Abstract
Apteronotidae is one of the oldest families from the order Gymnotiformes, and is composed of 94 species that can be found from Panama to northern Argentina. Some groups such as Apteronotus present taxonomic problems, mainly related to interspecific morphological similarity. In the present study, two species belonging to Apteronotus albifrons complex from different hydrographic basins were cytogenetically analyzed in order to define chromosomal structure and propose evolutionary trends for the group. Apteronotus albifrons presented 2n = 24 (14 m + 2sm + 8a) FN = 40 and Apteronotus caudimaculosus 2n = 24 (12 m + 2sm + 10a) FN = 38; this difference in karyotypic formulae can be explained by pericentric inversion involving metacentric and acrocentric chromosomes. The karyotypic similarity between these two species indicates a chromosomal diversification, characterized by changes in karyotype formulae, which were considered an excellent cytotaxonomic marker to discriminate A. caudimaculosus of other populations of Apteronotus albifrons complex.
Introduction
Apteronotidae is probably one of the oldest families from the order Gymnotiformes. According to Santana (Citation2007) the ancestor from which the 94 current species diversified (Eschmeyer and Fong Citation2016) arose about 11.8 million years ago. This fishes are popularly named sarapós, ituís or ghost knifefishes, and have a remarkable morphological variation in skull bones and jaws due to trophic specializations or sexual dimorphism (Crampton Citation2007; Albert and Crampton Citation2009). Members of Apteronotidae can be found from Panama to the Rio de La Plata in Argentina, with major diversity in Orinoco and Amazonas rivers (Ferraris Citation2007).
There are few phylogenetic reconstructions in Apteronotidae, and as Crampton (Citation2011) pointed out, it is still necessary to produce more robust phylogenetic trees. Some groups remain taxonomically confused, as is the case of Apteronotus albifrons. According to Albert (Citation2001), A. albifrons is, among apteronotids, the most widely distributed species, occurring from the Napo River basin (Ecuador), Orinoco, Tocantins and La Plata basin. Santana (Citation2007) hypothesized that A. albifrons represents a species complex consisting of six species: A. albifrons, A. maximilliani, A. spurrellii, A. jurubidae, A. mariae and A. caudimaculosus. The presence of two pale bands on the tail, which until then was a distinctive feature of only one species, is currently considered a synapomorphy for this monophyletic clade.
Although Apteronotidae are the most species rich family of the order Gymnotiformes, few cytogenetic studies were performed in the group. Currently karyotype information is available for: Apteronotus albifrons (2n = 24) (Howell Citation1972; Almeida-Toledo et al. Citation1981; Mendes et al. Citation2012), A. bonapartii (2n = 52) (Almeida-Toledo et al. Citation2007), Parapteronotus hasemanni (2n = 52), Sternachorhampus muelleri (2n = 32) and Sternachogiton preto (2n = 52) (Silva et al. Citation2014).
In this study a cytogenetic analysis was performed in Apteronotus albifrons and A. caudimaculosus from different hydrographic basins of Brazil. The purpose of this work is to compare the karyotype structure of these two species, aiming to finding karyotypic differences that are useful for a cytotaxonomic approach in this group of fish that is currently considered a species complex.
Material and methods
In this study, we performed cytogenetic analyses in Apteronotus caudimaculosus (five females and one male) (Figure ) collected in Parana River (Parana River basin) (21°06′10.26ʺ S, 51°47′14.01ʺ W) and A. albifrons (three females) (Figure ) from Bom river (Parana river basin) (23°52′08.77ʺ S, 51°38′49.01ʺ W). The collection of specimens was authorized by ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) license no. 1947869. After processing and subsequent fixation of the material, all specimens were deposited in the Museum of Zoology at the State University of Londrina (Vouchers: A. caudimaculosus MZUEL 15963 and A. albifrons MZUEL 15964).
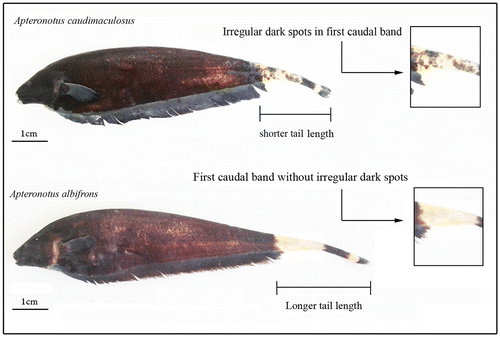
Figure 1. Species of Apteronotus albifrons complex analyzed here, highlighting the main morphological characteristics which distinguish them.
