Abstract
In order to produce an autotetraploid population of anise hyssop (Agastache foeniculum L.), different concentrations of antimitotic agents including colchicine (0, 5000, 12,500 and 17,500 μM), oryzalin (0, 10, 50 and 100 μM) and trifluralin (0, 10, 50 and 100 μM) were used in three experiments. In the first, the apical meristem of the seedlings at the emergence of two true type leaves stage was treated with the antimitotic agents. In the second and third experiments, the antimitotic agents were used to treat seeds and seedlings (immersing seedlings into antimitotic solutions) for 6, 12 and 24 h. The survival rates were recorded six weeks post treatment. The polyploidy induction was confirmed using morphological and physiological indices, stomatal characteristics, flow cytometric analysis, and chromosome count in the diploid and tetraploid plants. Different antimitotic agents showed significant effects on the survival rate. By increasing the concentration of the agents, the survival rate significantly (p ≤ 0.05) decreased. Maximum percentage of tetraploid plants (20%) was obtained from seeds treated with 100 μM oryzalin for 24 h, whereas the maximum amount (16%) of tetraploid induction of apical meristems and seedlings was obtained by 17,500 μM colchicine and 50 μM trifluralin, respectively. In the polyploid plants, stomatal size and density, chloroplast number, morphological features (leaf length and width, distance between the nodes, leaf area, plant height, fresh and dry weight, and spikes length) and physio-biochemical characteristics (net photosynthesis, protein content, catalase and peroxidase activity) increased significantly (p ≤ 0.05).
Introduction
Ploidy manipulation is considered as a valuable tool in genetic improvement of many plants (Lopez-Pujol et al. Citation2004). Polyploidy has been used in horticulture as a breeding tool to enhance ornamental characteristics such as plant size, leaf thickness, flower size, and width-to-length ratio of leaves (Schwantes et al. Citation1977; Kermani et al. Citation2003; Ahmadi et al. Citation2013). In most plant species, artificial polyploidy has often been accompanied by increased cell size, leading to larger reproductive and vegetative organs (Adaniya and Shira Citation2001). It also affects the quality and quantity of important secondary metabolites in medicinal plants (Dhawan and Lavania Citation1996). From a horticultural perspective, polyploidy is important for breeding ornamental species to produce plants with thicker and bigger leaves and flowers, and in fruit trees, for production of seedless fruits, which are more attractive to consumers (Chen et al. Citation2006). There are different methods for induction of polyploidy in plants by antimitotic agents such as treatment of seeds (Hanzelka and Kobza Citation2001; Quan et al. Citation2004), flower buds (Wu et al. Citation2007), apical meristems (Lavania and Srivastava Citation1991; Saharkhiz Citation2006), roots (Taira et al. Citation1991), and in vitro techniques (Roy et al. Citation2001; Kermani et al. Citation2003; Khosravi et al. Citation2008). Natural polyploidy is present within the Lamiaceae family, for example within Thymus loscosii (Lopez-Pujol et al. Citation2004), and Glechoma hederacea (Wide’n and Wide’n Citation2000), and in Lavandula genus (Upson and Andrews Citation2004) because they have very small sized chromosomes. There are several types of chemical inducers used in polyploid induction, e.g. colchicine, oryzalin (3,5-dinitro-N4,N4-dipropylsulfanilamide), trifluralin [2,6-dinitro-N,N-din-propyl-4-trifluomethyl aniline], amiprophosmethyl, and N2O (Wannakrairoj and Wondyifraw Citation2013). All these chemicals are known to bind to the tubulin dimers, preventing the formation of microtubules, and, hence, spindle fibers during cell division (Hansen and Andersen Citation1996). Thereby, they prevent the migration of chromatids to opposite poles. This process is known as mitotic slippage, besides, the non-occurrence of the cytokinesis (Petersen et al. Citation2003). Among the antimitotic chemicals, colchicine and oryzalin are the most commonly used in plant species (Ojiewo et al. Citation2007; Dhooghe et al. Citation2011). Van Tuyl et al. (Citation1992) reported that oryzalin was effective in lily (Lilium) to induce polyploidy and could be considered as an alternative for colchicine. In many plants, oryzalin and trifluralin are more effective for stable polyploidy induction, have an increased survival rate, and are used at lower concentrations than colchicine (Ganga and Chezhiyan Citation2002; Zlesak et al. Citation2005). Anise hyssop (Agastache foenculum) is a perennial medicinal and spice plant native to the north America and belongs to the Lamiaceae family (Audit et al. Citation1976; Ojiewo et al. Citation2007). Anise hyssop is suitable to be used as a cut flower, or as potpourri, with flowers that dry nicely to navy blue. This plant is a valuable source of nectar for honeybee forage or as a herb for seasoning food and in flavoring liqueurs. The essential oil of anise hyssop has been found to possess antimicrobial and antifungal properties (Fong Citation2008). Chromosome counts for this genus suggest a basic number of x=9 (Verhoeven et al. Citation1990). The aims of the present investigation were: (1) to compare the effect of different concentrations of colchicine, oryzalin and trifluralin on the polyploid induction of Agastache foeniculum Kuntze; (2) to pre-select the polyploid plants according to their morphological and physiological changes; and (3) to verify the ploidy level of the pre-selected plants using chromosome counts and flow cytometric analysis.
