Abstract
Quizalofop-p-ethyl (QPE) is a phenoxy herbicide which is used in agriculture to control weeds. The cytotoxic effects of QPE on pollen germination and pollen tube group in Hyacinthus orientalis were investigated and compared to the control. Experiments were carried out different QPE concentrations (0.125 M and 0.25 M). It was found that in vitro pollen germination and tube growth of H. orientalis was affected by QPE treatments; in particular, the highest QPE concentration caused changes in the morphological features of H. orientalis. The most potent QPE reduced pollen germination by threefold. Morphological abnormalities in the pollen tube were also observed.
1. Introduction
Rapidly increasing world population is regarded as one of the most important problems for mankind. A decline in agricultural areas plays an important role. Therefore, an evaluation of the available land for obtaining maximum yield has become a major goal. Pesticides can be used in plants against harmful organisms to improve agricultural productivity. However, the side-effects of an excessive use of these chemicals, incorrect application have started creating serious environmental problems (Tort et al. Citation2005).
Pesticides are known to pose serious health threats to living organisms and plants such as photosynthesis and transpiration are carried out; cause morphological, anatomical and physiological changes; inhibit pollen germination and pollen tube formation (He et al. Citation1995). Herbicides have been used in some agricultural fields in Turkey such as with beetroot, cabbage, canola, carrots, cauliflower, chickpeas, cucumbers, faba beans, lentils, onions, potatoes, pumpkins, and tomatoes (Bukun Citation2004; Tiryaki et al. Citation2004; Uludağ et al. Citation2006). Some farmers use herbicides at the recommended field dose (RFD) (Table ). We based the doses applied in our study on the doses applied in agriculture.In one study, the sub pod treatments consisted of two applications (10 and 15 days after sowing) in the main plot and post emergence of the herbicide QPE at 0.01, 0.005, 0.015, and 0.02 mM and an absolute control (Mahakavi et al. Citation2014). We determined the EC50 values for QPE on Glycine max in our previous study (Aksoy et al. Citation2013). There is no specific standard application in agriculture and the dose varies depending on plant species and the plant development process (Table ). According to Table , the average application dose range of 0.7–2 l ha–1 is recommended.
Table 1. Recommended field dose for QPE.
Pollen grains are simple structures of plants. Pollen tube formation is a simple model of growth and development (Taylor and Hepler Citation1997). In recent years, pollen germination, pollen tube development and pollen tube length have been used to determine the importance of the cytoskeleton in cell growth and differentiation and germination rate (Ma et al. Citation2000; Dillon and Zobel, Citation1957; Stairs and Troendle Citation1967; Mautinho et al., Citation2001.)
Hyacinthus orientalis is a perennial flower that belongs to the Asparagaceae family. It is native to southwestern Asia, southern and central Turkey. QPE is a herbicide used to control weeds. The aim of this study is to determine the effect of some herbicides on the pollen germination and pollen tube growth in H. orientalis.
2. Materials and methods
The End EC (5% quizalofop-p-ethyl, Agrogeneral, Izmir, Turkey) was used as an herbicide with QPE active ingredient. Pollen of H. orientalis was used as a test material because of the widespread occurrence of this ornamental plant in homes in Turkey. Pollen grains were collected at anthesis from opened flowers of H. orientalis. Pollen was germinated in liquid media containing 10% sucrose, 0.01% boric acid, and 0.03% CaCl2 and three microscopic slides were used for each treatment. QPE solutions and culture medium at the same volumes were used. First, 50 μl liquid media was dropped on microscopic slides. Then, 50 μl QPE solution was dropped on to slides. QPE solutions prepared with distilled water were 0.125 M and 0.25 M. For the control group, 50 μl distilled water was dropped instead of QPE solutions. Pollen was shed in this liquid culture media under an Olympus light microscope with aid of a hygiene pin (Olympus light microscope, Tokyo, Japan). The microscopic slides on which pollen was shed were placed in Petri dishes with a moist filter paper lining the lower plate, serving as an improvised humidity chamber. Then, the Petri dishes were settled in incubator at 22 ± 2°C for 1, 2, 3, 4, 5 and 24 h under dark conditions. After incubation the lamella was closed. Pollen tubes were stained with aceto orcein (Ünal Citation1986). Photographs were taken with the help of an Olympus photomicroscope. Statistical analyses of data were carried out using SPSS for Windows version 16.0 statistical software (SPSS Inc., Chicago, IL, USA). The lengths of 10 germinated pollen tubes randomly chosen from each of four different observation sections were measured and the averages were recorded for the statistical analysis. Statistically significant differences between the groups were compared using one-way analysis of variance (ANOVA) and Duncan’s test. The data are displayed as means ± standard deviation (SD), and p-values less than 0.05 are considered statistically significant.
