Abstract
The problem of the invasion of the invasive species Ambrosia artemisiifolia L. is particularly important for Romania and other countries. Besides the fact that this species is a strong allergen, its presence in agricultural crops can aggravate the growth and development of plants, just like other weeds. The purpose of this study was to investigate the allelopathic effect of different concentrations of aqueous extracts obtained from the above-ground part (leaves and stems) and from the underground part (roots) of Ambrosia artemisiifolia on the germination and growth of Zea mays. Furthermore, for the first time, we have conducted a study of the cytotoxic and genotoxic effects of these extracts in meristematic cells of Zea mays. All extracts, and in particular 10% above-ground extract, significantly reduced the germination of the seeds and had a different effect on the growth of maize seedlings. Also, these extracts reduced the mitotic index (MI) and induced chromosomal alterations such as multinucleated cells, C-metaphases and sticky, ring, fragment, and bridge-type chromosomes, thus revealing a strong cytotoxic and genotoxic effect on maize.
Introduction
The invasion of foreign species is recognized globally as one of the greatest threats to biodiversity, the economy and even human health (Lambdon et al. Citation2008; Bullock et al. Citation2013). Plant invasions can result from e.g. trade, transport, tourism and global climate change (Anastasiu and Negrean Citation2007).
Ambrosia artemisiifolia (ragweed) was first reported in Romania in 1908 in Orsova (south-western Romania), and later it was found that this species has spread in many localities throughout Romania (Hodisan and Morar Citation2008).
The competitiveness and reproductive potential of this species in maize fields has been shown by various studies (Weaver Citation2001; Simard and Benoit Citation2012).
Current research suggests that the invasion of A. artemisiifolia is due to a strong allelopathic effect. The allelopathic effect of plants depends on the receptor species and their susceptibility to allelochemicals (Soltys et al. Citation2013). Various bioactive compounds with allelochemical potential have been isolated from ragweed roots – for example, terpenes, polyacetylenes and sitosterol (Wang et al. Citation2005). Bruckner (Citation1998) suggests that the chemical constituents responsible for the allelopathic capacity of the species Ambrosia artemisiifolia are phenoloids and terpenoids.
In the germination tests performed by Lehoczky et al. (Citation2011), aqueous plant extracts of Ambrosia artemisiifolia (seeds or shoots) have a strongallelopathic effect, reducing the germination and growth rate of roots and seedlings of maize, wheat, rye and oat.
Considering the invasion of this species in maize cultures and the absence of data on its mutagenic potential through cytogenetic bio-tests, the purpose of this paper was to describe the effects of ragweed aqueous extracts on seed germination, root growth and shoots, chromosome structure and the cell cycle to Zea mays.
Materials and methods
Plant material and extracts preparation
Ragweed plants (Asteraceae) were collected in July from Craiova city, Romania. The plant material was divided into above-ground parts (stems and leaves) and underground parts (roots). Aqueous extracts were obtained by immersing the dried and fresh material, 5 g and 10 g, in 100 ml of distilled water. After 24 h, the extracts were filtered through filter paper. The control was moistened with distilled water.
Maize seeds were disinfected with 2% sodium hypochlorite solution for 5 min, and then washed with distilled water several times. Twenty seeds were placed in plastic casks on double filter paper, three repetitions for each extract concentration at a temperature of 22 ± 2°C.
Microscopic preparations
After being collected at a length of 1 cm, the meristematic roots were processed through fixation stage (in a fixative solution containing 30 ml ethyl alcohol and 10 ml acetic acid for 24 h), a hydrolysis stage (first, in 5 ml HCl 1 N for 5 min and then in HCl 50% consisting of equal parts of HCl and distilled water for 16 min at room temperature) and a colouring stage with Schiff reagent prepared in accordance with the method proposed by Darlington and LaCour (Citation1963). For this, 3 ml of Schiff reagent were added on the Zea mays meristematic tissues and when the tissues turned purple-bluish, five temporary microscopic preparations were made, for each of the variants of the experiment, and they were visualized in the digital microscope MBL 2000 (manufactured by KRÜSS Optronic, Hamburg, Germany).
Statistical analyses
The percentage of germination was calculated using the formula:
After seven days, root and shoot length were measured. The obtained data were analysed statistically with one-way analysis of variance (ANOVA) and differences between treatment means were compared using the LSD-test at a probability level of 0.05% (Botu and Botu Citation1997).