Material and methods
Chromosome doubling method
The investigation consisted of three subsequent experiments in order to induce polyploidy in anise hyssop (Agastache foeniculum). In all experiments, different concentrations of colchicine (0, 5000, 12,500 and 17,500 μM), oryzalin (0, 10, 50 and 100 μM) and trifluralin (0, 10, 50, and 100 μM) were used to treat different explants.
Apical meristem treatment
In the first experiment, the seeds were planted in trays that were filled with peat moss:perlite:soil (1:1:1). When the seedlings were at the stage of emergence of two true leaves, the apical meristems were treated with drops of the mentioned concentrations of colchicine, oryzalin and trifluralin every morning for three successive days. Immediately after the treatments, the seedlings were covered with polyethylene plastic to avoid fast evaporation of the solutions. Then the plastic sheet was removed and the seedlings were grown in a 16 h light period at 24–27°C and 63% humidity. This experiment was conducted on a completely randomized design with five replications. Each replicate consisted of 10 plants.
Seed treatment
In the second experiment, different concentrations of colchicine, oryzalin and trifluralin were used to treat the seeds by soaking them in the antimitotic solutions for 6, 12 or 24 h. In each treatment, 50 seeds were used, and Tween “20” was added as surfactant for better efficiency of the treatments. The treated seeds were washed with distilled water and planted in the trays filled with peat moss:perlite:soil (1:1:1) in greenhouse under 16 h light period, 27–24°C day/night temperature and 63% humidity. This experiment was arranged in a factorial based on a completely randomized design with five replications.
Seedling treatment
In the third experiment, the seeds were planted in planting trays. At the emergence of two true leaves stage, the seedlings were immerged in the above mentioned concentrations of colchicine, oryzalin and trifluralin for 6, 12 or 24 h. In each treatment, 50 seedlings were used. The treated seedlings were washed with distilled water and carefully planted in the pots filled with a mixture of leaf mold:sand:loam soil (1:1:1). The pots were put in greenhouse with 16 h light period, 27–24°C day/night temperature and 63% humidity. This experiment was a factorial arrangement based on a completely randomized design with five replications.
Flow cytometry
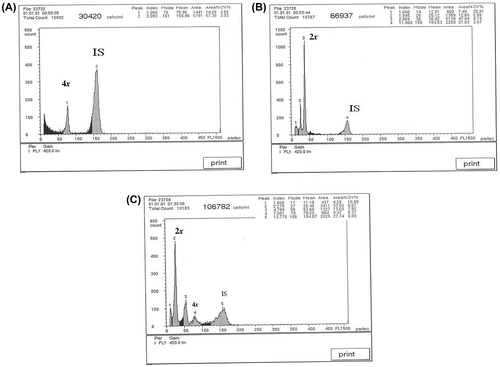
Ploidy was assessed by flow cytometry (FCM) using the same procedures, reagents and cytometer employed by Yokoya et al. (Citation2000). Petroselinum crispum “Champion Moss Curled” (2n=2x=22; 2C DNA amount=4.46 pg) was used as an internal calibration standard, and 4′,6-diamidino-2-phenylindole (DAPI) was used as the fluorochrome (Yokoya et al. Citation2000). Nuclei of anise hyssop leaves were mostly at the G1 (pre-replicative) stage of cell division, and only few of them were at the G2 (post-replicative) stage so that mixoploids could be identified in the FCM histograms by the presence of two large peaks.
Size and density of stomata
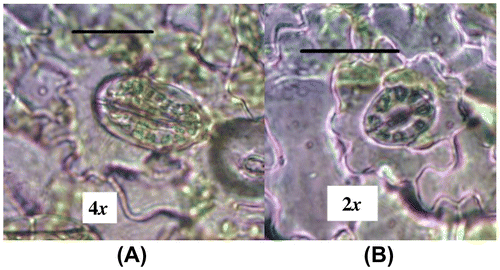
For this purpose, six diploid plants (control) and six plants of tetraploid plants were randomly selected. Measurement and scoring were performed for four well-expanded leaves of each plant. Three samples of epidermal cells were obtained from lower surface by nail varnish technique. A small area of the abaxial side of leaves was covered with a thin layer of clear nail polish and left to dry. Then, it was removed with a pair of fine tip forceps. The polish strips were mounted on a microscope slide and then evaluated for the density and size of leaf stomata under the light microscope (Olympus BX40, Shinjuku, Tokyo, Japan) at 40× and 100× magnification (Hamill et al. Citation1992; Saharkhiz Citation2006; Ghani et al. Citation2014).
Chloroplast number per guard cells
To study the chloroplast number in the stomatal guard cells, samples of the epidermal layer from the abaxial side of 10 diploid control plants and tetraploid plants’ leaves were obtained. The epidermal layer was stained with 1% Lugol’s iodine solution and observed by light microscope at 1000 × magnification (Guimarães and Stotz Citation2004; Omidbaigi et al. Citation2010a). The stomatal characteristics and chloroplast number were measured in the middle leaves from each of the tested branches both in the control and putative tetraploids (Omidbaigi et al. Citation2010b).