3. Results
Pollen germination in H. orientalis continued until the end of the 24th hour. The germination rate reached 78% at the end of the 24th hour in the control group. But germination rate decreased 24% and 20 at the end of 24th hour in the 0.125 M and 0.25 M concentration of QPE respectively. Maximum germination was noted in the 24th hour. The germination rate reached as high as 44% in the second hour in the control group. After the third hour, increases in the germination rate were less (Table ). Minimum germination was noted in the 0.25 M concentration of QPE at all treatment times, but there was not a big difference in the decreasing effect between the two concentrations of QPE.
Table 2. Germinated pollen rate over time.
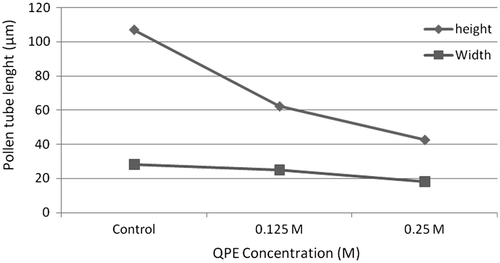
QPE at concentrations 0.125 and 0.25 had a slight effect on pollen germination but had more effect on pollen tube growth. At QPE concentrations of 0.125 M and 0.25 M, the germination rates were 24% and 20% and the tube lengths were 62.19 μm and 42.62 μm; these values were 78% and 106.83 μm of the control respectively at the end of the 24th hour. With an increase of QPE concentration, the pollen germination and pollen tube length exhibited an almost linear decline. The length of the pollen tube increased until the 24th hour, which was the last observation time and reached an average value of 106.83 μm at the end of the 24th hour for the control group. It was 42.62 μm with the treatment of 0.25 M QPE (Figure ).
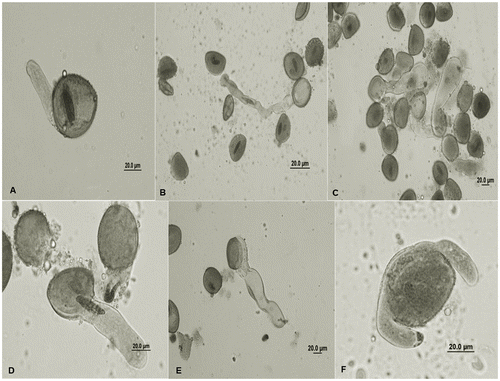
When the highest concentrations of the QPE solutions are considered in comparison with the control group, abnormality of pollens are given in Figure . Along with the start of germination, the exine layer thinned, the bulb started to appear due to swelling and ruptures in the germinal zone and the nutrients (proteins, starch grains) left the pollen. It was identified that among the pollens with high germination rates on the first day of incubation, the length of the pollen tubes was up to two times that of pollen stem on the first day of germination. During the observations of germination, a few cases of abnormal tube growth were determined, such as: elbow formation on pollen tube (Figure (B)), swelling at the tip of the tube (Figure (C)), weak pollen tubes (Figure (D)), expanded pollen tube and vacuolization (Figure (E)) and weak and undulating tubes and swelling (Figure (F)).
Figure 2. The change on pollen tube growth in H. orientalis after 24 h: (A) control; (B) elbow formation on pollen tube, 0.125 M QPE; (C) swelling at the tip of the tube, 0.125 M QPE; (D) weak pollen tubes, 0.25 M QPE; (E) expanded pollen tube and vacuolization, 0.25 M QPE; (F) weak and undulating tubes and swelling, 0.25 M QPE. Abbreviations: QPE: quizalofop-p-ethyl.
In vitro pollen germination was slow and started after transfer to the medium between 1 and 2 h. Some abnormal pollen tubes were observed. These abnormalities were found in 4.34% pollens of the control group, 82.46% and 84.67% pollens of 0.125 M and 0.25 M QPE treatment respectively at the end of the 24th hour (Table ). Effects of QPE on pollen germination, tube growth and morphology of H. orientalis in vitro are shown in Table . When the abnormality rate was 4.41% in the control group at the end of 24th hour, this rate was 84.19% in the 0.25 M. Over time abnormality rate increased in all concentrations and the control group.
Table 3. Effects of QPE on abnormality of pollen rate and morphology of H. orientalis over time.
4. Discussion
In vitro germination of pollen has been used as a powerful tool for genetic, physiological, biochemical and cytochemical studies for a wide range of plant species belonging to different families (Heslop-Harrison and Heslop-Harrison Citation1992). Pollen germination and pollen tube growth showed a decline with an increase of the herbicide concentration. The use of pesticides in agricultural areas increases the plant yield; however, some chemical substances may result in pollution in nature and health problems. The effects of pesticides used in high concentrations have been investigated by various studies. A number of studies were carried out to determine the harmful effects of pesticides (Soriano Citation1984; El-Khodary et al. Citation1989). The determination of pollen germination criteria is of considerable importance for the investigation of the impacts of yield-enhancing hormonal practices in breeding facilities (seed orchards, clone parks, etc.) and environmental pollution (radiation, etc.) on pollen biology (Caliskan et al. Citation2009).
