Abstract
Vampyrum spectrum is the largest bat species found in the New World. Few cytogenetic data are available on the species, although the diploid chromosome number is known from a specimen captured in Central America in 1970. The present study provides further data on the cytogenetics of V. spectrum, based on the techniques of C banding, impregnation with silver nitrate (AgNORs), staining with chromomycin A3 (CMA3) and fluorescent in situ hybridization (FISH) with probes for 18S rDNA and telomeric sequences. The species presented 2n = 30 chromosomes and 56 autosomal arms. The C banding revealed blocks of constitutive heterochromatin in the pericentromeric regions of all the chromosomes and subterminal blocks on the short arms of chromosome pairs 5, 6, 8 and 9. The AgNORs were found in the secondary constrictions of the long arm of chromosome pair 1. The CMA3 revealed multiple bands in the chromosomes, which permitted the accurate identification of the different chromosome pairs that constitute the karyotype of the species. FISH with 18S rDNA probes revealed the presence of sites in chromosome pair 1, confirming the AgNOR data, while the telomeric probes revealed terminal sites in all the chromosomes of the karyotype. The study thus permitted the identification of specific regions of the chromosomes of this species not catalogued previously, providing important data for comparative studies with other chiropterans.
Keywords:
1. Introduction
The spectral bat, Vampyrum spectrum, is a species of the subfamily Phyllostominae, which is known to be represented by 17 species in Brazil (Nogueira et al. Citation2014). The bats of this clade form a diversified group, with body sizes varying from 10 g to 200 g (Vizotto and Taddei Citation1973). The species of this subfamily also vary considerably in their feeding niches, with insectivorous, frugivorous, omnivorous, nectarivorous, and carnivorous forms. Vampyrum spectrum is the largest bat found in the New World, with some individuals reaching a body weight of 200 g (Navarro and Wilson Citation1982), and a forearm length of 104–110 mm. The wingspan is generally 70–90 cm, but may reach one meter in some specimens (Nowak Citation1994).
In addition to its large size, the species can be recognized easily by its large, long and rounded ears, the robust snout, which is long and narrow, the absence of a tail, and a goblet-shaped nasal leaf (Reid Citation1997). In Brazil, V. spectrum is found in the Amazon, Pantanal, and Cerrado biomes (Marinho-Filho and Sazima Citation1998; Sousa et al. Citation2011). Despite its ample distribution, V. spectrum is one of the bat species listed as “near threatened” by the IUCN (International Union for Conservation of Nature), due to the fact that it invariably occurs at low densities, which makes its populations vulnerable to the effects of habitat fragmentation (IUCN Citation2016). Given its rarity, very few data are available on this species. The only chromosomal data available for V. spectrum are the diploid number, based on the conventional staining of samples obtained from an individual collected in Central America (Baker and Hsu Citation1970).
In the Americas, the first cytogenetic studies of bats were conducted in the 1960s, when Baker and Patton (Citation1967) analyzed specimens of 32 species captured in the USA. In Brazil, the first study was that of Yonenaga et al. (Citation1969), which focused on eight bat species from the southeast of the country. Since this early research, cytogenetic studies have grown progressively, and banding techniques have been applied widely to chromosomal studies of bats (Baker and Bickham Citation1980; Sotero-Caio et al. Citation2013). Even so, the data are still relatively scant, considering the enormous diversity of bats found in Brazil (Varella-Garcia and Taddei Citation1989; Moratelli and Morielle-Versute Citation2007). In fact, basic karyotypes have been defined for only 54 (Moratelli and Morielle-Versute Citation2007; Garcia and Pessôa Citation2010; Almeida et al. Citation2016) of the 178 bat species known to occur in Brazil (Nogueira et al. Citation2014).
