Abstract
The purpose of this paper was to investigate the proliferative and genotoxic activity of juices from fruits of Psidium cattleianum, also known as strawberry guava, using the Allium cepa test. Fruits were collected and frozen for subsequent juice preparation at a concentration of 125 g l−1. The treatments consisted of T1: distilled water (negative control), T2: juice of yellow strawberry guava collected in Arroio Grande, T3: juice of yellow strawberry guava collected in Palma, T4: juice of red strawberry guava collected at UFSM, and T5: 1% glyphosate solution (positive control). Eight thousand cells per treatment were analysed and the mitotic indices (MI) were calculated. The juice samples were analysed by high-performance liquid chromatography (HPLC). Statistical analyses were performed using the chi-square and Tukey tests (p < 0.05). The results show irregularities in the cell division, mainly during metaphase, and an antimitotic effect in addition to an increase in the MI in treatments containing strawberry guava juice. Using HPLC, epicatechin was predominant in T2 and T4, and isoquercitrin in T3. Overall, strawberry guava has proliferative and genotoxic effects on the cell division of A. cepa, as well as an antimitotic action preventing the mitotic spindle from forming.
Introduction
As well as the large quantity of plants whose fruits have preventive and antioxidant properties, there is a wide range of fruit plants that are used for medicinal purposes. They are used as teas in global and local folk medicine, and their fruits are consumed because of their medicinal potential. The study of these plants is important to minimize their negative effects on public health and their diversity needs to be further investigated in natural ecosystems, because only a small percentage of their active principles have been properly studied. Thus, many benefits remain unknown (Guerra and Nodari Citation2007).
Among these plants is Psidium cattleianum Sabine (strawberry guava). It is known in Brazil as araçá, araçá-vermelho, araçá-rosa, araçá-amarelo and araçá-de-coroa (Lorenzi Citation1992). The fruits of this plant can be either yellow or red. Sousa and Sobral (Citation2007) studied the systematics of these two morphotypes, considering them as “yellow fruit morphotype” (or “yellow morphotype”) and “red fruit morphotype” (or “red morphotype”). The different morphotypes have some differences in leaf size and chemical composition (Varella et al. Citation2007), size, habit and natural occurrence.
Strawberry guava fruits can be consumed in natura or as juice, jam, jelly and ice cream (Haminiuk et al. Citation2006). The levels of vitamin C are four times higher than in citrus fruits. Besides fruit and wood, their roots, bark and leaves are used for infusions in folk medicine (Medina Citation2009).
According to Volpato (Citation2005), several isolated plant compounds, previously believed to have medicinal properties, are genotoxic and cytotoxic and linked to tumour development. An example is nephrotoxicity caused by plant species containing saponins and terpenes. Plant bioassays can be used to detect genotoxicity, serving as a preliminary assessment of environmental hazards. Mutagenic agents, for instance, may be detected cytologically by cell inhibition, metaphase disruption, numerical and structural chromosomal abnormalities ranging from fragmentation to perturbations in mitotic spindle, and in all subsequent mitotic phases (Tedesco and Laughinghouse IV Citation2012).
Developed by Levan (Citation1938), the evaluation method used for detecting chromosomal abnormalities in Allium cepa roots is validated by the International Programme on Chemical Safety (IPCS, WHO) and the United Nations Environment Programme (UNEP) as an efficient method for in situ testing and genotoxicity monitoring of environmental substances (Cabrera and Rodriguez Citation1999; Bagatini et al. Citation2007; Fachinetto et al. Citation2007; Frescura et al. Citation2013). Leme and Marin-Morales (Citation2009) emphasize the importance of using A. cepa to detect different classes of environmental pollutants, such as metals, pesticides, aromatic hydrocarbons, textile stains, and products used for disinfecting potable water.
Teixeira et al. (Citation2003) obtained similar results using tests with onion root meristematic cells, rat bone marrow cells and human lymphocytes, supporting the A. cepa test for cytogenetic studies.
The aim of our paper is to determine phenolic compounds, as well as to examine the proliferative and genotoxic activity of P. cattleianum juice by observing potential chromosomal damage in addition to the inhibition of cell division in A. cepa root-tip meristematic cells, which may suggest other types of cellular damage in human cells.
