Abstract
The present study investigates the cytogenetic effects of the fungicide Royal Flo on mitotic cell division in maize (Zea mays L.) root cells. The maize grains were treated with various fungicide concentrations (50, 70, and 80%) for 20, 24 and 48 h exposure times. The results obtained indicate that the fungicide Royal Flo had some cytogenetic effects, by reducing the mitotic index and inducing various cytologic and chromosomal anomalies. These effects manifested differently, their intensity being proportional to the concentration and exposure time. The most frequent chromosomal anomalies were bridges, fragments and binucleated cells, but sticky chromosomes, rings chromosomes and cells with a micronucleus have also been seen. This study proves that the fungicide Royal Flo, even when administered in smaller quantities than generally recommended, reduces the germination and the mitotic index of maize cells and induces a large number of chromosomal anomalies, which suggests its toxic, mutagenic potential. Consequently, we should identify the optimal concentrations for each agricultural species, so that the efficacy of the fungicide does not diminish and, at the same time, its cyto-genotoxic potential is kept low.
Introduction
Farming Production costs are increased due to direct losses due to pathogens reducing the number of plants in the field, and indirect losses, including replanting costs (Baniani et al. Citation2016). To reduce and control pests and pathogenic agents which can harm seeds or plants, the seeds must be treated or disinfected with various chemical substances.
Fungicides, like all pesticides, affect human health and the environment, thus their effects should be evaluated (Adams and Moss Citation2008). The first and simplest step for evaluating the toxicity of a fungicide is at the level of the plant, by using test plants and assessing cytotoxic effects at the cellular level.
The widespread use of fungicides in agriculture to combat plant diseases has led to the appearance of a large number of commercial products on the market, but their over-use can create a range of environmental problems.
Maize seeds are generally treated with a fungicide before being sold, to protect the seeds from fungal infections (Munkvold and OMara Citation2002).
Royal Flo is a contact fungicide from the group of dithiocarbamates, approved in Romania and having an excellent effect on maize, wheat, sunflower and rape seeds in neutralizing a large range of diseases transmitted by seeds or from soil. The fungicide has the effect of blocking germination and suppressing mycelium growth for a large number of fungi. In maize, it is used to prevent infection by Fusarium spp. (fusariosis) and Phytium spp. (seedlings falling over, i.e. damping-off), but also with mould, common blight, cob and panicle blight in maize.
Dithiocarbamate fungicides are a group of chemical products that have been widely used in agriculture for a long time. The worldwide consumption of dithiocarbamates used as fungicides is between 25,000 and 35,000 t per year (Srivastava and Singh Citation2013).
There is no information in the literature regarding the effects of the fungicide Royal Flo on plants, but existing studies on other fungicides or herbicides show that they induce chromosomal anomalies in various plants (Bonciu Citation2012; Maity, Citation2014; Pulate and Tarar Citation2014; Aksoy et al. Citation2015). Extensive use of fungicides based on Thiram (the active substance of the fungicide Royal Flo) can have adverse effects on human cells (Santovito et al. Citation2012), the hepatic system (Dalvi et al. Citation1984) and the reproductive system (Bretveld et al. Citation2006).
The purpose of this study is to investigate the cytological and genotoxic effects induced by the fungicide Royal Flo in meristematic tissues of maize. We have chosen this topic because this fungicide is frequently over-used by farmers and toxicity effects for the environment can be very serious.
Materials and methods
Plant material
The biological material (Zea mays L, the Olt hybrid) was supplied by the SCDA Simnic Agricultural Research Station of Craiova. The fungicide chosen for study was Royal Flo (active substance Tiram, 480 g l–1), produced by Chemtura Corporation (Philadelphia, PA, USA). This fungicide is often used in Romania in growing maize, usually applied in a 100% concentration for 24 h.
Maize grains were sterilized with a 2% solution of NaClO for 5 min, and then they were washed several times with distilled water. Three concentrations of fungicide were obtained by dilution with distilled water (concentrations of 50, 70 and 80%). The seeds were soaked in the solutions with these concentrations for 20, 24 and 48 h, respectively, after being put in plastic casseroles on filter paper, at a temperature of 22 ± 2 °C. The control samples were treated with distilled water.
Microscopic preparations
After four days, the tips of the roots were collected and put in small glass bottles in a fixative solution containing ethyl alcohol and acetic acid (3:1) for 24 h, followed by meristematic tissue colouring using the Feulgen-Rossenbeck method, to visualize the chromosomes with an optical microscope. For this, the meristematic tissues were first hydrolysed for 5 min in HCl 1 N, and then they were put in HCl 50% (consisting of equal parts of HCl and distilled water) for 16 min at room temperature.