More advanced techniques, based on the physical mapping of DNA sequences, using FISH (fluorescent in situ hybridization), amplified from the genome of the species, have been used increasingly for the study of the cytogenetics of Neotropical bats, with promising results (Pieczarka et al. Citation2005; Faria et al. Citation2009; Gomes et al. Citation2010, Citation2016; Sotero-Caio et al. Citation2013). The present study provides the first detailed cytogenetic analysis of Vampyrum spectrum from the Brazilian Cerrado savanna biome, based on both conventional banding and molecular techniques.
2. Material and methods
Nova Xavantina is located in the valley of the das Mortes River, in the Araguaia basin, in eastern Mato Grosso, central Brazil. The vegetation of this region is typical of the Cerrado, ranging from grassland to woodland and forest formations (Piaia Citation1999). The region’s climate is tropical humid, or Aw in the Köppen classification (Vianello and Alves Citation2012), with a dry season between April and September, and a rainy season between October and March (Marimon et al. Citation2003).
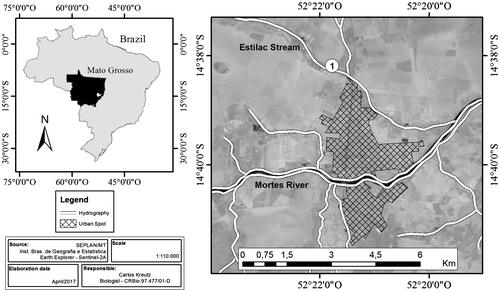
The cytogenetic analyses were conducted on samples from a male specimen of Vampyrum spectrum, captured in 2010, collected under license number 18276–1 from IBAMA/SISBIO/MT (Sousa et al. Citation2011), in an area of Cerradão woodland (14°38′14ʺ S, 52°21′49ʺ W) within the Brazilian Cerrado savanna biome (Figure ). The specimen was placed in a holding cage until the subsequent morning, when the cytogenetic procedures were conducted. Following the extraction of samples, the animal was fixed and deposited in the scientific collection of Chiroptera at the Nova Xavantina campus of Mato Grosso State University, under catalog number RM 123.
Figure 1. Collecting locality of the specimen of Vampyrum spectrum, in the municipality of Nova Xavantina, Mato Grosso, Brazil.
The chromosomal preparations were obtained directly from the bone marrow following the protocol described by Morielle-Versute et al. (Citation1996). The diploid number and fundamental number of autosomal chromosomes were determined using Giemsa staining. The procedure of Howell and Black (Citation1980) was adapted for the determination of the nucleolus organizer regions (AgNORs). The blocs of constitutive heterochromatin were visualized using the technique of Sumner (Citation1972), with minor adjustments: all solutions (HCl 2 N, barium hydroxide and 2×SSC) at 42 °C and stained with propidium iodide. Schweizer (Citation1980) approach was used to stain the sample with base-specific CMA3 fluorochrome to highlight the regions rich in C and G. FISH used the 18S rDNA probe, amplified with the primers 18SA 5′ CCGCTTTGGTGACTCTTGAT 3′ and 18SB 5′ CCGAGGACCTCACTAAACCA 3′ (Gross et al. Citation2010). The primers TelA- 5′ TTA GGG 3′ and TelB- 5′ CCC TAA 3′ (Ijdo et al. Citation1991) were used for the telomeric DNA, based on the protocol described by Pinkel et al. (Citation1986). The chromosomes were documented under an Olympus BX5 microscope (Tokyo, Japan) and the karyotypes were mounted in Photoshop Cs6 portable, edition free.