Materials and methods
Sampling
Strawberry guava fruits were collected between February and March 2014 in three different areas: yellow-skinned fruits were collected in Palma (29°42′2.0ʺS 53°37′55.5ʺW) and Arroio Grande (29°40′27.7ʺS 53°38′19.9ʺW) – two districts of the municipality of Santa Maria in the state of Rio Grande do Sul (Brazil). Red-skinned fruits were collected at the Federal University of Santa Maria in Santa Maria (29°43′20.0ʺS 53°43′13.9ʺW). Plant material is deposited in the SMDB herbarium of the Department of Biology at UFSM (SMDB no. 15065, 15064 and 15063, respectively).
Preparation
Fresh strawberry guavas were washed and chopped. Fruit skins and seeds were not removed. They were sorted into three 25 g portions according to sampling area and placed in a freezer. The frozen fruits were then mixed with 200 ml of distilled water in a 600-W blender at speed 3 for 1 min. Finally, the mixtures were sieved and poured into 50 ml plastic cups where the previously rooted onion bulbs (in distilled water) were placed for 24 h at room temperature.
Analysis of juices by high performance liquid chromatography
A sample of each juice at a concentration of 125 g l−1 was separated and analysed by high performance liquid chromatography (HPLC) with diode array detection (HPLC-DAD), in the Phytochemical Research Laboratory, Department of Industrial Pharmacy, UFSM.
Chemical, apparatus and general procedures
All chemicals were of analytical grade: methanol, acetic acid, gallic acid, chlorogenic acid, caffeic acid, and ellagic acid purchased from Merck (Darmstadt, Germany). Rutin, epicatechin, catechin, isoquercitrin, quercetin, quercitrin and kaempferol were acquired from Sigma Chemical Co. (St Louis, MO, USA). HPLC was carried out with a Shimadzu Prominence Auto Sampler (SIL-20A) system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps, connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software.
Quantification of compounds by HPLC
Chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm × 150 mm) packed with 5 μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% of (B) for 2 min, 25% (B) for 10 min, then 40, 50, 60, 70 and 80% (B) each for 10 min, following Peroza et al. (Citation2013) with slight modifications. Strawberry guava (Arroio Grande, Palma and UFSM) juice and mobile phase were filtered through a 0.45 μm membrane filter (Millipore, Billerica, MA, USA) and then degassed by ultrasonic bath prior to use. The juices were analysed at a concentration of 125 g l−1. The flow rate was 0.6 ml min–1, injection volume 40 μl and the wavelengths were 254 nm for gallic acid, 280 nm for catechin and epicatechin, 327 nm for caffeic, chlorogenic and ellagic acids, and 365 nm for rutin, quercetin, quercitrin, isoquercitrin and kaempferol.
Stock solutions of reference standards were prepared in the HPLC mobile phase at a concentration range of 0.050–0.250 mg ml–1 for quercetin, quercitrin, isoquercitrin, kaempferol, catechin and epicatechin; and 0.030–0.450 mg ml–1 for ellagic, gallic, chlorogenic and caffeic acids. Chromatography peaks were confirmed by comparing the retention time with those of reference standards and by DAD spectra (200–500 nm). The calibration curves were as follows: gallic acid: Y = 12739x + 1186.9 (r = 0.9994); chlorogenic acid: Y = 11976x + 1206.5 (r = 0.9997), caffeic acid: Y = 12573x + 1270.3 (r = 0.9996), ellagic acid: Y = 13062x + 1269.3 (r = 0.9990), catechin: Y = 11968x + 1347.1 (r = 0.9995), epicatechin: Y = 12763x + 1269.5 (r = 0.9993), quercetin: Y = 13184x + 1256.1 (r = 0.9999), isoquercetin: Y = 11985x + 1359.7 (r = 0.9996), quercitrin: Y = 13065x + 1249.6 (r = 0.9993), rutin: Y = 13583x + 1267.5 (r = 0.9998) and kaempferol: Y = 13423x + 1153.2 (r = 0.9998). All chromatography operations were carried out at room temperature and in triplicate.