Morphological characteristics
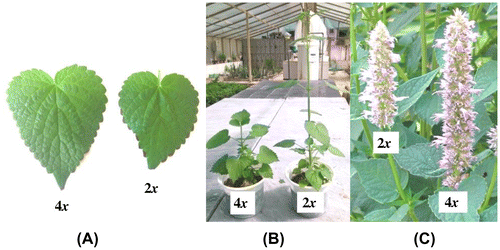
The morphological characteristics such as leaf length and width, size of internodes, leaf area, plant height, fresh and dry weight, and flower spike were compared between 10 tetraploid and diploid plants. These parameters were evaluated with caliper and digital balance.
Physiological characteristics
Protein content was determined following the method of Aebi (Citation1984). Catalase (CAT) and peroxidase (POD) activity was measured according to the method of Chance and Maehly (Citation1955). Chlorophyll index was measured with a SPAD-502 (Konica Minolta Sensing Inc., Tokyo, Japan) and net photosynthesis by using photosynthometer (HCM, Walz, Germany). Ten samples of tetraploid and diploid plants were selected to measure these features. The morphological and physiological parameters evaluated 16 weeks after the polyploidization treatments.
Chromosome count
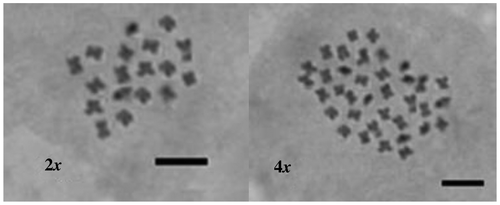
Plants that had 0.5–1.0 cm long roots were pretreated with 0.002 M 8-hydroxyquinoline (8-HQ) for 4 h at room temperature. The roots were then washed in distilled water for 5 min. The root tips were fixed in Carnoy solution (ethyl alcohol and acetic acid, 3:1) at room temperature for 24 h. The samples were rinsed twice with distilled water, and stored in 70% ethanol at 4°C for further cytological analysis. The roots were hydrolyzed in 1 N HC1 at 60°C for 12 min, and then squashed on slides containing a drop of 1% acetocarmine staining solution (Sakhanokho et al. Citation2009). A photomicroscope (Olympus BX40) was used for chromosome observations.
Statistical analysis
Mean data were determined using a one-way (apical meristem) and two-way (seed and seedling) analysis of variance (ANOVA). Significant differences among the treatments were detected using Duncan’s multiple range test (DMRT) at the 0.01 or 0.05 level of probability. The statistical differences among the means of the traits of control and autotetraploid plants were computed by the t-test.
Results and discussion
Apical meristem treatment
ANOVA results showed that different antimitotic chemical concentrations had significant effects (p<0.01) on the percentage of survival and tetraploid induction from the apical meristems of the plants. The highest percentage of survival was observed in the control (96%) and 5000 μM colchicine treatment (98%), and the lowest percentage of survival (54.00%) was detected in 17,500 μM colchicine and 100 μM trifluralin. The maximum percentage of diploid (98.00%), mixoploid (34.00%) and tetraploid (16.00%) was observed by application of 5000 μM colchicine, 50 μM oryzalin and 17500 μM colchicine, respectively (Table ).
Table 1. Influence of different concentrations of antimitotic agents on the survival rate and ploidy level of the apical meristems.
Seed treatment
The results showed that the different antimitotic chemicals concentrations and duration times significantly (p<0.01) affected the survival rate and tetraploid induction in anise hyssop. Table illustrates that the highest (98.00%) and the lowest (56.00%) survival rates were observed in the control and the seed treatment with 50 μM oryzalin for 24 h, respectively. The highest percentage was of diploid (100.00%), mixoploids (24.00%) and tetraploids (20.00%) observed in the control (6 h), 100 μM trifluralin (24 h) and 100 μM oryzalin (24 h), respectively (Table ).
Table 2. Influence of different concentrations of antimitotic agents on the survival rate and ploidy level of the plant seeds and seedlings.
Seedling treatment
Different antimitotic concentrations and duration periods had significant (p<0.01) effect on the survival rate and tetraploid induction in the seedlings. The seedlings that were not treated with antimitotic chemicals (controls) had the highest percentage of survival and diploid, whereas the lowest percentage of survival (24.00%) was observed in the seedlings treated with 17500 μM colchicine for 24 h (Table ). The maximum percentage of mixoploid (40.00%) and tetraploid (16.00%) induction was observed by applying 100 μM oryzalin for 12 h and 50 μM trifluralin for 12 h, respectively.
Flow cytometry
FCM was applied to screen the potential polyploids. Ploidy level was estimated by comparing the peak position of the G1 nuclei of a standard plant (parsley) with the peak position of the G1 nuclei of the unknown sample. In the present investigation, putative tetraploids had a double amount of DNA, confirming their tetraploidy (Figure ). Ploidy level was confirmed by chromosome counting.
Stomatal characteristics
The t-test analysis showed significant differences (p<0.01) in the stomatal length and diameter, stomatal density and chloroplast number in the guard cells of diploid and tetraploid plants (Table ). Stomatal diameter, length and density increased significantly in tetraploid plants (Figures and ).
Table 3. Morphophysiological characteristics of the diploid and tetraploid plants.
Chloroplast number
There was a significant difference between the guard cell chloroplast numbers of the diploid and tetraploid plants (Figure ). The guard cells of the diploid plants had an average number of 11.16 ± 0.98 chloroplasts whereas those of the tetraploids had an average number of 20.16 ± 3.18 chloroplasts (Table ).