5. Conclusions
In conclusion, it is found that in vitro pollen germination and tube growth of H. orientalis was affected by herbicide treatments. The herbicide used in our treatments led to some changes in the morphological features of H. orientalis pollen tubes and decreased pollen germination rate. Our studies show that in vitro pollen germination and tube growth of H. orientalis was affected by QPE treatments, and in particular the highest QPE concentration caused cytotoxic effects such as changes in the morphological features and several abnormalities in pollen tube growth. Pollen germination rates decreased with increasing concentrations.
H. orientalis is a perennial flowering plant and it is native to central Turkey. Wide application of herbicides for the control of weeds in agricultural practices is a potential threat to the genetic constitution of plants like H. orientalis. These results indicate that QPE may represent a potential danger to the genetic material of exposed living organisms by causing damage to plants and all living organisms. Therefore, it is necessary to test the genotoxic and cytotoxic effects of herbicides on plants and other systems before considering their applications for agricultural purposes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Kocaeli University under Research Project grant number [BAP 2009/40].
References
- Aksoy O, Deveci A, Kızılırmak S, Akdeniz GB. 2013. Phytotoxic effect of Quizalofop-P-Ethyl on Soybean (Glycine max L.). J of Biol and Environ Sci. 7(19):49–55.
- Bukun B. 2004. Critical periods for weed control in cotton in Turkey. Weed Res. 44(1):404–412.10.1111/j.1365-3180.2004.00415.x
- Caliskan B, Colgecen H, Pehlivan S. 2009. Pollen characteristics and in vitro pollen germination of Cedrus libani A. Rich. Afr J Biotechnol. 8(21):5696–5701.
- Dillon ES, Zobel BJ. 1957. A simple test for viability of pine pollen. J Forest. 55(1):31–32.
- El-Khodary S, Habib A, Haliem A. 1989. Cytological effect of the herbicide Garlon-4 on root mitosis of Allium cepa. Cytologia. 54(3):465–472.
- He Y, Wetzstein HY, Palevitz BA. 1995. The effects of a triazole fungicide, propiconazole, on pollen germination, tube growth and cytoskeletal distribution in Tradescantia virginiana. Sex Plant Reprod. 8(4):210–216.
- Heslop-Harrison J, Heslop-Harrison Y. 1992. Intracellular motility, the actin cytoskeleton and germinability in the pollen of wheat (Triticum aestivum L.). Sex Plant Reprod. 5(4):247–255.
- Ma LG, Fan QS, Yu ZQ, Zhou HL, Zhang FZ, Sun DY. 2000. Does Aluminum inhibit pollen germination via extracellular calmodulin? Plant Cell Physiol. 41(3):372–376.10.1093/pcp/41.3.372
- Mahakavi T., Bakiyaraj R., Baskaran L., Rashid N., Ganesh S.K. 2014. Effect of herbicide (Quizalofop-p-ethyl) on growth, photosynthetic pigments, enzymes and yield responses of Blackgram (Vigna mungo L.) Int Lett of Nat Sci. 4(9):58-65.
- Mautinho A, Camacho L, Haley A, Pais MS, Trewavas A, Malho R. 2001. Antisense perturbation of protein function in living pollen tubes. Sex Plant Reprod. 14(1):101–104.10.1007/s004970100086
- Soriano JD. 1984. Herbicides induced chromosomal aberrations and inheritance of digenic seedling mutation in Sorghum. Cytologia. 49(1):201–207.
- Stairs GR, Troendle V. 1967. Male bud pollen radiosensitivity in selected conifer species. Silva Genetica. 18(1):61–64.
- Taylor LP, Hepler PK. 1997. Pollen germination and tube growth. Annu Rev of Plant Physiol and Plant Mol Biol. 48(1):461–491.10.1146/annurev.arplant.48.1.461
- Tiryaki O, Yücel Ü, Sezen G. 2004. Biodegradation of trifluralin in Harran soil. J of Environ Sci and Health Part B. 39(5):747–756.10.1081/LESB-200030847
- Tort N, Öztürk I, Güvensen A. 2005. Effects of some fungicides on pollen morphology and anatomy of tomato (Lycopersicon esculentum Mill.). Pak J Bot. 37(1):23–30.
- Uludağ A, Uremis I, Ulger AC, Çakır B, Aksoy E. 2006. The use of maize as replacement crop in trifluralin treated cotton fields in Turkey. Crop Prot. 25(3):275–280.10.1016/j.cropro.2005.05.005
- Unal M. 1986. A Comparative cytological study on compatible and incompatible pollen tubes of Petunia hybrida. Istanbul Univ Fen Fak Mecm Seri B. 51(1):1–12.