3. Results
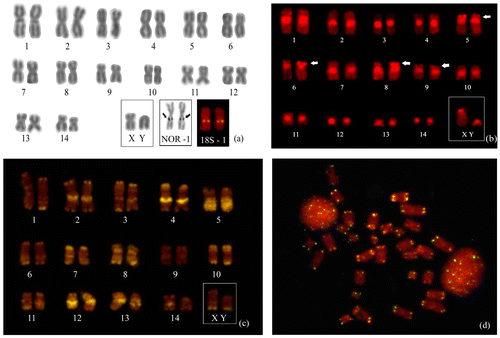
The cytogenetic analyses confirmed the diploid number (2n) of 30 chromosomes for Vampyrum spectrum, including 28 autosomal chromosomes (24 m + 4sm) and a pair of sexual chromosomes made up of a medium submetacentric X and a small acrocentric Y (Figure (a)). A total of 56 autosomal arms (FN = 56) were detected. The nucleolus organizer regions and the 18S rDNA were identified in chromosome pair 1, the largest of the complement, in a region that coincides with the secondary constrictions (Figure (a), box). Blocs of constitutive heterochromatin were identified in the pericentromeric regions of all the chromosomes and in the subterminal blocs of the short arms of chromosome pairs 5, 6, 8 and 9 (Figure (b)). The chromomycin A3 technique revealed multiple transversal bands that contributed to the accurate identification of the different pairs of chromosomes (Figure (c)). FISH with the probes for the telomeric DNA revealed the presence of small sites in all the terminal portions of all the chromosomes (Figure (d)).
Figure 2. (a) Karyotype of Vampyrum spectrum (2n = 30 and NA = 56). The boxes highlight the sexual chromosomes, AgNORs, and FISH with the 18S rDNA probe. (b) Karyotype with C banding, showing blocs of pericentromeric heterochromatin in all the chromosomes and in the subterminal regions of pairs 5, 6, 8 and 9 (arrowheads). (c) Karyotype based on the CMA3 technique. (d) Metaphase following fluorescent in situ hybridization with a telomeric DNA probe, showing blocs of repetitive DNA (TTAGGG)n in the telomeric regions of all the chromosomes.
4. Discussion
Baker and Hsu (Citation1970) presented the first data on the characteristics of the chromosomes of Vampyrum spectrum, describing a karyotype with 2n = 30 and FN = 56 in a specimen from Central America. This study also identified an XX/XY system of sexual chromosomes, with a medium subtelocentric X chromosome and a small, submetacentric Y chromosome. These findings were reconfirmed in the present study, for the male specimen captured in the Cerrado savanna of central Brazil. In addition to the basic data obtained by Giemsa staining, the present study provided data on the AgNORs, C bands, CMA3, and FISH with 18S rDNA and telomeric probes.
Many cytogenetic studies of bats have focused on species of the family Phyllostomidae (Varella-Garcia and Taddei Citation1989; Pieczarka et al. Citation2005; Moratelli and Morille-Versute Citation2007; Faria et al. Citation2009; Garcia and Pessôa Citation2010; Gomes et al. Citation2010, Citation2016; Sotero-Caio et al. Citation2013). Diploid numbers in this family vary from 2n = 14 for Vampyressa melissa to 2n = 46 for Macrotus waterhousii (Baker Citation1970), and the number of autosomal arms range from FN = 20 in Tonatia bidens (Tavares et al. Citation2015) to FN = 68 in Micronycteris megalotis (Baker Citation1970). The most common diploid numbers are 2n = 30 and 32, and the most frequent complements of autosomal arms are 56 and 60 (Baker Citation1970; Varella-Garcia et al. Citation1989; Moratelli and Morielle-Versute Citation2007).
While considerable interspecific variation is found in bat karyotypes, intraspecific variation is relatively rare. To date, in fact, intraspecific variation has only been observed in four species, and the most striking case is Uroderma bilobatum, in which 13 distinct karyomorphs have been identified (Silva et al. Citation2005; Moratelli and Morielle-Versute Citation2007). In the case of Vampyrum spectrum, while the two specimens studied to date are separated by a considerable geographic distance, the macro structure of their karyotypes is identical. Overall, the cytogenetic data available for the different families of the Microchiroptera, including the species V. spectrum, indicate a high degree of conservatism in their karyotypes, which probably reflects a slow rate of chromosomal evolution (Varella-Garcia et al. Citation1989; Souza and Araújo Citation1990; Morielle-Versute et al. Citation1996; Gomes et al. Citation2010; Sotero-Caio et al. Citation2013).