Morphological differences between the diploid and tetraploid plants
The morphological and physiological parameters were evaluated 16 weeks after the polyploidization treatments. Morphological variations were observed between the diploid and tetraploid plants (Table ). The tetraploid plants had larger leaf length (57.23 ± 0.43 mm), leaf width (46.14 ± 0.90 mm), leaf area (22.16 ± 0.98 mm−2) and flower spike length (16.82 ± 0.25 cm) compared to the diploid plants (Table ). Moreover, the tetraploids had higher chlorophyll index (64.16 ± 1.39), fresh weight (12.28 ± 0.39 g) and dry weight (1.34 ± 0.08 g) compared to the diploids (Table ). Shorter stem length (85.98 ± 0.59 mm) and internode (11.99 ± 0.38 mm) were observed in the tetraploids compared to the diploids (Table and Figure ).
Physiological variations between the diploid and tetraploid plants
Induction of polyploidy significantly affected the enzyme activity and protein content. Table illustrates that the tetraploid plants have the highest CAT activity (2.32 ± 0.18 μmol min−1 mg−1 protein), POD activity (01.17 ± 0.02 μmol min−1 mg−1 protein) and protein content (0.39 ± 0.03 mg g−1 FW). However, net photosynthesis was increased (9.38 μmol Co2 m−2s−1) in the tetraploid plants (Table ).
Chromosome observation
Ploidy levels of the diploid (2n=2x=18) and tetraploid (2n=4x=36) plants were confirmed by the chromosome count of the root tips (Figure ). Determination of chromosome number is difficult in Agastache foeniculum because of the thickness of the roots and very small sizes of the chromosomes.
Discussion
In the present study, increasing the concentration of antimitotic chemicals significantly decreased the percentage of plant survival. The highest tetraploid induction (16%) and the lowest survival rate (54%) from the apical meristems was attained by 17,500 μM colchicine treatment. Applying colchicine at 0.1–2.5% concentrations on the apices of cotyledons for 2–7 days was very successful in inducing polyploidy in Pelargonium × hortorum (Jadrna et al. Citation2010). Colchicine application has been effective for chromosome doubling of many crops including feverfew (Tanacetum parthenium L.) (Saharkhiz Citation2006), dragonhead (Dracocephalum moldavica L.) (Omidbaigi et al. Citation2010b) and basil (Ocimum basilicum L.) (Omidbaigi et al. Citation2010a; Malekzadeh Shafaroudi et al. Citation2012). Colchicine had irreversible effects on cell division and growth and was also effective in micronucleus formation (Temel and Gozukirmizi Citation2015). In the seed treatment, the highest rate of chromosome doubling (20%) was achieved when the explants were treated with 100 μM oryzalin for 24 h, which was associated with the lowest survival rates (56%). Higher concentration of antimitotic chemicals and longer duration of treatment reduced the survival rate but increased tetraploid induction. In the seed treatment experiments with different antimitotic chemicals, oryzalin was more effective compared to trifluralin and colchicine, which resulted in 20% tetraploid induction. Oryzalin inhibits mitosis activity and is one of the chemicals used for chromosome doubling in lily (Van Tuyl et al. Citation1992), potato (Verhoeven et al. Citation1990), tobacco (Ramulu et al. Citation1991), rose (Kermani et al. Citation2003) and many others. Several reports show that oryzalin is more effective in polyploidy induction than colchicine (Fong Citation2008). This is supported by the findings of Van Tuyl et al. (Citation1992) in lily, and Wannakrairoj and Wondyifraw (Citation2013) in Aframomum corrorima. In the seedling treatment, oryzalin and trifluralin were more effective than colchicine for polyploid induction. The maximum percentage of tetraploid induction (16%) was achieved with the treatment containing 50 μM trifluralin for 12 h. The lowest survival rate (24%) was observed in the seedlings treated with 17,500 μM colchicine for 24 h. Li-hong et al. (Citation2014) and Zlesak et al. (Citation2005) reported that trifluralin was more effective in polyploid induction in Anthurium andraeanum and Rosa chinensis var. minima. Lower survival rates may be due to a physiological disturbance caused by spindle inhibitors, resulting in a reduced rate of cell division (Swanson Citation1957). The results showed that stomatal size and chloroplast number increased in the tetraploid plants but stomatal density decreased. The stomatal length and diameter also increased as the ploidy level was increased. Therefore, tetraploids had larger stomata compared to the control progenitors. Similar results were observed in Aframomum corrorima (Wannakrairoj and Wondyifraw Citation2013), Mentha mozaffarianii (Ghani et al. Citation2014), and Ocimum basilicum (Omidbaigi et al. Citation2010a). However, the stomatal number per unit was significantly reduced by increasing the ploidy level due to increased stomatal size. High ploidy levels resulting in lower stomatal density have previously been confirmed in Mentha mozaffarianii (Ghani et al. Citation2014), feverfew (Saharkhiz Citation2006), and Tagetes erecta (Sajjad et al. Citation2013). Our findings are further in agreement with the observations in Salvia hains (Hosseini Grouh et al. Citation2011), Humulus lupulus (Roy et al. Citation2001), Zizyphus jujube (Gu et al. Citation2005) and Dracocephalm moldavica (Omidbaigi et al. Citation2010a). Conversely, Kim and Kim (Citation2003) in Cymbidium hybrid and Kerdsuwan and Te-chato (Citation2012) in Rhynchostylis gigantean