The actual colouring phase followed, with Schiff reagent prepared in accordance with the method proposed by Darlington and LaCour (Citation1963), based on the following components: fuchsine (1 g), distilled water (200 ml), hydrochloric acid (30 ml) and potassium metabisulphite (3 g). To obtain the colouring, after removing the hydrolysing solution, 3 ml of Schiff reagent were added on the meristematic tissues. After about 45 min, when the tissues turned purple-bluish, several temporary microscopic preparations were made, for each of the variants of the experiment, and they were visualized in the digital microscope MBL 2000 (manufactured by KRÜSS Optronic, Hamburg, Germany). The microscope has a zooming power of 4×, 10×, 20× and 60×; the field illumination of the microscope is adjustable, and the condenser has a double lens.
Statistical analyses
Five microscopic preparations were made for each variant of the experiment, and 500 cells per variant were visualized. The mitotic index, the index of the chromosomal anomalies and the germination percentage were calculated using the following formulae (Rehman et al. Citation1998):
Mitotic index (MI%) = total number of cells in division / total number of analysed cells × 100;
Chromosomal abnormalities index (AI%) = total number of aberrant cells / total number of cells in division × 100;
Germination (G%) = number of seeds germinated / total number of seeds × 100.
Statistical analysis was done using MS Excel 2007 (Botu and Botu Citation1997). The mean and standard deviation (SD) were calculated for the MI. The relationship between the MI and the frequency of abnormal cells was investigated through the Pearson correlation analysis, with the significance being shown for P5% and P1%.
Results
The cytoxicity and genotoxicity potential of the fungicide Royal Flo was estimated by observing cytological parameters, i.e. the mitotic index and the number and percentage of chromosomal anomalies.
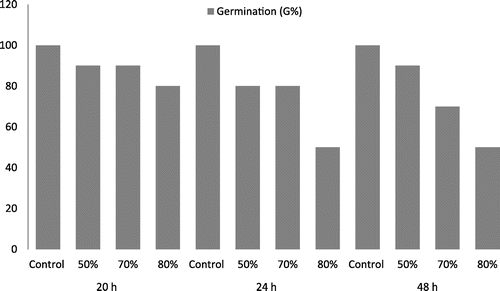
The results led to the conclusion that the treatment of maize seeds with the Royal Flo fungicide, depending on the concentration and exposure time, negatively influenced seed germination (Figure ). The maximal percentage of germination (100%) was reached by control samples, and the lowest value (50%) corresponded to an 80% fungicide concentration, for both 24 and 48 h exposure times.
Figure 1. The decrease of percentage of germination (G%) to Zea mays L. meristematic roots treated with fungicide Royal Flo at different concentrations and with different exposure times.
As shown in Table , cell division was also influenced by the fungicide treatment. The highest value of the mitotic index (MI) was reported for control samples without chromosomal anomalies (for all three exposure times). Also, various types and percentages of chromosomal anomalies, depending on the treatment variant, were identified in the meristematic cells of treated maize. The anomaly frequency was proportional to the reduction of the mitotic index (r = –0.897).
Table 1. The cytogenetic effects induced by the fungicide Royal Flo on the meristematic roots to Zea mays L.
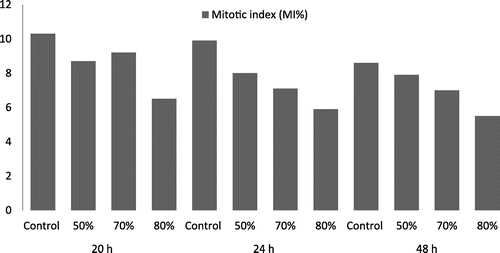
The fungicide Royal Flo was found to reduce the mitotic index, irrespectively of the concentration or exposure time, compared to the control batch. We can infer that there is a direct correlation between the increase of exposure time, fungicide concentration and mitotic activity reduction (Figure ).
Figure 2. The decrease of mitotic index (MI%) in Zea mays L. seeds treated with fungicide Royal Flo.
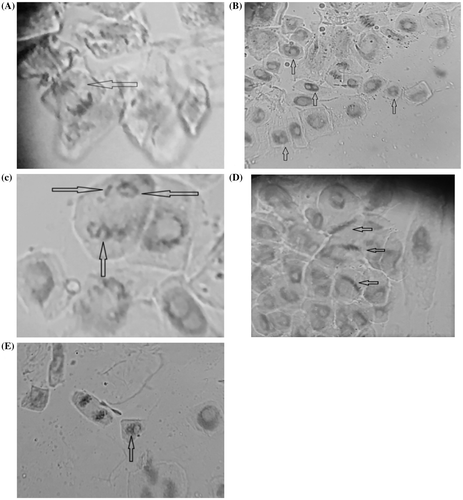
The most frequently identified anomalies were bridges (Figure (A)) and binucleated cells (Figure (B)), but ring chromosomes and fragments (Figure (C)), sticky chromosomes (Figure (D)) and cells with a micronucleus (Figure (E)) were also noticed.