The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve (Boligon et al. Citation2013).
Treatments and analysis criteria
The onion bulbs were divided into five groups of four bulbs, each corresponding to one treatment, and subsequently placed in distilled water to root. After rooting, one group was kept in distilled water to serve as the negative control, another group was placed in a 1% glyphosate solution to serve as the positive control, and the remaining groups were transferred into treatments containing the three different strawberry guava juices for 24 h.
Genotoxicity of the strawberry guava juices was performed according to the following (T) treatments:
| • | T1: negative control in distilled water; | ||||
| • | T2: yellow strawberry guava juice (125 g l−1, Distrito de Arroio Grande – DAG –Santa Maria, RS, Brazil); | ||||
| • | T3: yellow strawberry guava juice (125 g l−1, Distrito de Palma – DP – Santa Maria); | ||||
| • | T4: red strawberry guava juice (125 g l−1, Universidade Federal de Santa Maria – UFSM – Santa Maria); | ||||
| • | T5: positive control in a 1% glyphosate solution. | ||||
After treatment, rootlets were removed from the bulbs of all groups, fixed in ethanol/acetic acid (3:1 v/v) for 24 h, and stored in 70% (v/v) ethanol under refrigeration. The root tips were hydrolysed in 1 N HCl for 5 min and then washed in distilled water for slide preparation. Under stereomicroscopy, the root caps were removed and the meristematic zone was subsequently stained with 2% acetic-orcein, smashed with a glass rod and covered with a coverslip. Two slides were prepared for each bulb and these were analysed using a light microscope. A thousand cells were counted blindly for each slide, summing 2000 cells per bulb and 8000 cells per treatment. The mitotic indices were calculated based on the percentage of cell division. Apart from cell division stages, irregularities such as chromosome breakage, lost or laggard chromosomes, bridges and micronucleus were also observed.
Statistical analysis
The experimental design was completely randomized. The comparison between mitotic indices and chromosomal alterations was performed by the chi-square (χ2) test (p < 0.05) using BIOESTAT 5.0 (Ayres et al. Citation2007). The results obtained by HPLC were analysed by the Tukey test (p < 0.05), using Assistat®, beta 7.7.
Results
In Table , we present the cell division analysis of Allium cepa root-tips, showing the total number of cells in interphase and other stages of mitosis, as well as the mitotic indices.
Table 1. Number of meristematic cells of Allium cepa roots analysed at interphase and other stages of the cell cycle, including irregularities, as well as the mitotic indices (MI) for each treatment.
Chromosomal breakage, lost chromosomes, a micronucleate cell (Figure (a)), bridges (Figure (b)) and cells in apoptosis (Figure (c)) were observed (Table ). However, most irregularities were observed in metaphase, during which chromosomes were not aligned on the equatorial plate as in proper cell division. Instead, they were scattered randomly throughout the cell in a disorganized fashion (Figure (d–g)), though similar, since the juice caused an interruption in the cell cycle that enabled chromosome counting.
Figure 1. Irregularities found in the cells of Allium cepa under the treatments with Psidium cattleianum juices. (a) Cell with micronucleus treated with yellow strawberry guava juice 125 g l−1 (Distrito de Palma) – T3. (b) Cell with bridges in telophase treated with red strawberry guava juice 125 g l−1 (Universidade Federal de Santa Maria) – T4. (c) Cells in apoptosis treated with the positive control – T5. Cells with irregular metaphases: (d, e) Treatment with yellow strawberry guava juice 125 g l−1 (Distrito de Arroio Grande) – T2; (f) treatment T3; (g) treatment T4. Scale: 10 μm.
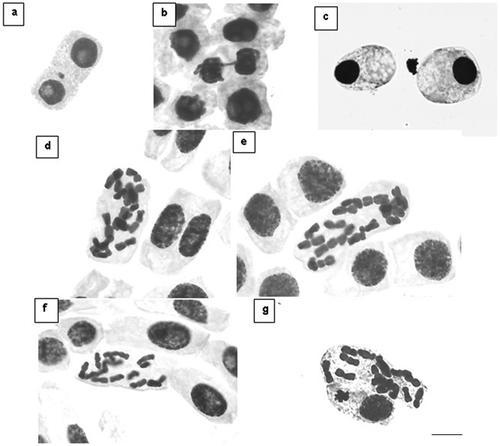
Table 2. Number and type of chromosomal irregularities found in Allium cepa root tips submitted to the distinct treatments and mean cell with alterations.