observed higher stomatal density by increasing the ploidy levels. In the present study, the number of chloroplasts in stomatal guard cells increased by increase in polyploid induction, which is in agreement with a previous study on basil (Ocimum basilicum L.) (Omidbaigi et al. Citation2010a). It is expected that high ploidy levels are correlated with higher numbers of chloroplasts; however, it is also related to plant species and tissues that are used to measure the chloroplast number (Hosseini Grouh et al. Citation2011). Kerdsuwan and Te-chato (Citation2012) reported that the chloroplast numbers of tetraploid plants of Rhynchostylis gigantean were lower than that of diploid plants, indicating a negative correlation between chloroplast number and ploidy level. In this experiment, tetraploids had larger leaf length, leaf width, leaf area and spike length but shorter plant height and internode space. Rhododendron tetraploids, which were induced by oryzalin, had larger leaves, flowers and pollen compared with diploids (Contreras et al. Citation2007). Tetraploid watermelons had greater leaf area, and larger flower ovaries and seeds as well as a thicker rind (Jaskani et al. Citation2005). Similar results were previously reported in feverfew (Saharkhiz Citation2006), Mentha mozaffarianii (Ghani et al. Citation2014) and Dracocephalm moldavica (Omidbaigi et al. Citation2010a). Also tetraploid plants had higher chlorophyll index, as well as fresh and dry weights compared to diploids. Increasing the ploidy level caused more chlorophyll content in Festuca arundinacea (Joseph et al. Citation1981) and strawberry (Murti et al. Citation2012). Abdoli et al. (Citation2013) reported that the chloroplast number in guard cells, amount of chlorophyll, width and thickness of leaves were increased in tetraploid plants of Echinacea purpurea than that of diploid plants. But the results of Bagheri and Mansouri (Citation2015) showed that the chlorophyll content was not changed by polyploidy induction in Cannabis sativa plants. The slower rate of growth in polyploid plants is related to the reduced rate of cell division (Eigsti Citation1947), less growth hormone (Larsen and Mintung Citation1950) and the low activity of metabolites in tetraploids (Larsen and Mintung Citation1950). In the present work, tetraploids had higher fresh and dry weights compared to diploids; whereas the dry weight of Vicia villosa (Tulay and Unal Citation2010) and Anthurium andraeanum (Chen Citation2011) was higher in diploids than in tetraploids. These results show that ploidy level significantly affected the net photosynthesis rate. In this regard, Joseph et al. (Citation1981) reported that the net photosynthesis was significantly increased by the ploidy levels of 4x, 6x, 8x and 10x allopolyploid series of tail fescue (Festuca arundinacea). They suggested that ribulose bisphosphate carboxylase (RuBPCase) may represent a marker for increasing the net photosynthesis. Polyploidization appeared to increase the selective allocation of total protein for synthesis of RuBPCase; however, there was also a range for carboxylase content among the genotypes within a given ploidy level. Beysel (Citation1957) reported that the net photosynthesis was increased by increasing the ploidy level in Beta vulgaris. Similar results were also observed with tetraploid ryegrass plants (Garrett Citation1978). However, in some species, the photosynthetic rate decreased with polyploidization (Hall et al. Citation1978; Albuzio et al. Citation1978).
In the present study, the activity of enzymes (CAT and POD) and protein content were increased in the tetraploid plants. Enhancement in enzyme activity with increased ploidy level has been confirmed by some researchers (Maroni and Plaut Citation1973; Shafieizargar et al. Citation2013; Zhang et al. Citation2010). Polyploidization increases vigor through not only stomatal features but also antioxidant capacity (Deng et al. Citation2012; Talukdar Citation2014). Riddle et al. (Citation2010) reported that polyploid induction changed physiological functions or gene expression. As the chromosome number increased, DNA content and enzyme activity per cell were also increased. In contrast, unchanged and even reduced enzyme levels have also been described in some studies (Audit et al. Citation1976; Schwantes et al. Citation1977). Our findings showed that the protein content of the induced tetraploid plants increased. However, Joseph et al. (Citation1981) reported that the total protein was not increased significantly by increasing the ploidy level in Festuca arundinacea.
In the present study, chromosome counting and flow cytometric analysis demonstrated the induction of tetraploid individuals (2n = 4x =36) from diploid anise hyssop plants. Moghbel et al. (Citation2015) showed that the DNA content was increased by polyploidy induction in safflower and licorice plants. The basic method used for ploidy estimation was FCM, which is a quick, reliable and widely used technique for identifying ploidy in plants (Yang et al. Citation2006; Urwin et al. Citation2007). FCM reduces the time to determine ploidy level in safflower and licorice plants (Moghbel et al. Citation2015). The reliability of FCM has been proved in cytological analysis (Gu et al. Citation2005). Although chromosome counting is a direct method for ploidy analysis, the FCM analysis facilitates the screening for ploidy levels after treatment with different antimitotic chemicals quickly and efficiently.