Discussion
The results indicated that the increase in Royal Flo fungicide concentration reduces maize seed germination. The same results have been obtained in studies on other plants, such as Vigna mungo (Maity Citation2014) and Triticum aestivum (Marini et al. Citation2011), but they are contrary to the ones obtained in Zea mays by Alrajhi (Citation2014) who reported a stimulation of germination after treatment with the fungicides Dithane M-45 and Amistar.
The mitotic index reflects the frequency of cell division, its value being acknowledged as a measure of cytotoxicity for any living organism; if its value decreases below 22% of the control value, the decrease will have lethal effects on the tested organism (Antonsie–Wiez Citation1990).
Chromosomal anomalies are considered concrete proof of pesticide genotoxicity. Thus, Dimitrov et al. (Citation2006) claim that the test of chromosomal aberrations detects the clastogenic activity, both quantitatively and qualitatively, whereas the micronucleus test detects both the clastogenic effects and the deterioration of the mitotic apparatus.
The high number of chromosomal anomalies identified in the maize meristematic tissues treated with the Royal Flo fungicide indicates that the meristematic cells of Zea mays are highly sensitive to the genotoxicity phenomenon induced by pesticides. This is suggested by the direct correlation between the fungicide concentration, exposure time and the frequency of chromosomal anomalies. On the other hand, the appearance of various types of chromosomal anomalies could be accounted for by the differentiated assimilation of the pesticide by the meristematic cells of Zea mays, leading to the variable affectation of that species’ genome. However, the simple presence of chromosomal anomalies suggests the genotoxic potential of the fungicide Royal Flo in maize, even if the concentrations and the exposure time are more reduced than those recommended. That is why to avoid a potential risk to the health of humans, animals and the environment, pesticides should be selected so as to have the lowest impact in terms of genotoxicity effects, manifested through the appearance of cell anomalies, in general, and chromosomal ones, in particular.
Similar results were found in other studies on various fungicides. For example, the treatment of corn seeds with the Sportak fungicide showed that the mitotic index frequency in the meristematic cells of the root decreased and the chromosomal abnormalities frequency increased at all concentrations (Aksoy et al. Citation2015). Sutan et al. (Citation2014) reported that the use of the Ridomil fungicide for Allium cepa L. caused a decrease of the mitotic index and the appearance of chromosomal anomalies, which suggests an aneugenic potential.
The appearance of chromosomal anomalies similar to those identified in the present study, resulting from treatment with fungicide, has been reported by other authors as well (Haiba et al. Citation2011; Pulate and Tarar Citation2014; Selvaraju et al. Citation2015).
The lowest frequency was seen in the case of cells with micronuclei; similar results were obtained by Fisun and Rasgele (Citation2009) when using the fungicide Raxil for Allium cepa. On the other hand, the highest frequency of chromosomal abnormalities was observed in bridge-type chromosomes. Jabee et al. (Citation2008) consider that the presence of the chromosomes bridges could be due to their breakage and merger.
Even though several studies have reported the appearance of sticky chromosomes, some authors consider that the biochemical basis and the primary cause of the phenomenon remain unknown (Paglıarını Citation2000).
The appearance of chromosomal anomalies in plants is generally regarded as a potential danger, even if, sometimes, chemical mutagens (pesticides included) create variability.
The toxic effects induced by the Royal Flo fungicide to the species Zea mays L. may be much more serious, but undetectable with our current research devices, and that is why this research should be continued.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adams MR, Moss MO. 2008. Food microbiology and public health. In: Food Microbiology Royal Society of Chemistry. 3rd ed. Cambridge (UK): RSC Publishing. p. 158–176.
- Aksoy O, Asuman A, Tütünoğlu B. 2015. Genotoxic effects of the fungicide Sportak on Zea mays. Fresenius Environ Bull. 24(2):552–559.
- Alrajhi AMH. 2014. Effects of Amistar and Dithane M-45, a systemic fungicide, on growth parameters and antioxidative enzymes of maize (Zea mays L.). RRJBS. 3(4):13–19.
- Antonsie-Wiez D. 1990. Analysis of the cell in the root meristem of Allium cepa under the influence of Ledakrin folia. Histochem Cytobiol. 28(1–2):79–95.
