cancer cell lines, because many Heracleum species exhibited considerable anticarcinogenic activity (Bahadori et al. Citation2016).
4. Conclusions
The results of this study show that H. sosnowskyi juice did not exhibit genotoxicity at concentration 1 ml l–1. Considerable genotoxic effects of H. sosnowskyi juice at concentrations of 10–300 ml l–1 were observed. The mitotic index was significantly decreased at 5–300 ml l–1 concentrations of H. sosnowskyi juice. Phase indexes were modified at 10–300 ml l–1 concentrations of H. sosnowskyi juice. Formation of micronuclei via nuclear budding were detected at 100 ml l–1 and 300 ml l–1 concentrations of H. sosnowskyi juice. The total frequency of mitotic abnormalities (chromosome lagging), chromosome aberrations (fragments, bridges) and micronuclei were significantly increased at 10–300 ml l–1 concentrations of H. sosnowskyi juice.
Thus H. sosnowskyi juice exhibits both clastogenic and aneugenic activity in plant cells. However, aneugenic activity is more pronounced.
Acknowledgments
The author would like to express gratitude to Vladimir B. Verbitsky, Tatiana S. Pesnya, Alexander S. Pesnya, Sergey E. Bolotov and Inna M. Prokhorova for their helpful advice and notes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abderrahman SM, Shbailat SJ. 2014. Genotoxic and cytotoxic effects of Artemisia herba-alba on mammalian cells. Caryologia. 67(4):265–272.10.1080/0144235X.2014.974355
- Abyshev AZ, Denisenko PP. 1973. The coumarin composition of Heracleum sosnowskyi. Chem Nat Compd. 9(4):515–516.10.1007/BF00568643
- Achary VM, Parinandi NL, Panda BB. 2013. Calcium channel blockers protect against aluminium-induced DNA damage and block adaptive response to genotoxic stress in plant cells. Mutat Res. 751(2):130–138.10.1016/j.mrgentox.2012.12.008
- Adamakis IS, Panteris E, Cherianidou A, Eleftheriou EP. 2013. Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L. Mutat Res. 750(1–2):111–120.10.1016/j.mrgentox.2012.10.012
- Arshad M, Siddiqui S, Daoud A. 2016. In vitro anti-proliferative and apoptotic effects of ethanolic extract of Cissus quadrangularis. Caryologia. 69(2):128–132.10.1080/00087114.2015.1136542
- Bahadori MB, Dinparast L, Zengin G. 2016. The genus Heracleum: a comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Comp Rev Food Sci Food Saf. doi:10.1111/1541-4337.12222
- Bakare AA, Adeyemi AO, Adeyemi A, Alabia OA, Osibanjo O. 2012. Cytogenotoxic effects of electronic waste leachate in Allium cepa. Caryologia. 65(2):94–100.10.1080/00087114.2012.709786
- Barbério A, Voltolini JC, Mello MLS. 2011. Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology. 20(4):927–935.10.1007/s10646-011-0602-8
- Beyaz R, Alizadeh B, Gürel S, Özcan SF, Yildiz M. 2013. Sugar beet (Beta vulgaris L.) growth at different ploidy levels. Caryologia. 66(1):90–95.10.1080/00087114.2013.787216
- Bogucka-Kocka A, Smolarz H, Kocki J. 2008. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia. 79(7–8):487–497.10.1016/j.fitote.2008.07.002
- Celik TA, Aslanturk OS. 2009. Investigation of cytotoxic and genotoxic effects of Ecballium elaterium juice based on Allium test. Methods Find Exp Clin Pharmacol. 31(9):591–596.
- Constantin MJ, Owens ET. 1982. Introduction and perspectives of plant genetic and cytogenetic assay. Mutat Res. 99(1):1–12.10.1016/0165-1110(82)90027-6
- Dixit V, Prabha R, Chaudhary BR. 2013. Effects of EMS and SA on meiotic cells and thymoquinone content of Nigella sativa L. cultivars. Caryologia. 66(2):178–185.10.1080/00087114.2013.823295
- Falistocco E. 2016. Cytogenetic characterization of cultivated globe artichoke (Cynara cardunculus var. scolymus) and cardoon (C. cardunculus var. altilis). Caryologia. 69:1–4.10.1080/00087114.2015.1109935
- Fenech M. 2000. The in vitro micronucleus technique. Mutat Res. 455(1-2):81–95.10.1016/S0027-5107(00)00065-8
- Firuzi O, Asadollahi M, Gholami M, Javidnia K. 2010. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 122(1):117–122.10.1016/j.foodchem.2010.02.026
- Fiskesjo G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102(1):99–112.