HPLC fingerprinting of strawberry guava juices (Figure (a–c)) revealed the presence of the gallic acid, catechin, chlorogenic acid, caffeic acid, ellagic acid, epicatechin, rutin, quercitrin, isoquercitrin, quercetin, and kaempferol (Table ).
Figure 2. Chromatographic profile of Psidium cattleianum juices: (a) Distrito de Arroio Grande (DAG); (b) Distrito de Palma (DP); (c) Universidade Federal de Santa Maria (UFSM); UV detection at 327 nm. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), ellagic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), isoquercitrin (peak 9), quercetin (peak 10), and kaempferol (peak 11).
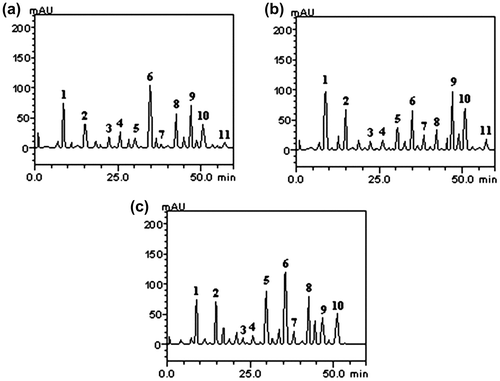
Table 3. Composition of phenolic compounds in Psidium cattleianum juice of three different accessions using high performance liquid chromatography (HPLC).
Discussion
By analysing the cell division, we found cell irregularities in the positive control, unlike the negative control (Table ). The amount of cells in division was slightly higher in the treatments containing juice. For instance, the mitotic index (MI) in T3 and T4 was 5.69% and 5.49% respectively, thus differing significantly from the positive and negative control whose MI was 3.04% for both. On the other hand, the MI = 4.86% in T2 represented a significant difference as compared to T3 and T4, as well as in relation to the positive and negative controls. Treatments T3 and T4 (consisting of different varieties, i.e. the yellow-skinned and the red-skinned strawberry guava) had similar results. Due to genetic variability between the samples of P. cattleianum and possible different edaphoclimatic factors (altitude, temperature, precipitation and stress), treatments T3 and T2 (both of the yellow variety) had different results.
These results agree with Coelho et al. (Citation2017), where aqueous extracts from Echinodorus grandifloras (Cham & Schltdl.) Micheli (6 g l−1) and Sagittaria montevidensis Cham. & Schlecht (6 and 24 g l−1) also stimulated the cell division of A. cepa root-tips, at the same time as increased chromosomal aberrations.
On the other hand, our results disagree with Medina et al. (Citation2011), where extracts from seedless strawberry guava significantly reduced cell proliferation in breast and colon cancer, regardless of the genotypic testing and the solvent used for extraction (water or acetone). Furthermore, in vivo studies by Costa et al. (Citation2008) using the hydroalcoholic extract of P. cattleianum leaves in Mus musculus (mice) cells, found that the compounds present in the extract did not have genotoxic or mutagenic effects on some cell types. A study by Teixeira et al. (Citation2003) that evaluated leaf infusions of Psidium guajava in vivo and in vitro found that the infusions did not cause significant alterations in the root cells of A. cepa, in rat cells, or in human lymphocytes. Studies by Silva et al. (Citation1995) found that there are mutagenicity data in Psidium guajava and that there are compounds such as rutin, tannins, flavonoids, sesquiterpenic alcohol and triterpenoid acids.
The significant number of irregularities found in our treatments was unexpected, since they contained juices made from fruits that can be consumed in natura. Furthermore, the irregularity levels were higher in the treatments containing juice than in the positive control.