- Baniani E, Arabsalmani M, Farahani E. 2016. Effect of seeds treatment with fungicides and insecticides on germination and vigurity, abnormal root producing and protection of cotton seedling. Int J Life Sci Sci Res. 2(5):519–530.
- Bonciu E. 2012. Cytological effects induced by Agil herbicide to onion. J Horticult For Biotechnol. 16(1):68–72.
- Botu I, Botu M. 1997. Statistical parameters used in the evaluation of the research of fruit plants. In: Methods and techniques of research in pomiculture. Rm. Valcea (RO): Conphys Publishing. p. 213–236.
- Bretveld RW, Thomas CM, Scheepers PT, Zielhuis GA, Roeleveld N. 2006. Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod Biol Endocrin. 4:30. doi:10.1186/1477-7827-4-30.10.1186/1477-7827-4-30
- Dalvi RR, Robbins TJ, Williams MK, Deoras DP, Donastorg F, Banks C. 1984. Thiram-induced toxic liver-injury in male sprague-dawley rats. J Environ Sci Health B. 19 (8–9):703–712. 10.1080/03601238409372458
- Darlington CD, LaCour LF. 1963. Methoden der Chromosomen Utersuchungen [Methods of chromosomes investigation]. Stuttgard: Frankische Verlagshandlung. W. Keller und Ca.
- Dimitrov B, Gadeva P, Benova D, Bineva M. 2006. Comparative genotoxicity of the herbicides Roundup, Stomp and Reglone in plant and mammalian test systems. Mutagenesis. 21(6):375–382. 10.1093/mutage/gel044
- Fisun K, Rasgele PG. 2009. Genotoxic effects of Raxil on root tips and anthers of Allium cepa L. Caryologia. 62(1):1–9. 10.1080/00087114.2004.10589659
- Haiba AAA, El-Hamid NRA, El-Hady EAAA, Al-Ansary ERMF. 2011. Cytogenetic effect of insecticide Telliton and fungicide Dithane M-45 on meiotic cells and seed storage proteins of Vicia faba. J Am Sci. 7(1):19–25.
- Jabee F, Ansari MYK, Shahab D. 2008. Studies on the eff ect of maleic hydrazide on root tip cells and pollen fertility in Trigonella foenum-graecum L. Turk J Bot. 32(5):337–344.
- Maity SK. 2014. Effects of Dithane M-45 (a fungicide) on root meristem of Vigna Mungo (L.) Hepper. IJAREAS. 3 (4):1–6.
- Marini N, Tunes LM, Silva JI, Moraes DM, Olivo F, Cantos AA. 2011. Carboxim Tiram fungicide effect in wheat seeds physiological quality (Triticum aestivum L.). Revista Brasileira de Ciências Agrárias. 6(1):17–22. 10.5039/agraria
- Munkvold GP, OMara JK. 2002. Laboratory and growth chamber evaluation of fungicidal seed treatments for maize seedling blight caused by Fusarium species. Plant Dis. 86(2):143–150. 10.1094/PDIS.2002.86.2.143
- Paglıarını MS. 2000. Meiotic behaviour of economically important plant species: the relationship between fertility and male sterility. Genet Mol Biol. 23(4):997–1002.
- Pulate PV, Tarar JL. 2014. Cytogenetic effect of systemic fungicide Calixin on root meristem cells of Allium cepa L. Int J Life Sci. 2(4):341–345.
- Rehman S, Harris PJC, Bourne WF. 1998. Effect of pre-sowing treatment with calcium salts, potassium salts, or water on germination and salt tolerance of acacia seeds. J Plant Nutr. 21(2):277–285. 10.1080/01904169809365402
- Santovito A, Cervella P, Delpero M. 2012. Chromosomal aberrations in cultured human lymphocytes treated with the fungicide. Thiram Drug Chem Toxicol. 35(3):347–351. 10.3109/01480545.2011.627862
- Selvaraju S, Vasanth M, Rajarajan R, Muralidharan R, Raghupathy V. 2015. Genotoxic effects of Carbendezim (fungicide) on the root apical meristems of Allium cepa L. RJPP. 7(1):29–33.
- Srivastava P, Singh A. 2013. Induction of chromosomal aberrations by Carbamate fungicide in fish Clarius batrachus (Asian Catfish). School J Agric Sci. 3(11):487–491.
- Sutan NA, Popescu A, Mihaescu C, Soare LC, Marinescu MV. 2014. Evaluation of cytotoxic and genotoxic potential of the fungicide Ridomil in Allium cepa L. Analele Stiintifice ale Universitatii „Al. I. Cuza” Iasi s. II a. Biologie vegetala. 60(1):5–12.