Studies of the distribution of the constitutive heterochromatin have revealed the presence of blocs of C bands in telomeric and interstitial regions, and, in some cases, whole chromosomes. In general, however, the most common pattern is the presence of C bands in the pericentromeric regions of all the chromosomes (Varella-Garcia and Taddei Citation1989; Tavares et al. Citation2015). The data on V. spectrum confirm this typical pericentromeric distribution of the constitutive heterochromatin.
In the case of the AgNORs, Baker and Hsu (Citation1970) did not even provide data on the location of the secondary constrictions in V. spectrum. In most bat species, the AgNORs and 18S rDNA are found in only one chromosome pair, which has been considered to be the ancestral condition in the family Phyllostomidae (Morielle-Versute and Varella-Garcia Citation1996; Santos and Souza Citation1998).
The CMA3 fluorochrome has been used to produce multiple bands, similar to R bands, in a number of different vertebrates (Santos and Souza Citation1998), revealing specific patterns of banding that can be used to distinguish the chromosome pairs (Sumner Citation1990). In bats, however, this technique has been applied only in studies of Desmodus rotundus and Diphylla ecaudata (Moratelli and Morielle-Versute Citation2007). Santos and Souza (Citation1998) observed the pattern of chromosomal banding produced by CMA3 in Carollia perspicillata, and Tavares et al. (Citation2015) observed the same pattern in Tonatia bidens and T. saurophyla. The results of these studies permitted the accurate identification of the different pairs of homologous chromosomes in these species. In this context, the results of the CMA3 banding of V. spectrum presented here provide further evidence of this pattern in bats. It was possible to identify conclusively all the homologous chromosome pairs, as well as the sexual pair, despite the lack of a female karyotype for comparison.
The telomeric probes revealed only (TTAGGG)n sites in the telomeres of V. spectrum. No evidence was found of the presence of these sequences outside these regions, which might indicate some structural modification of the karyotype (Meyne et al. Citation1990). However, these authors also note that the identification of possible telomeric sites in pericentromeric or interstitial regions may be accounted for by the fact that these sequences are components of the satellite DNA of some mammalian species.
Vampyrum spectrum is difficult to capture. In Mato Grosso, the only previous record of the species was obtained 62 years ago in the Pantanal wetlands (Vieira Citation1955). In addition to being the second record of the species in Mato Grosso, the specimen analyzed in the present study was the first to be recorded in the Cerrado biome of the Brazilian Midwest (Sousa et al. Citation2011). The classification of the species by the IUCN as “near threatened” also reinforces the need for a better understanding of the biology of Vampyrum spectrum — as in the case of the cytogenetic data presented here — to guarantee the development of adequate conservation measures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors are grateful to the Brazilian Coordination for Higher Education Training (CAPES) for providing the first author with a graduate stipend, the Mato Grosso State Research Foundation [process 738631/2008] and National Counsel of Technological and Scientific Development (CNPq) [process 4469252014-4].
References
- Almeida B, Novaes RLM, Aguieiras M, Souza RF, Esbérard CEL, Geise L. 2016. Karyotype of three Lonchophylla species (Chiroptera, Phyllostomidae) from Southeastern Brazil. Comp Cytogenet. 10(1):109–115.
- Baker RJ. 1970. Karyotypic trends in bats. In: Wimsatt WA, editor. Biology of bats. New York (NY): Academic Press; p. 65–97.
- Baker RJ, Bickham JW. 1980. Karyotypic evolution in bats: evidence of extensive and conservative chromosomal evolution in closely related taxa. Syst Zool. 29(3):239–253.
- Baker RJ, Hsu TC. 1970. Further studies on the sex-chromosome systems of the American leaf-nosed bats (Chiroptera Phyllostomatidae). Cytogenetics. 9(2):131–138.