Before undergoing euthanasia (48 h), the specimens received an intraperitoneal injection of 2 ml of Broncho-vaxom (bacterial lysate) to trigger an inflammatory process and hence increase the number of renal cells in mitotic division (Molina et al. Citation2010). The mitotic chromosomes were obtained according to Bertollo et al. (Citation1978) and classified according to the arm ratio proposed by Levan et al. (Citation1964). The heterochromatin pattern was determined using the C-banding technique suggested by Sumner (Citation1972) with slight modifications briefly: in the staining phase Giemsa was replaced with 0.7 μl propidium iodide (50 μg ml–1) (Lui et al. Citation2012).
The number and localization of the major rDNA was determined using fluorescence in situ hybridization (FISH) performed according to Pinkel et al. (Citation1986). A rDNA 18S probe was obtained by polymerase chain reaction (PCR) amplification of the DNA of Prochilodus argenteus Spix & Agassiz, 1829 (Hatanaka and Galetti Jr. Citation2004) and biotin labeled by nick translation.
Results and discussion
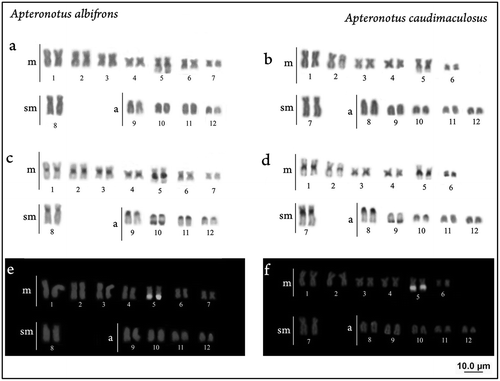
In both species 2n = 24 chromosomes was observed; however, small changes were detected in karyotype formulae and fundamental number. A. albifrons shows 14 m + 2sm + 8a and FN = 40 (Figure (a)), as already described for specimens from the Amazon basin (Howell Citation1972, Almeida-Toledo et al. Citation1981) and Upper Parana river (Mendes et al. Citation2012). In A. caudimaculosus the karyotypic formulae is 12 m + 2sm + 10a and FN = 38 (Figure (b)). This structural difference in chromosomes morphology between these two species can be explained by pericentric inversion involving metacentric and acrocentric pairs, and it is the only karyotypic difference observed.
Figure 2. Karyotypes: (a), (b) after conventional staining (Giemsa); (c), (d) after C banding (e), (f) after FISH with rDNA 18S.
Pericentric inversions play a major role in the karyotypic diversification of neotropical fishes, as already reported for species of Doradidae (Milhomem et al. Citation2008), Loricariidae (Takagui et al. Citation2014), Apteronotidae (Silva et al. Citation2014) and others. This structural rearrangement frequently contributes to the process of speciation (Feder and Nosil Citation2009), especially when such populations are in geographically isolated hydrographic basins, as described for Loricariichthys anus (Takagui et al. Citation2014). Alternatively the species may be in the same basin but in very distant locations; this can make genetic flow impossible and facilitate the fixation of the karyotype differences resulting from chromosomal rearrangement, as observed in the species of Apteronotus albifrons complex analyzed here.
Small variations in the chromosomal formula have already been reported for A. albifrons (de Almeida Toledo et al. Citation1981), including the presence of potential B-chromosomes in the population inhabiting the Parana River (Mendes et al. Citation2012). The occurrence of various cytotypes (different in the diploid number and/or karyotype formula) in the same species is coherent with the existence of a species complex (Kavalco et al. Citation2009; Laridondo Lui et al. Citation2010) and could not be considered a strong support for the taxonomic distinction of two species.