In normal conditions, cell division in plant tissues, such as in vivo grown roots, is continuous, although the spindle formation and, consequently, cell division can be inhibited by antimitotic agents, such as hydroxyquinoline, colchicines or paradichlorobenzene. Ekong et al. (Citation2014) compared paradichlorobenzene, hydroxyquinoline, and colchicine pre-treatment methods and found them all to be effective in inhibiting the mitotic spindle and stopping the chromosomes during metaphase in A. cepa roots. This result can also be obtained using the well-known pre-treatment technique with ice-cold water (Osalou et al. Citation2013).
The estimated time for inhibition of cell division and consequent metaphase in chromosomes for counting can vary among species. Ortiz et al. (Citation2013) demonstrated that native species of the genus Arachis required a 3-h pre-treatment in a 0.002 M solution of 8-hydroxiquinoline. Fachinetto and Tedesco (Citation2009) used paradichlorobenzene 15% for 10 h in order to achieve metaphase arrest in Hyptis mutabilis (Rich.) Briq. Osalou et al. (Citation2013) pre-treated the roots by placing plants at 0 °C in melting ice, inside a refrigerator at 4 °C for 24 h.
Considering the circumstances in which our A. cepa test was undertaken, we found that treatments containing the different varieties of strawberry guava juice performed similarly to the antimitotic inhibitors mentioned above. Our results demonstrate that the mitotic chromosomes in metaphase were properly arranged, allowing chromosome counting. This result is not due to using juices made from frozen fruits, since the A. cepa test was carried out at room temperature. Thus strawberry guava juices are believed to contain a substance whose antimitotic effect is similar to that of colchicine, which is a mutagenic agent that leads to doubling of chromosomes. Taking into account that the temperature of the juices was not responsible for the antimitotic effect, we believe that strawberry guava juice compounds caused this effect.
The analysis of juices by HPLC revealed a predominance of epicatechin (peak 6) followed by gallic acid (peak 1) and isoquercitrin (peak 9) in T2. Epicatechin was also the main compound in T4, followed by ellagic acid (peak 5) and quercetin (peak 8). Isoquercitrin was predominant in T3, followed by gallic acid. These findings show that there is a difference of chemical composition between yellow and red fruits, as Biegelmeyer et al. (Citation2011) found. According to Biegelmeyer et al. (Citation2011), red strawberry guava fruits contained higher levels of polyphenols and flavonoids than yellow strawberry guava fruits. Studies by Medina et al. (Citation2011) demonstrated that fresh strawberry guava pulp contained high levels of phenolic compounds, which were successfully extracted with acetone.
Phenolic compounds, such as flavonoids (quercetin and isoquercetin), are beneficial due to free radical scavenging activity (Decker Citation1997). Epicatechin is another natural flavonoid that has antioxidant and anti-carcinogenic properties (Bianchi and Antunes Citation1999). Luximon-Ramma et al. (Citation2003) observed a high antioxidant potential in strawberry guava fruits in terms of total phenols, proanthocyanidins, flavonoids and level of vitamin C.
It was not possible to specifically identify which component was responsible for difficulty in the formation of the spindle fibres, resulting in a large number of cells with well-spread metaphase chromosomes. Using the Allium cepa test, Oyeyemi and Bakare (Citation2013) found that aqueous extracts of Spondias mombin L., Nymphea lotus L., and Luffa cylindrica (L.) M. Roem. are spindle inhibitors. This may be responsible for the popular success of these plants in the traditional management of cancer.
Considering that access to medical resources in Brazil is often scarce, the scientific understanding of effects caused by medicinal plants and their use as alternative therapy is vital, since these resources are readily accessible to the public and cheap. These studies can also prevent and reduce “over-dosing” due to inappropriate use (Alvarenga et al. Citation2007).
We were surprised by the results of our study, which demonstrates a new method of obtaining mitotic metaphase cells, i.e. without any chemical substances. These results are important for public health, since strawberry guava fruits are consumed in natura and as frozen pulps. Therefore, further studies are needed to determine which compounds are responsible for the antimitotic activity caused by these fruits on cell division.