As for cytological analysis, for each variant of the experiment, 500 cells were visualized. The mitotic index was calculated using the following formula:
All the results were expressed as the mean of three replicates per treatment ± standard error (SE).
Results
Table presents the variance analysis (ANOVA) for the studied characters, which shows that among the various treatments used there are statistically significant differences for the percentage of germination and root length, but not for shoot length (p = 0.05).
Table 1. Variance analysis (ANOVA) of studied traits of Zea mays L.
Table shows the effects of various concentrations of Ambrosia artemisiifolia aqueous extracts (5 and 10%) obtained from different parts of the plant on the germination and growth of maize seedlings. From these results it was observed that all treatment variants (T2, T3, T4, T5) determined a significant (p < 0.05) reduction of seed germination against the control (T1) (Table ).
Table 2. Comparison of the percentage of germination (% G), the shoot length and root length.
Generally a similar behaviour was observed on shoot growth, but it was statistically insignificant. Regarding root growth, only the T3 treatment significantly reduced the growth of the roots compared to the control (p = 0.05).
Table presents the results of the effects of Ambrosia artemisiifolia extracts on the mitotic index and the cell division phases in Zea mays. A significant reduction (p = 0.05) of the mitotic index compared to the control was observed in all treatments (T2–T5). The mitotic index (MI) reached the minimum value of 5.8 ± 0.69% compared to the control value of 12.8 ± 0.58% for the extract from above-ground parts 10% (T3).
Table 3. Mitotic index (MI) and number of cells in interphase and mitosis stages of root meristems of Zea mays.
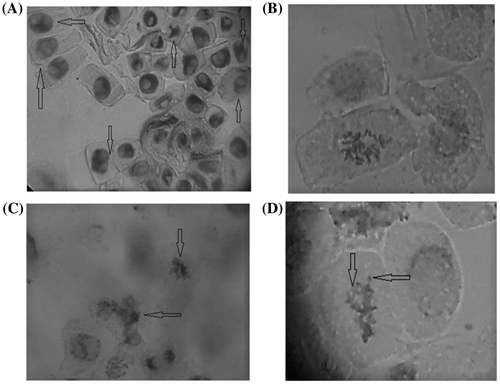
Table shows the different types of chromosomal alterations induced by Ambrosia artemisiifolia aqueous extracts in corn: multinucleated cells (Figure (a)), C-metaphases and sticky, ring, fragment, and bridge chromosomes (Figure (b–d)). The total number of cells with chromosomal alterations was significantly higher in all treatments (T2–T5) than in the control (p = 0.05). The number of modified cells reached the maximum value of 33 compared to the control value of 2, in the case of treatment with 10% extract from above-ground parts.
Table 4. Number of cells with chromosomal alterations in the root meristem of Zea mays.
Discussion
The reduction of germination percentage is the most sensitive parameter that can be used to determine the phytotoxicity levels of the components of the used extract (Valerio et al. Citation2007).
Our results have demonstrated the phytotoxic effects of Ambrosia artemisiifolia aqueous extracts as a result of the reduction of seed germination percentage and even corn root growth depending on the concentrations and parts used.
Bruckner (Citation1998) stated that the main phytotoxic compounds responsible for the allelopathic capacity of A. artemisiifolia L. are phenolic compounds and terpenes. Kong et al. (Citation2007) reported that Ambrosia trifida inhibited the growth of wheat (T. aestivum), the allelochemicals involved in this process being carotene-type sesquiterpenes.
Wang and Zhu (Citation1996) reported that the aqueous extract of the A. artemisiifolia aerial parts significantly inhibited the germination and primary growth of corn, soy, wheat and rice cultures, and the aqueous extract from the roots had no effect on seed germination, but had a different effect on primary crop growth.
Buzhdygan and Baglei (Citation2016) have reported controversial results, namely that the aqueous extract of Ambrosia artemisiifolia significantly stimulated seed germination and shoot length in Hordeum vulgare L. but significantly inhibited the germination and length of the roots in Helianthus annuus. The extract also significantly inhibited the length of Triticum aestivum roots.