- Fiskesjo G. 1993. The Allium cepa test in wastewater monitoring. Environ Toxicol Water Qual. 8(3):291–298.10.1002/(ISSN)1098-2256
- Frescura VD, Kuhn AW, Laughinghouse IVHD, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66(2):138–144.10.1080/00087114.2013.821832
- Frescura VD, Laughinghouse IVHD, Tedesco SB. 2012. Antiproliferative effect of the tree and medicinal species Luehea divaricata on the Allium Cepa cell cycle. Caryologia. 65(1):27–33.10.1080/00087114.2012.678083
- Grant WF. 1994. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 310(2):175–185.10.1016/0027-5107(94)90112-0
- Jakubowicz O, Żaba C, Nowak G, Jarmuda S, Żaba R, Marcinkowski JT. 2012. Heracleum Sosnowskyi Manden. Ann Agric Environ Med. 19(2):327–328.
- Jakubska-Busse A, Sliwinski M, Kobylka M. 2013. Identification of bioactive components of essential oils in Heracleum sosnowskyi and Heracleum mantegazzianum (apiaceae). Arch Biol Sci. 65(3):877–883.10.2298/ABS1303877J
- Karaismailoglu MC. 2014. Evaluation of potential genotoxic effect of trifluralin in Helianthus annuus L. (sunflower). Caryologia. 67(3):216–221.10.1080/0144235X.2014.974348
- Karaismailoglu MC. 2016. Investigation of the potential toxic effects of prometryne herbicide on Allium cepa root tip cells with mitotic activity, chromosome aberration, micronucleus frequency, nuclear DNA amount and comet assay. Caryologia. 68(4):323–329.
- Kibrik BS, Prokhorova IM, Pesnya DS. 2013. Mutagenic and antimitotic activity of the immunomodulatory drug tubosan. Pharm Chem J. 47(5):261–263.10.1007/s11094-013-0941-2
- Klancnik K, Drobne D, Valant J, Dolenc Koce J. 2011. Use of a modified Allium test with nanoTiO2. Ecotox Environ Saf. 74(1):85–92.10.1016/j.ecoenv.2010.09.001
- Kuhn AW, Tedesco M, Laughinghouse IVHD, Flores FC, de Bona da Silva C, do Canto-Dorow TS, Tedesco SB. 2015. Mutagenic and antimutagenic effects of Eugenia uniflora L. by the Allium cepa L. test. Caryologia. 68(1):25–30.10.1080/00087114.2014.998525
- Kumari M, Mukherjee A, Chandrasekaran N. 2009. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 407(19):5243–5246.10.1016/j.scitotenv.2009.06.024
- Leme DM, Marine-Morels MA. 2009. Allium cepa test in environmental monitoring: a review on its applications. Mutat Res. 682(1):71–81.10.1016/j.mrrev.2009.06.002
- Leme DM, Marin-Morales MA. 2008. Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water—A case study. Mut Res. 650(1):80–86.10.1016/j.mrgentox.2007.10.006
- Lindberg HK, Wang X, Jarventaus H, Falcka GCM, Norppa H, Fenech M. 2007. Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutat Res. 617(1–2):33–45.10.1016/j.mrfmmm.2006.12.002
- Malikov VM, Saidkhodzahaev AI. 2004. Coumarins: plants, structure, properties. J Nat Compd. 34(2):202–264.
- Mishyna M, Laman N, Prokhorov V, Maninang JS, Fujii Y. 2015. Identification of octanal as plant growth inhibitory volatile compound released from Heracleum sosnowskyi fruit. Nat Prod Commun. 10(5):771–774.
- Modallal N, Abderrahman SM, Papini A. 2008. Cytogenetic Effect of Arum maculatum Extract on the Bone Marrow Cells of Mice. Caryologia. 61(4):383–387.