- Baker RJ, Patton JL. 1967. Karyotypes and karyotypic variation of north American vespertilionid bats. J Mammal. 48(2):270–286.
- Faria KC, Marchesin SR, Moreira PR, Beguelini MR, Morielle-Versute E. 2009. New insights into telomeric DNA sequence (TTAGGG)n location in bat chromosomes. Genet Mol Res. 8(3):1079–1084.
- Garcia JP, Pessôa LM. 2010. Karyotipc composition of bats from the Brazilian nuclear power plant, state of Rio de Janeiro. Chiropt Neotrop. 16(1):617–628.
- Gomes AJB, Rodrigues LRR, Rissino JD, Nagamachi CY, Pieczarka JC. 2010. Biogeographical karyotypic variation of Rhinophylla fischerae (Chiroptera: Phyllostomidae) suggests the occurrence of cryptic species. Comp Cytogenet. 4(1):79–85.
- Gomes AJB, Nagamachi CH, Rodrigues LRR, Benathar TCM, Ribas TFA, O’Brien PCM, Yang F, Ferguson-Smith MA, Pieczarka JC. 2016. Chromosomal phylogeny of Vampyressine bats (Chiroptera, Phyllostomidae) with description of two new sex chromosome systems. BMC Evol Biol. 16(1):1–11.
- Gross MC, Schneider CH, Valente GT, Martins C, Feldberg E. 2010. Variability of 18S rDNA locus among Symphysodon fishes: chromosomal rearrangements. J Fish Biol. 76(5):1117–1127.
- Howell WN, Black DA. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Cell Mol Life Sci. 36(8):1014–1015.
- Ijdo JW, Wells RA, Baldini A, Reeders ST. 1991. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 19(17):4780.
- IUCN. 2016. Vampyrum spectrum. The IUCN Red List of Threatened Species 2008: e.T22843A9395576 [cited 2016 Apr 31]. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T22843A9395576.en
- Marimon BS, Felfli MJ, Lima ES, Pinheiro-Neto J. 2003. Padrões de distribuição de species na mata de galeria do córrego bacaba, Nova Xavantina, Mato Grosso, em relação a fatores ambientais [Distribution patterns of species in the gallery forest of the bacaba stream, Nova Xavantina, Mato Grosso, in relation of environmental factors]. Heringer. 12(1):1–108.
- Marinho-Filho J, Sazima I. 1998. Brazilian bats and conservation biology: a first survey. In: Kunz TH, Racey PA, editors. Bat biology and conservation. Washington (DC): Smithsonian Institution; p. 282–294.
- Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OG, Wiley JE, Wurster-Hill DH, Yates TL, Moyzis RK. 1990. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 99(1):3–10.
- Moratelli R, Morielle-Versute E. 2007. Métodos e Aplicações da citoenética na taxonomia de morcegos brasileiros [Methods and applications of cytogenetics in the taxonomy of Brazilian bats]. In: Reis NR, Peracchi AL, Pedro WA, Lima IP, editors. Morcegos do Brasil [Brazilian bats]. Londrina: Nelio Roberto dos Reis; p. 197–228.
- Morielle-Versute E, Varella-Garcia M, Taddei VA. 1996. Karyotypic patterns of seven species of molossid bats (Molossidae, Chiroptera). Cytogenet Cell Genet. 72(1):26–33.
- Navarro D, Wilson DE. 1982. Vampyrum spectrum. Mammalian Species. 184(1):1–4.
- Nogueira MR, Lima IP, Moratelli R, Tavares VC, Gregorin R, Peracchi AL. 2014. Checklist of Brazilian bats, with comments on original records. Check List. 10(4):808–821.
- Nowak RM. 1994. Walker’s bats of the world. Chicago (IL): The Johns Hopkins University Press; p. 287
- Piaia II. 1999. Geografia de Mato Grosso [Geography of Mato Grosso]. 2nd ed. Cuiabá: EDUNIC; p. 207.