Allelochemicals modify the mitotic index and suppress the synthesis of hormones, affecting germination and plant growth (Rice Citation1984). The cytotoxic and genotoxic effect of extracts from other invasive plants has been tested by several authors using various plant species for testing, but this is the first report on the effect of aqueous extracts of Ambrosia artemisiifolia on maize meristematic roots.
The mitotic index (MI) is an acceptable measure of cytotoxicity assessment in all living organisms (Smaka-Kincl et al. Citation1996). The cytotoxic level can be determined by increasing or decreasing this mitotic index.
According to Sharma (Citation1983) the cytotoxic threshold is the concentration that causes a mitotic depression of 50% compared to the control. In our study, we noticed that this cytotoxic threshold was exceeded only in the T3 treatment with 10% extract from above-ground parts, which means that this mitotic depression is the main cause of the drastic reduction of germination percentage and growth of maize roots. However, the cytotoxic effects of ragweed were evident in all treatments as a result of the significant reduction of MI.
Sousa and Viccini (Citation2011) explain the reduction of MI as a result of the interphase nucleation division, thus stopping the onset of prophase, and thus the division of cells.
We have noticed that plant parts and different concentrations of ragweed extract have induced different types of chromosomal alterations at different stages of cell division, increasing the total percentages of abnormalities. The presence of multinucleated cells is due to failure in cell plate formation for already bi-nucleated cells. The incomplete anaphase or unequal distribution of chromosomes to daughter cells leads to the production of polyploid cells (Grant Citation1978).
The presence of the C-metaphase suggests that some allelochemicals of the extracts act on the mitotic spindle apparatus, probably interfering with the polymerization and depolymerization of microtubules (Seth et al. Citation2008). Fiskesjó (Citation1993, Citation1997) explains that C-metaphases are formed due to the complete inactivation of spindle division, and stickiness is considered a common sign of toxic effects on chromosomes, which probably leads to cell death.
Other authors also reported the occurrence of these types of chromosomal alterations in maize or other plant species, indicating the cytogenetic effects of the used agents (Abderrahman Citation1998; Frescura et al. Citation2013; Bonea and Bonciu Citation2017; Kundu and Ray Citation2017).
Low values of the mitotic index in maize roots indicate the presence of cytotoxic substances in Ambrosia aqueous extracts, and the increase in the percentage of chromosomal alterations indicates the genotoxic effects of these extracts.
In conclusion, this paper provides evidence of the cytotoxicity and genotoxicity of Ambrosia artemisiifolia in the Zea mays meristematic roots, confirming the efficacy of the cytological tests in cytotoxicity monitoring. The growth of Ambrosia artemisiifolia in association with the Zea mays culture can lead to a drastic reduction in seed germination of this crop. Therefore, this invasive species should be controlled/monitored more closely in maize crops.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abderrahman SM. 1998. Cytogenetic effects of Peganum harmala extract on maize root tips. Cytologia. 63(3):283–291.10.1508/cytologia.63.283
- Anastasiu P, Negrean G. 2007. Invadatori vegetali în România/Plant invaders in Romania. Bucharest: University of Bucharest Publishing; p. 12–13.
- Bonea D, Bonciu E. 2017. Cytogenetic effects induced by the fungicide Royal Flo to maize (Zea mays L.). Caryologia. 70(3):195–199.10.1080/00087114.2017.1318232
- Botu I, Botu M. 1997. Statistical parameters used in the evaluation of the research of fruit plants. In Conphys, editors. Methods and techniques of research in pomiculture. Rm. Valcea (RO): Conphys Publishing; p. 213–236.
- Bruckner DJ. 1998. The allelopathic effect of ragweed (Ambrosia artemisiifolia L.) on the germination of cultivated plants. Novenytermeles. 47(6):635–644.
- Bullock JM, Chapman D, Schafer S, Roy D, Girardello M, Haynes T, Beal S, Wheeler B, Dickie I, Phang Z, et al. 2013. Assessing and controlling the spread and the effects of common ragweed in Europe. Final report: ENV.B2/ETU/2010/0037, Natural Environment Research Council, UK; p. 456.
- Buzhdygan OY, Baglei OV. 2016. Developmental traits in grassland and agricultural plants under the influence of ragweed. Biological Systems. 8(2):202–207.
- Darlington CD, LaCour LF. 1963. Methods of the chromosome investigations. Stuttgart: French Publishing firm. W. Keller and Ca.