Conclusion
In conclusion, the present investigation for the first time proposed a model for induction of polyploidy in anise hyssop. The results showed that different treatments had significant effects on survival rate and polyploidy induction. Increasing the concentration of antimitotic chemicals decreased the plants’ survival rate. In the apical meristem treatment, maximum percentage of tetraploid induction (16%) was observed by 17,500 μM colchicine treatment. The seeds treated with 100 μM oryzalin for 24 h showed the maximum rate of polyploid induction (20%). The seedlings treated with 50 μM trifluralin for 12 h showed the maximum rate (16%) of tetraploid plants. By increasing the ploidy level, the stomatal features as well as the morphophysiological characteristics of the plant markedly changed. Moreover, the techniques of polyploid induction and screening of putative polyploid plants, developed in the present work, could facilitate the breeding of the new varieties of anise hyssop, or be considered as models for polyploidy manipulation in other medicinal and ornamental plants. Overall, anise hyssop hardly responses to antimitotic chemicals for tetraploidy induction. For further investigation, in vitro polyploid induction of this species or others of the same genus by antimitotic chemicals is recommended.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors wish to extend their thanks and appreciation to Shiraz University, Research and Technology Council, for financial support.
References
- Abdoli M, Moieni A, Naghdi Badi H. 2013. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant. 35(7):2075–2083.10.1007/s11738-013-1242-9
- Adaniya S, Shira D. 2001. In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci Hort. 88(4):277–287.10.1016/S0304-4238(00)00212-0
- Aebi H. 1984. Catalase in vitro. Methods in Enzymology. 105(1):121–126.10.1016/S0076-6879(84)05016-3
- Ahmadi T, Jafarkhani Kermani M, Mashayekhi K, Hasanloo T, Shariatpanahi ME. 2013. Comparing plant morphology, fertility and secondary metabolites in Rosa hybrida cv iceberg and its chromosome-doubled progenies. Int Res J of App and Bas Sci. 4(12):3840–3849.
- Albuzio A, Spettoli P, Cacco G. 1978. Changes in gene expression from diploid to autoploid status of Lycopersicon esculentum. Physiol Plant. 44(2):77–80.10.1111/ppl.1978.44.issue-2
- Audit I, Deparis P, Flavln M, Rosa R. 1976. Erythrocyte enzyme activities in diploid and triploid salamanders' (Pleurodeles waltlii) of both sexes. Biochem Genet. 14(9):759–769.10.1007/BF00485339
- Bagheri M, Mansouri H. 2015. Effect of induced polyploidy on some biochemical parameters in Cannabis sativa L. Appl Biochem Biotechnol. 175(5):2366–2375.10.1007/s12010-014-1435-8
- Beysel D. 1957. Assimilations and atmungsmessungen an diploiden and polyploiden. Zuckerrüben. 27(1):261–272.
- Chance B, Maehly AC. 1955. Assay of catalases and peroxidase. Methods in Enzymology. 2(1):764–775.10.1016/S0076-6879(55)02300-8
- Chen C. 2011. Induction of Anthurium andraeanum ‘‘Arizona’’ tetraploidby colchicine in vitro. Euphytica. 181(2):137–145.10.1007/s10681-010-0344-3
- Chen LP, Wang YJ, Zhao M. 2006. In vitro induction and characterization of tetraploid Lychnis senno Siebold et Zucc. Hort Sci. 41(3):759–761.
- Contreras RN, Ranney TG, Tallury SP. 2007. Reproductive behaviour of diploid and allotetraploid Rhododendron L. ‘Fragrant Affinity’. Hort Sci. 42(1):31–34.
- Deng B, Du W, Liu C, Sun W, Tian S, Dong H. 2012. Antioxidant response to drought, cold and nutrient stress in two ploidy levels of tobacco plants: low resource requirement confers polytolerance in polyploids? Plant Grow Regu. 66(1):37–47.
- Dhawan OP, Lavania UC. 1996. Enhancing the productivity of secondary metabolites via induced polyploidy. Euphytica. 87(2):81–89.10.1007/BF00021879
- Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J. 2011. Mitotic chromosome doubling of plant tissues in vitro. A review. From the issue entitled "Special Issue: in Vitro Ploidy Manipulation in the Genomics Era". Plant Cell Tiss Org. 104(3):359–373.10.1007/s11240-010-9786-5
- Eigsti OJ. 1947. The pollen tube method for making comparisons of differences in mitotic rates between diploid and tetraploid. Genet. 32(1):32–58.
- Fong SW. 2008. In vitro induction of polyploidy in nepenthes gracilis [ MSc thesis]. KualaLumpur, Malaysia: Facultyof Science, University of Malaya.
- Ganga M, Chezhiyan N. 2002. Influence of the antimitotic agents colchicine and oryzalin on in vitro regeneration and chromosome doubling of diploid bananas (Musa spp). J Hort Sci and Biot. 77(5):572–575.10.1080/14620316.2002.11511540
- Garrett MK. 1978. Control of photorespiration at RuBP carboxylase/oxygenase level in ryegrass cultivars. Nature. 274(1):913–915.10.1038/274913a0
- Ghani A, Neamati SH, Azizi M, Saharkhiz MJ, Farsi M. 2014. Artificial autotetraploidy induction possibility of two iranian endemic mint (Mentha mozaffarianii) ecotypes. Not Sci Biol. 6(2):185–191.