The C banding reveals heterochromatin blocks in pericentromeric regions and adjacent in the secondary constriction in both species (Figure (c) and (d)). This pattern was also observed in specimens of A. albifrons from Upper Parana river system (Mendes et al. Citation2012), with the exception of the terminal block detected in the short arm of pair 11. On the other hand, some specimens of this population have totally heterochromatic supernumerary chromosomes; this polymorphism was not observed in A. albifrons specimens from Bom River and in A. caudimaculosus. Silva et al. (Citation2014) described a similar pattern for Sternachorhampus muelleri, with heterochromatin in pericentromeric regions and in NORs, whereas Parapteronotus hasemanni and Sternachogiton preto showed a higher amount of heterochromatin forming large blocks to the full extent of the short arm in different chromosome pairs.
The major rDNA were detected by FISH with rDNA 18S probes in subterminal position on pair 6 in A. albifrons (Figure (e)) and pair 5 in A. caudimaculosus (Figure (f)). Single NORs in terminal position were also related in others populations of A. albifrons (Mendes et al. Citation2012), Parapteronotus hasemanni and Sternachogiton preto (Silva et al. Citation2014). The knowledge about the organization of rDNA sites in Apteronotidae is scarce, which makes impossible to delineate evolutionary trends.
According to Silva et al. (Citation2014) the basal karyotype for Apteronotidae is probably composed of 50 or 52 chromosomes, considering that the frequency of these diploid numbers is high in sister group Gymnotidae (Gymnotus + Eletrophorus) which is also the oldest group among Gymnotiformes (Albert Citation2001). In Apteronotus 2n = 52 is probably a plesiomorphic condition as already described to Apteronotus sp. and A. bonapartti (Almeida-Toledo et al. Citation2007). Thus, the reduction in the number of chromosomes in Apteronotus albifrons and A. caudimaculosus, besides being a remarkable feature of this group, would also be a derivative condition originated from centric fusions. The presence of 2n = 24 chromosomes and the pair bearing of NORs can be considered a cytogenetic character diagnostic for A. albifrons complex, as well as the presence of two pale bands on the tail represent a morphological synapomorphy for the group (Santana Citation2003).
Cytogenetic data reveal a high similarity between A. albifrons and A. caudimaculosus, but divergences in karyotypic formulae may be evidence of a process of karyotypic diversification. Santana (Citation2003) found a few morphological diagnostic characters for A. caudimaculosus, in particular the presence of dark spots in first caudal light band and the shorter tail length in the first species. Thus, the presence of a distinct karyotype formula originated from pericentric inversion, besides differentiating A. caudimaculosus from the other populations of A. albifrons, is also an important cytotaxonomic character which confirms that A. caudimaculosus is a valid species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors are grateful to CAPES for their financial support.
Acknowledgments
The authors are grateful to the Universidade Estadual de Londrina (UEL), Centro de Ciências Biológicas (CCB), Departamento de Biologia Geral for providing the laboratory infrastructure to carry out this work, and ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade), for permitting the collection of biological material.
References
- Albert JS. 2001. Species diversity and phylogenetic systematic of American knifefishes (Gymnotiformes, Teleostei). Vol.190. Miscellaneous Publications Museum of Zoology, University of Michigan; p. 1–127.
- Albert JS, Crampton WGR. 2009. A new species of electric knifefish, genus Compsaraia (Gymnotiformes: Apteronotidae) from the Amazon River, with extreme sexual dimorphism in snout and jaw length. Syst Biodivers. 7:81–92.10.1017/S1477200008002934
- Almeida-Toledo LF, Foresti F, Toledo-Filho SA. 1981. Constitutive heterochromatin and nucleolus organizer in the knifefish, Apteronotus albifrons (Pisces, Apteronotidae). Experientia. 37:953–954.10.1007/BF01971773
- Almeida-Toledo, LF, Daniel-Silva, MFZ, Moyses, CB, Foresti, F. 2007. Chromosome variability in Gymnotiformes (Teleostei: Ostariophysi). In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish cytogenetics. Vol. 1. Enfield (NH): Science Publishers. p. 17–39.
- Bertollo LAC, Takahashi CS, Moreira-Filho O. 1978. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Revista Brasileira de Genetica. 1:103–120.
- Crampton, WGR. 2007. Diversity and adaptation in deep channel Neotropical electric fishes. In: Sebert P, Onyango DW, Kapoor BG, editors. Fish life in special environments. Vol. 1. Enfield (NH): Science Publishers. p. 283–339.