- Fiskesjó G. 1993. Allium test I: A 2–3 day plant test for toxicity assessment by measuring the mean root growth of onions (allium cepa L.). Environ Toxicol Water Qual. 8(4):461–470.10.1002/(ISSN)1098-2256
- Fiskesjö G. 1997. Allium test for screening chemicals; evaluation of cytological parameters. In: Wang Wuncheng, Gorsuch Joseph W, Hughes Jane S, editors. Plants for environmental studies. New York, NY: Lewis Publishers; p. 307–333.10.1201/9781420048711
- Frescura VD, Kuhn AW, Laughinghouse IV HD IV, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66(2):138–144.10.1080/00087114.2013.821832
- Grant WF. 1978. Chromosome aberrations in plants as a monitoring system. Environ Health Perspect. 27:37–43.10.1289/ehp.782737
- Hodisan N, Morar G. 2008. The spread of the invasive species Ambrosia artemisiifolia L. in Romania, between 2005-2007. Bulletin UASVM. Agriculture. 65(1):129–134.
- Kong CH, Wang P, Xu XH. 2007. Allelopathic interference of Ambrosia trifida with wheat (Triticum aestivum). Agric Ecosyst Environ. 119(3-4):416–420.10.1016/j.agee.2006.07.014
- Kundu LM, Ray S. 2017. Mitotic abnormalities and micronuclei inducing potentials of colchicine and leaf aqueous extracts of Clerodendrum viscosum Vent. in Allium cepa root apical meristem cells. Caryologia. 70(1):7–14.10.1080/00087114.2016.1254452
- Lambdon PW, Pysek P, Basnou C, Hejda M, Arianoutsou M, Essl F, Jarosí V, Pergl J, Winter M, Anastasiu P, et al. 2008. Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs. Preslia. 80(2):101–149.
- Lehoczky E, Szabo R, Nelima MO, Nagy P, Beres I. 2011. Allelopathic effects of ragweed (Ambrosia artemisiifolia L.) on cultivated plants. Commun Agric Appl Biol Sci. 76(3): 545–549.
- Rice L. 1984. Allelopathy. 2nd ed. New York, NY: Academic Publishers; p. 424.
- Seth CS, Misra V, Chauhan LK, Singh RR. 2008. Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and comet assay approach. Ecotoxicol. Environ. Saf. 71(3):711–716.10.1016/j.ecoenv.2008.02.003
- Sharma CBSR. 1983. Plant meristems as monitor sof genetic toxicity of environmental chemicals. Curr Sci. 52:1000–1002.
- Simard M-J, Benoit DL. 2012. Potential pollen and seed production from early- and late-emerging common ragweed in corn and soybean. Weed Technol. 26(03):510–516.10.1614/WT-D-11-00178.1
- Smaka-Kincl V, Stegnar P, Lovka M, Toman Mihael J. 1996. The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutat Res. 368(3-4):171–179.10.1016/S0165-1218(96)90059-2
- Soltys D, Krasuska U, Bogatek R, Gniazdowska A. 2013. Allelochemicals as bioherbicides - present and perspectives. In Herbicides – Current Research and Case Studies in Use. Price AJ, Kelton JA, editors; Rijeka, Croatia: InTech Publisher; p. 517–542. CC BY
- Sousa SM, Viccini LF. 2011. Cytotoxic and genotoxic activity of Achillea millefolium aqueous extracts. Braz J Pharmacog. 21(1):98–104.10.1590/S0102-695X2011005000022
- Valerio ME, García JF, Peinado FM. 2007. Determination of phytotoxicity of soluble elements insoils, based on a bioassay with lettuce (Lactuca sativa L.). Sci Total Environ. 378(1-2):63–66.10.1016/j.scitotenv.2007.01.007
- Wang D, Zhu X. 1996. Research on allelopathy of Ambrosia artemisiifolia. Acta Ecologica Sinica. 16(1):11–19.
- Wang P, Liang W, Kong C, Jiang Y. 2005. Allelopathic potential of volatile allelochemicals of Ambrosia trifida L. on other plants. Allelopath J. 15(1):131–136.
- Weaver SE. 2001. Impact of lamb’s-quarters, common ragweed and green foxtail on yield of corn and soybean in Ontario. Can J Plant Sci. 81(4):821–828.10.4141/P01-057