In summary, Psidium cattleianum fruits cause proliferative and genotoxic effects on the Allium cepa cell division, which may be valid in other types of eukaryotic cells. We found that strawberry guava juice can also be used as pre-treatment for metaphase arrest in plant cytogenetics due to their antimitotic properties. However, caution is required regarding the indiscriminate use of fruits and juices, despite beneficial effects of compounds such as epicatechin.
Declaration of interest
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to Dr Margareth Linde Athayde (in memoriam), Professor in the Department of Industrial Pharmacy, for her valuable collaboration.
References
- Alvarenga AL, Schwan RF, Dias DR, Schwan-Estrada KRF, Bravo-Martins CEC. 2007. Atividade antimicrobiana de extratos vegetais sobre bactérias patogênicas humanas. Rev Bras Plantas Med. 9:86–91.
- Ayres M, Ayres Júnior M, Ayres DL, Santos AA. 2007. BioEstat: aplicações estatísticas nas áreas das ciências biomédicas. Belém: Ong Mamiraua.
- Bagatini MD, Silva ACF, Tedesco SB. 2007. Uso do sistema teste de Allium cepa como bioindicador de genotoxicidade de infusões de plantas medicinais. Rev Bras Farmacogn. 17(3):444–447.10.1590/S0102-695X2007000300019
- Bianchi MLP, Antunes LMG. 1999. Radicais livres e os principais antioxidantes da dieta. Rev Nutr. 12:123–130.10.1590/S1415-52731999000200001
- Biegelmeyer R, Andrade JMM, Aboy AL, Apel MA, Dresch RR, Marin R, Raseira MDCB, Henriques AT. 2011. Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianum var. lucidum) strawberry guava fruit. J Food Sci. 76:C991–C996.10.1111/jfds.2011.76.issue-7
- Boligon AA, Kubiça TF, Mario DN, Brum TF, Piana M, Weinblen R. 2013. Antimicrobial and antiviral activity-guided fractionation from Scutia buxifolia Reissek extracts. Acta Physiol Plant. 35:2229–2239.10.1007/s11738-013-1259-0
- Cabrera GL, Rodriguez DMG. 1999. Genotoxicity of soil from farmland irrigated with wastewater using three plant biossays. Mutat Res. 426:211–214.10.1016/S0027-5107(99)00070-6
- Coelho APD, Laughinghouse HD IV, Kuhn AW, Boligon AA, Canto-Dorow TS, Silva ACF, Tedesco SB. 2017. Genotoxic and antiproliferative potential of extracts of Echinodorus grandiflorus and Sagittaria montevidensis (Alismataceae). Caryologia. 70(1):82–91.10.1080/00087114.2016.1275932
- Costa TDA, Vieira S, Andrade SF, Maistro EL. 2008. Absence of mutagenicity effects of Psidium cattleyanum Sabine (Myrtaceae) extract on peripheral blood and bone marrow cells of mice. Genet Mol Res. 7(3):679–686.10.4238/vol7-3gmr475
- Decker EA. 1997. Phenolics: prooxidants or antioxidants? Nutr Rev. 55:396–407.
- Ekong NJ, Akpan GA, Udo IJ. 2014. Comparative effects of colchicine, 8-hydroxyquinoline and paradichlorobenzene on arm ratio of mitotic chromosomes of Allium cepa L. Int J Med Plant Altern Med. 2:21–26.
- Fachinetto JM, Bagatini MD, Durigon J, da Silva ACF, Tedesco SB. 2007. Efeito anti-proliferativo das infusões de Achyrocline satureioides DC (Asteraceae) sobre o ciclo celular de Allium cepa. Revista Brasileira de Farmacognosia. 17:49–54.
- Fachinetto JM, Tedesco SB. 2009. Número cromossômico, análise meiótica e estimativa da viabilidade polínica em populações de Hyptis mutabilis (Rich.) Briq. Rev Bras Plant Med. 11:110–116.10.1590/S1516-05722009000100017
- Frescura VD, Kuhn AW, Laughinghouse IV HD, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66:138–144.10.1080/00087114.2013.821832
- Guerra MP, Nodari RO. 2007. Biodiversidade: aspectos biológicos, geográficos, legais e éticos. In: Simões CMO. (Org.), Farmacognosia: da planta ao medicamento. 6th ed. Florianópolis: UFRGS, Porto Alegre; UFSC. p. 13–28.