- Gu XF, Yang AF, Meng H, Zhang JR. 2005. In vitro induction of tetraploid plants from diploid Zizyphus jujube Mill. Cv Zhanhua Plant Cel Rep. 24(11):671–676.10.1007/s00299-005-0017-1
- Guimarães RL, Stotz HU. 2004. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection1. Plant Physiol. 136(3):3703–3711.10.1104/pp.104.049650
- Hall NP, Keys AJ, Merrettr MJ. 1978. Ribulose-1,5-diphosphate carboxylase protein during flag leaf senescence. J Exp Biol. 29(1):31–37.
- Hamill SD, Smith MK, Dodd WA. 1992. In vitro induction of banana autotetraploids by colchicine treatment of micro-propagated diploids. Aust J Bot. 40(6):887–896.10.1071/BT9920887
- Hansen NJP, Andersen SB. 1996. In vitro chromosome doubling potential of colchicine, oryzalin, trifluralin and APM in Brassica napus microspore culture. Euphytica. 88(2):159–164.10.1007/BF00032447
- Hanzelka P, Kobza F. 2001. Genome induced mutation in Challistephus chinensis 1: effect of colchicine application on the early plant development. Zahradnictvi Hort Sci. 28(1):15–20.
- Hosseini Grouh MS, Meftahizade H, Lotfi N, Rahimi V, Baniasadi B. 2011. Doubling the chromosome number of Salvia hains using colchicine: evaluation of morphological traits of recovered plants. J Medi plant Rese. 5(19):4892–4898.
- Jadrna P, Plavcova O, Kobza F. 2010. Morphological changes in colchicine-treated Pelargonium × hortorum L.H. Bailey greenhouse plants. Horti Sci (Prague). 37(1): 27–33.
- Jaskani MJ, Kwon SW, Kim DH. 2005. Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica. 145(3):259–268.10.1007/s10681-005-1644-x
- Joseph MC, Randall DD, Nelson CJ. 1981. Photosynthesis in polyploid tall fescue. Plant Physiol. 68(4):894–898.10.1104/pp.68.4.894
- Kerdsuwan N, Te-chato S. 2012. Effects of colchicine on survival rate, morphological, physiological and cytological characters of chang daeng orchid (Rhynchostylis gigantean var. rubrum Sagarik) In Vitro. Int J Agric Technol. 8(4):1451–1460.
- Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J, Sieber VK. 2003. Oryzalin-induced chromosome doubling in Rosa and its eVect on plant morphology and pollen viability. Theor Appl Gen. 107(7):1195–1200.10.1007/s00122-003-1374-1
- Khosravi P, Jafarkhani Kermani M, Nematzade GA, Bihamta MR. 2008. Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica. 160(2):267–275.10.1007/s10681-007-9571-7
- Kim MS, Kim JS. 2003. Chromosome doubling of a Cymbidium hybrid with colchicine treatment in meristem culture. [Online]; [cited 2007 Jan 16]. Available from: www. Biolo.aichiedu.ac.jp/ NlOC2003porter/10KoreaCym.pdf.
- Larsen P, Mintung S. 1950. Growth promoting and growth retarding substances in pollen from 2n and 3n apple varieties. Bot Gaz. 3(1):436–447.10.1086/335614
- Lavania UC, Srivastava S. 1991. Enhanced productivity of tropane alkaloids and fertility in artificial autotetraploids of Hyoscyamus niger L. Euphytica. 52(2):73–77.
- Li-hong C, Jia-jia P, Zhao W, Kai Z, Su-xia Y, Jun M, Chun L. 2014. Studies on polyploid induction in anthurium andraeanum using oryzalin, trifluralin and colchicine. Acta Hort Sin. 41(11):2275–2280.
- Lopez-Pujol J, Bosch M, SimonJ Blanche C. 2004. Allozyme diversity in the tetraploid endemic Thymus loscosii (Lamiaceae). Ann of Bot. 93(3):323–332.10.1093/aob/mch039
- Malekzadeh Shafaroudi S, Ghani A, Habibi M, Amiri A. 2012. The study of autotetraploidy induction in Basil (Ocimum basilicum) by colchicine treatment. Iran J Hort Sci. 25(4):461–469.
- Maroni G, Plaut W. 1973. Dosage compensation in Drosophila melanogaster triploids. II. Glueose~6-phosphate dehydrogenase activity. Genet. 74(2):331–342.
- Moghbel N, Khalili Borujeni M, Bernard F. 2015. Colchicine effect on the DNA content and stomata size of Glycyrrhiza glabra var.glandulifera and Carthamus tinctorius L. cultured in vitro. J of Gen Eng and Biot. 13(1):1–6.10.1016/j.jgeb.2015.02.002
- Murti RH, Kim HY, Yeoung YR. 2012. Morphological and anatomical characters of ploidy mutants of strawberry. Int J Agr Biolo. 14(2):204–210.
- Ojiewo CO, Murakam KI, Masinde PW, Agong SG. 2007. Polyploidy breeding of African nightshade (Solanum section Solanum). Int J Plant Breed. 1(1):10–21.
- Omidbaigi R, Mirzaee M, Hassani ME, Sedghi Moghadam M. 2010a. Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int J Plant Prod. 4(2):87–98.
- Omidbaigi R, Yavari S, Hassani ME, Yavari S. 2010b. Induction of autotetraploidy in dragonhead (Dracocephalum moldavica L.) by colchicine treatments. J Fru Orn Plant Res. 18(1):23-35.