- Mosham MH, Sharififar F, Dehghana GR, Ameri A. 2009. Bioassay screening of the various extracts of fruits of Heracleum Persicum desf. using brine shrimp cytotoxicity assay. Iranian J Pharmac Res 8(1):59–63.
- Nielsen C, Ravn HP, Nentwig W, Wade M. (eds.) 2005. The Giant Hogweed Best Practice Manual. Guidelines for the management and control of an invasive weed in Europe. Forest and Landscape Denmark, Hoersholm. 44 pp.
- Olorunfemi D, Duru E, Okieimen F. 2012. Induction of chromosome aberrations in Allium cepa L. root tips on exposure to ballast water. Caryologia. 65(2):147–151.10.1080/00087114.2012.711676
- Olorunfemi DI, Duru L, Olorunfemi OP. 2015. Genotoxic effects of bilge water on mitotic activity in Allium cepa L. Caryologia. 68(4):265–271.10.1080/00087114.2015.1032574
- Panda KK, Achary VM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB. 2011. In vitro biosynthesis, genotoxicity bioassay of silver nanoparticles using plants. Toxicol In Vitro. 25(5):1097–1105.10.1016/j.tiv.2011.03.008
- Pesnya DS, Romanovsky AV. 2013. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutat Res. 750(1–2):27–33.10.1016/j.mrgentox.2012.08.010
- Pesnya DS. 2013. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia. 66(3):275–281.10.1080/00087114.2013.852342
- Prokhorova IM, Kibrik BS, Pavlov AV, Pesnya DS. 2013. Estimation of mutagenic effect and modifications of mitosis by silver nanoparticles. Bull Exp Biol Med. 156(2):255–259.10.1007/s10517-013-2325-8
- Prokhorova IM, Kovaleva MI, Fomicheva AN, Babanazarova OV. 2008. Spatial and temporal dynamics of mutagenic activity of water in lake Nero. Inl Water Bio J. 1(1):1–25.
- Rezaee R, Behravan E, Behravan J, Soltani F, Naderi Y, Emami B, Iranshahi M. 2014. Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem Toxicol. 37(2):144–148.10.3109/01480545.2013.834352
- Rzymski P, Klimaszyk P, Poniedziałek B, Karczewski J. 2015. Health threat associated with Caucasian giant hogweeds: awareness among doctors and general public in Poland. Cutan Ocul Toxicol. 2015. 34(3):203–207.10.3109/15569527.2014.948685
- Sathak S, Manigandan P, Sekar Babu H, Sivaraj C, Sindhu S, Arumugam P. 2014. Antioxidant, antiproliferative activities and GC-MS analysis of methanol extract of leaves of Heracleum sprengelianum Linn. Intl J Pharma Bio Sci. 5(4):346–356.
- Satsyperova IF. 1984. Hogweed flora of the USSR - new fodder plants. Leningrad: Nauka; p. 223.
- Schimmer O. 1981. Vergleich der photomutagenen wirkungen von 5-MOP (Bergapten) und 8-MOP (Xanthotoxin) in Chlamydomonas reinhardii. Mutat Res. 89(4):283–296.10.1016/0165-1218(81)90109-9
- Ślusarczyk J, Dudek M, Wierzbicka M, Suchocki P, Kuraś M. 2014. Antimitotic effect of Selol and sodium selenate (IV) on Allium test cells. Caryologia. 67(3):250–259.10.1080/0144235X.2014.974353
- Sobieh SS, Kheiralla ZMH, Abeer AR, Naglaa ANYa. 2016. In vitro and in vivo genotoxicity and molecular response of silver nanoparticles on different biological model systems. Caryologia. 69(2):147–161.10.1080/00087114.2016.1139416
- Venturini S, Tamaro M, Monti-Bragadin C, Bordin F, Baccicchetti F, Carlassare F. 1980. Comparative mutagenicity of linear and angular furocoumarins in Escherichia coli strains deficient in known repair functions. Chem Biol Interact. 30(2):203–207.10.1016/0009-2797(80)90126-X
- Yourlova LYu, Chernyak DM, Koutovaya OP. 2013. Furocoumarins Heracleum sosnowskyi and Heracleum moellendorffi. Pac Med J. 2:91–93.
- Zimmet J, Ravid K. 2000. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp Hematol. 28(1):3–16.10.1016/S0301-472X(99)00124-1