- Crampton, WGR. 2011. An ecological perspective on diversity and distributions. In: Albert JS, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. vol. 1. Berkeley (CA): University of California Press. p. 165–189.10.1525/california/9780520268685.001.0001
- Eschmeyer WN, Fong JD. 2016. Species of fishes by family/subfamily. [cited 2016 Jun 14]. Available from: http://research.calacademy.org/research/ichthyology/catalog/Species>ByFamily.asp
- Feder JL, Nosil P. 2009. Chromosomal inversions and species differences: when are genes affecting adaptative divergence and reproductive isolation expected to reside whithin inversions? Evolution. 63:3061–3075.10.1111/evo.2009.63.issue-12
- Ferraris, CJ. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalog primary types. In: Zootaxa. Auckland: Magnolia Press.
- Hatanaka T, Galetti PM. 2004. Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica. 122:239–244.10.1007/s10709-004-2039-y
- Howell WM. 1972. Somatic chromosomes of the black ghost knifefish, Apteronotus albifrons (Pisces: Apteronotidae). Copeia. 1972:191–193.10.2307/1442803
- Kavalco KF, Brandão KO, Pazza R, Almeida-Toledo LF. 2009. Astyanax hastatus Myers, 1928 (Teleostei, Characidae): a new species complex within the genus Astyanax? Genet Mol Biol. 32(3):477–483.10.1590/S1415-47572009005000055
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Lui RL, Blanco DR, Margarido VP, Moreira Filho O. 2010. Chromosome characterization and biogeographic relations among three populations of the driftwood catfish Parauchenipterus galeatus (Linnaeus, 1766) (Siluriformes: Auchenipteridae). Braz Biol J Linn Soc. 99:648–656.10.1111/j.1095-8312.2009.01389.x
- Lui RL, Blanco DR, Moreira-Filho O, Margarido VP. 2012. Propidium iodide for making heterochromatin more evident in the C-banding technique. Biotech Histochem. 87(7):433–438.10.3109/10520295.2012.696700
- Mendes VP, Portela-Castro ALB, Júlio-Júnior HF. 2012. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 6:1–16.
- Milhomem SSR, Souza ACP, Nascimento AL, Carvalho JR, Feldberg E, Pieczarka JC, Nagamachi CY. 2008. Cytogenetic studies in fishes of the genera Hassar, Platydoras and Opsodoras (Doradidae, Siluriformes) from Jarí and Xingú Rivers, Brazil. Genet Mol Biol. 31:256–260.10.1590/S1415-47572008000200017
- Molina WF, Alves DEO, Araújo WC, Martinez PA, Silva MFM, Costa GWWF. 2010. Performance of human immunostimulating agents in the improvement of fish cytogenetic preparations. Genet Mol Res. 9:1807–1814.10.4238/vol9-3gmr840
- Pinkel D, Straume T, Gray J. 1986. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Nat Acad Sci USA. 83:2934–2938.10.1073/pnas.83.9.2934
- Santana CDCM. 2003. Apteronutus caudimaculosus n. sp. (Gymnotiformes: Apteronotidae), a sexually dimorphic black ghost knifefish from the Pantanal, Western Brazil, with a note on the monophyly of the A. albifrons species complex. Zootaxa. 252:1–11.10.11646/zootaxa.252.1
- Santana CDCM. 2007. Sistemática e Biogeografia da Família Apteronotidae Jordan 1900 (Otophysi: Gymnotiformes) [ Tesis]. Instituto Nacional de Pesquisas da Amazônia (INPA).
- Silva FHR, Pieczarka JC, Cardoso AL, Silva PC, Oliveira JA, Nagamachi CY. 2014. Chromosomal diversity in three species of electric fish (Apteronotidae, Gymnotiformes) from Amazon Basin. Genet Mol Biol. 37(4):638–645.10.1590/S1415-47572014005000018
- Sumner AMT. 1972. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 75:304–306.10.1016/0014-4827(72)90558-7
- Takagui FH, Venturelli NB, Dias AL, Swarça AC, Vicari MR, Fenocchio AS, Giuliano-Caetano L. 2014. The importance of pericentric inversions in the karyotypic diversification of the species Loricariichthys anus and Loricariichthys platymetopon. Zebrafish. 11(4):300–305.10.1089/zeb.2014.0985