- Petersen KK, Hagberg P, Kristiansen K. 2003. Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tiss Organ Cult. 73(2):137–146.10.1023/A:1022854303371
- Quan K, Guolu L, Qigao G, Xiaolin L. 2004. Polyploid induc-tion of Arctium lappa by colchicine. Plant Physiol Communi. 40(2):157–158.
- Ramulu KS, Verhoeven HA, Dijkhuis P. 1991. Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma. 160(2):65–71.10.1007/BF01539957
- Riddle NC, Jiang H, An L, Doerge RW, Birchler JA. 2010. Gene expression analysis at the intersection of ploidy and hybridity in maize. Theor Appl Gen. 120(2):341–353.10.1007/s00122-009-1113-3
- Roy AT, Leggett G, Koutoulis A. 2001. In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep. 20(6):489–495.
- Saharkhiz MJ. 2006. The effects of some environmental factors and ploidy level on morphological and physiological characteristics of feverfew (Tanacetum parthenium L.) medicinal ornamental plant [ PhD Thesis]. Iran: Tarbiat Modares University: 173.
- Sajjad Y, Jaskani MJ, Mehmood A, Ahmad I, Abbas H. 2013. Effect of colchicine on in vitro polyploidy induction in African marigold (Tagetes erecta). Pak J Bot. 45(3):1255–1258.
- Sakhanokho HF, Rajasekaran K, Kelley RV, Farjdj NI. 2009. Induced polyploidy in diploid ornamental ginger (Hedychiummuluense R. M. Smith) using colchicine and oryzalin. Hort Sci. 44(7):1809–1814.
- Schwantes MLB, Schwantes AR, Becak W. 1977. Electrophoretie studies on polyploid amphibians I. 6-Phosphoglueonate dehydrogenase (6-PGD). Comparative biochemistry and physiology part B: comparative biochemistry. Biochemistry. 55(4):393–396.
- Shafieizargar A, Awang Y, Juraimi AS, Othman R. 2013. Comparative studies between diploid and tetraploid Dez Orange [Citrus sinensis (L.) Osb.] under salinity stress. Astr J Crop Sic. 7(10):1436-1441.
- Swanson CP. 1957. Cytology and ctogenetics. Hoboken (NJ): Prentice Hall.
- Taira T, Shao ZZ, Hamawaki H, Larter EN. 1991. The effect of colchicine as a chromosome doubling agent for wheatrye hybrids as influenced by pH, method of application, and post-treatment environment. Plant Bree. 106(4):329–333.10.1111/pbr.1991.106.issue-4
- Talukdar D. 2014. Increasing nuclear ploidy enhances the capability of antioxidant defense and reduces chromotoxicity in Lathyrus sativus roots under cadmium stress. Tur J Bot. 38(3):696–712.10.3906/bot-1310-9
- Temel A, Gozukirmizi N. 2015. Cytotoxic effects of metaphase-arresting methods in barley. Cytol Genet. 49(6):382–387.10.3103/S0095452715060109
- Tulay E, Unal M. 2010. Production of colchicine induced tetraploids in Vicia villosa roth. Caryologia. 63(3):292–303.10.1080/00087114.2010.10589739
- Upson T, Andrews S. 2004. The genus Lavandula. 1st ed. Portland (OR): Timber Press.
- Urwin NAR, Horsnell J, Moon T. 2007. Generation and characterization of colchicine-induced autotetraploid Lavandula angustifolia. Euphytica. 156(1):257–266.10.1007/s10681-007-9373-y
- Van Tuyl JM, Meijer B, Van Dien MP. 1992. The use of oryzalin as an alternative for colchicine in in-vitro chromosome doubling of Lilium. The lily yearbook of North American Lily Society. 43(1):19–22.
- Verhoeven HA, Ramulu KS, Dijkhuis P. 1990. A comparison of the effects of various spindle toxins on metaphase arrest and formation of micronuclei in cellsuspension culture of Nicotiana plumbaginifolia. Planta. 182(3):408–414.10.1007/BF02411392
- Wannakrairoj S, Wondyifraw T. 2013. In vitro chromosome doubling in korarima [Aframomum corrorima (braun) p.c.m. jansen] using colchicine and oryzalin. Kaset J (Nat Sci). 47(5):684–694.
- Wide’n B, Wide’n M. 2000. Enzyme variation and inheritance in Glechoma hederacea (Lamiaceae), a diploidized tetraploid. Hereditas. 132(3):229–241.
- Wu HZ, Zheng S, He Y, Yan G, Bi Y, Zhu Y. 2007. Diploid female gametes induced by colchicine in oriental Lilies. Sci Hort. 141(1):50–53.10.1016/j.scienta.2007.04.004
- Yang XM, Cao ZY, An LZ, Wang YM, Fang XW. 2006. In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica. 152(2):217–224.
- Yokoya K, Roberts AV, Mottley J, Lewis R, Brandham PE. 2000. Nuclear DNA amounts in roses. Ann Bot. 85(4):557–561.10.1006/anbo.1999.1102
- Zhang XY, Hu CG, Yao JL. 2010. Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J Plant Physiol. 167(2):88–94.10.1016/j.jplph.2009.07.006
- Zlesak DC, Thill CA, Anderson NO. 2005. Trifluralin-mediated polyploidisation of Rosa chinensis minima (Sims) Voss seedlings. Euphytica. 141(3):281–290.10.1007/s10681-005-7512-x