- Pieczarka JC, Nagamashi CY, Obrien PCM, Yang F, Rens W, Barro RMS, Noronha RCR, Rissino JD, Oliveira EHC, Ferguson-Smith MA. 2005. Reciprocal chromosome painting between two South American bats: Carollia brevicauda and Phyllostomus hastatus (Phyllostomidae, Chiroptera). Chromosome Res. 13(4):339–347.
- Pinkel D, Straume T, Gray JW. 1986. Cytogenetic analysis using quantitative, high sensitivity, fluorescence hybridization. Proc Natl Acad Sci. 83(9):2934–2938.
- Reid FAA. 1997. Field guide to the mammals of Central America and southeast Mexico. New York (NY): Oxford University Press; 334 p.
- Santos N, Souza MJ. 1998. Characterization of the constitutive heterochromatin of Carollia perspicillata (Phyllostomidae, Chiroptera) using the base-specific fluorochromes, CMA3 (GC) and DAPI (AT). Caryologia. 51(1):51–60.
- Schweizer D. 1980. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenet Cell Genet. 27(2–3):190–193.
- Silva AM, Marques-Aguiar SA, Barros RMS, Nagamachi CY, Pieczarka JC. 2005. Comparative cytogenetic analysis in the species Uroderma magnirostrum and U. bilobatum (cytotype 2n = 42) (Phyllostomidae, Stenodermatinae) in the Brazilian Amazon. Genet Mol Biol. 28(2):248–253.
- Sotero-Caio CG, Volleth M, Gollahon LS, Fu B, Cheng W, Ng BL, Yang F, Baker RJ. 2013. Chromosomal evolution among leaf-nosed nectarivorous bats – evidence from cross-species chromosome painting (Phyllostomidae, Chiroptera). BMC Evol Biol. 13(276):1–12.
- Sousa RF, Kreutz C, Oliveira SL, Faria KC. 2011. Mammalia, Chiroptera, Phyllostomidae, Vampyrum spectrum (Linnaeus, 1758): first record for the Cerrado. Check List. 7(4):468–469.
- Souza MJ, Araújo MCP. 1990. Conservative pattern of the G-bands and diversity of C-banding partterns and NORs in Esternodermatinae (Chiroptera-Phyllostomatidae). Rev Bras Genét. 13(2):255–268.
- Sumner AT. 1972. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 75(1):304–306.
- Sumner AT. 1990. Chromosome banding. London: Unwin Hyman; 434 p.
- Tavares JR, Sousa TP, Silva JM, Venere PC, Faria KC. 2015. Cytogenetics and DNA barcoding of the round-eared bats, Tonatia (Chiroptera: Phyllostomidae): a new karyotype for Tonatia bidens. Zoologia. 32(5):371–379.
- Varella-Garcia M, Morielle-Versute E, Taddei VA. 1989. Survey of cytogenetic data on brazilian bats. Rev Brasil de Gené. 12(4):761–793.
- Varella-Garcia M, Taddei VA. 1989. Citogenética de Quirópteros: Métodos e Aplicações [Cytogenetics of Chiroptera: methods and applications]. Rev Bras Zool. 6(2):297–323.
- Vianello RL, Alves AR. 2012. Metereologia básica e aplicações [Basic meteorology and applications]. 2nd ed. Viçosa: UFV; 460 p.
- Vieira CCO. 1955. Lista remissiva dos mamíferos do Brasil [Remissive list of mammals of Brazil]. Arq Zool. 8(1):341–465.
- Vizotto LD, Taddei VA. 1973. Chave para a determinação de quirópteros brasileiros [Key to the determination of Brazilian bats]. Revista da Faculdade de Filosofia, Ciências e Letras, São José do Rio Preto – Boletim de Ciências. 1(1):1–72.
- Yonenaga Y, Pessoa OF, Lewis KR. 1969. Karyotypes of seven species of Brazilian bats. Caryologia. 22(1):63–80.