- Haminiuk CWI, Sierakowski MR, Vidal JRMB, Masson ML. 2006. Influence of temperature on the rheological behavior of whole araçá pulp (Psidium cattleianum Sabine). LWT - Food Sci Technol. 39(4):427–431.10.1016/j.lwt.2005.02.011
- Leme DM, Marin-Morales MA. 2009. Allium cepa test in environmental monitoring: A review on its application. Mutat Res. 682:71–81.10.1016/j.mrrev.2009.06.002
- Levan A. 1938. The effect of colchicine on root mitoses in AIlium. Hereditas. 24:471–486.
- Lorenzi H. 1992. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Nova Odessa: Plantarum.
- Luximon-Ramma A, Bahorun T, Crozier A. 2003. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J Sci Food Agric. 83(5):496–502.10.1002/(ISSN)1097-0010
- Medina AL. 2009. Atividade antioxidante e antimicrobiana de extratos de araçá (Psidium cattleianum) [Master diss]. Pelotas: Universidade Federal de Pelotas. p. 63.
- Medina AL, Haas APS, Chaves FC, Salvador M, Zambiazi RC, da Silva WP, Nora L, Rombaldi CV. 2011. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 128(4):916–922.10.1016/j.foodchem.2011.03.119
- Ortiz AM, Silvestri MC, Lavia GI. 2013. Karyotypic studies in wild species of Arachis (Leguminosae) belonging to sections Erectoides, Procumbentes and Rhizomatosae. Bol Soc Argent Bot. 48:295–300.
- Osalou AR, Rouyandezagh SD, Alizadeh B, Er C, Sevimay CS. 2013. A comparison of ice cold water pretreatment and α Bromonaphthalene cytogenetic method for identification of Papaver species. Scientific World J. Article ID 608650. 6 p. doi: 10.1155/2013/608650
- Oyeyemi IT, Bakare AA. 2013. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia. 66:360–367.10.1080/00087114.2013.857829
- Peroza LR, Busanello A, Leal CQ, Ropke J, Boligon AA, Meinerz D, Libardoni M, Athayde ML, Fachinetto R. 2013. Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro. Neurochem Res. 38:789–796.10.1007/s11064-013-0981-8
- Silva I, Franco SL, Molinari SL, Conegero CI, Miranda-Neto MH, Cardoso MLC, Sant’ana DMG, Iwanko NS. 1995. Noções sobre o organismo humano e a utilização de plantas medicinais. Cascavel: Assoeste Editora Educativa.
- Sousa LP, Sobral MEG. 2007. Morfotipos do Araçazeiro, Psidium cattleianum Sabine (Myrtaceae) no Estado do Paraná. In: Pedrosa-Macedo JH, DalMolin A, Smith CW (Orgs), O Araçazeiro: Ecologia e Controle Biológico. Curitiba: FUPEF. p. 19–28
- Tedesco SB, Laughinghouse IV HD. 2012. Bioindicator of genotoxicity: the Allium cepa test. In: Srivastava JK, editors. Environmental Contamination. Rijeka: InTech. p. 137–156. Available from: https://www.intechopen.com/books/environmental-contamination/bioindicator-of-genotoxicity-the-allium-cepa-test.
- Teixeira RO, Camparoto ML, Mantovani MS, Vicentini VEP. 2003. Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L. in in vivo assays. Genet Mol Biol. 26:551–555.10.1590/S1415-47572003000400021
- Varella MXS, Santos CAM, Duarte MR. 2007. Caracteres morfoanatômicos e constituintes químicos de folha de araçazeiro – morfotipos de fruto vermelho e amarelo. In Pedrosa-Macedo JH, DalMolin A, Smith CW (Orgs.), O Araçazeiro: Ecologia e Controle Biológico. Curitiba: FUPEF. p. 39–45
- Volpato AMM. 2005. Investigação do potencial antibacteriano de Calendula officinalis (Asteraceae) para seu emprego como fitoterápico [ PhD diss]. Curitiba: Universidade Federal do Paraná; p. 115.
