ABSTRACT
Detailed karyotypic investigation of five species (Q. hartwissiana Steven., Q. frainetto Ten., Q. macranthera subsp. syspirensis (C. Koch.) Menitsky, Q. virgiliana Ten., and Q. trojana Webb) belonging to the genus Quercus was carried out in order to contribute to the taxonomic relationships of the genus. The somatic chromosome number was found to be 2n = 24 in all taxa examined. Q. hartwissiana, Q. frainetto and Q. macranthera subsp. syspirensis showed similar karyotypes, consisting of 22 metacentric and 2 submetacentric chromosomes. The karyotype of Q. virgiliana included all metacentric chromosomes and finally Q. trojana showed the highest variation according to karyotypic description (14 m + 10 sm), haploid complement and other morphometric parameters. The study results showed similarity with previous reports in Turkey, different to European oaks.
Introduction
The genus Quercus L. (oaks) belongs to the Fagaceae family and includes over 500 woody plant species with high ecological and morphological diversity (Govaerts and Frodin Citation1998). Oak trees have high economic value as ornamentals and in the timber industry. Furthermore, they play important functional and structural roles in forest communities worldwide.
Oaks are dominant species which exhibit high phenotypic variation with natural hybrids in the northern hemisphere (Manos et al. Citation2001; Borazan and Babaç Citation2003). Oaks appear frequently as dominant trees in Anatolia and Mediterranean region of the northern hemisphere (Manos et al. Citation2001; Uslu and Bakış Citation2012).
Turkey is an important transitional region between the Asian and European continents. Its location and geomorphological structure increase the climatic effects and separate Turkey into different phytogeographic regions which influence species number and diversity (Uslu and Bakış Citation2012). Climatic changes in Turkey occurred especially at the beginning of the Holocene and these changes affected its geographical structure and the flora (Atalay Citation2005). Beside the phytogeographical regions, another factor affecting species diversity and distribution is the Anatolian Diagonal, which divides Anatolia into eastern and western parts (Davis Citation1971; Çıplak et al. Citation1993; Borazan and Babaç Citation2003).
Turkey is one of the richest countries according to species number, diversity and, in particular, endemic species (Yaltirik Citation1984). Eighteen oak species currently occur in Turkey, belonging to three subgeneric sections (Quercus, Cerris and Ilex) (Yaltirik Citation1984).
Section Ilex Loudon. is characterized by the evergreen trees and shrubs: Q. ilex L., Q. coccifera L. and Q. aucheri Jaub. et Spach. (Yaltirik Citation1984).
Cerris Loudon. is the second largest section and includes five species: Q. libani Olivier, Q. trojana Webb, Q. cerris L., Q. brantii Lindl. and Q. ithaburensis subsp. macrolepis (Kotschy) Hedge et Yalt. (Yaltirik Citation1984).
Section Quercus L. has the greatest number of species: Q. pontica C. Koch., Q. robur L., Q. hartwissiana Steven., Q. macranthera subsp. syspirensis (C. Koch.) Menitsky, Q. frainetto Ten., Q. petraea (Mattuschka) Lieb., Q. vulcanica (Boiss. Heldr. ex) Kotschy, Q. infectoria Oliver, Q. pubescens Willd. and Q. virgiliana Ten. (Yaltirik Citation1984).
Although vegetative characters are important and frequently preferred in the identification of oaks, these are considered as quite risky because of hybridization between oak taxa. Scientific interest has recently moved from classic description to biological understanding of oak evolution by means of molecular markers (Petit et al. Citation2003a; Oh and Manos Citation2008; Denk and Grimm Citation2010; Simeone et al. Citation2013). However, oak taxonomy is still problematic and under debate due to often insufficient diagnostic morphological characters (Denk and Grimm Citation2010; Simeone et al. Citation2013), weak reproductive barriers between species, wind pollination (Bacilieri et al. Citation1996; Manos et al. Citation1999; Borazan and Babaç Citation2003; Denk and Grimm Citation2010; Simeone et al. Citation2013) and the lack of investigations for each taxon such as ecological, historical and genetic descriptors (Simeone et al. Citation2013). These factors make the taxonomy of the genus Quercus problematic in Turkey, as well as globally.
Cytologic techniques are very important and widely used for determining taxonomy and polyploidy, but they are not frequently used for oaks because of difficulties in germination of acorns and the small size of chromosomes in trees like oaks (D’emerico et al. Citation1995; Chokchaichamnankit et al. Citation2008).
In the present paper, karyotype analyses of Q. hartwissiana, Q. frainetto, Q. macranthera subsp. syspirensis, Q. virgiliana belonging to section Quercus, and Q. trojana belonging to section Cerris were described from different locations in Turkey.
Chromosome analyses of all species from the section Ilex in Turkey have been completed in a previous study (Aykut et al. Citation2011). Together with the present study, the karyotypes of all species of section Quercus and Cerris are made available, except Q. pontica from section Quercus and Q. brantii from section Cerris (Aykut et al. Citation2008, Citation2011; Yılmaz 2017). The cytological analysis of the 16 oak taxa from three section occurring in Turkey will increase the available knowledge on Quercus biology, contribute to assessing taxonomic relationships with the Eurasian species, and facilitate further studies on oak chromosome structure, function and activity.
Materials and methods
Acorns belonging to five oak species were collected from different localities in Turkey. The studied species and their locations are listed in .
Table 1. Species, sections and localities of studied species.
Acorns were put in a refrigerator at 4°C and observed regularly for germination. Germinated roots with lengths of 2–10 mm were excised and put into small glass bottles and pretreated in α-monobromonaphthalene for 16 h at 4°C.
Root tip meristems after α-monobromonaphthalene treatment were fixed overnight with 3:1 ethanol:acetic acid. After fixation, root tips were stored in 70% alcohol at 4°C until use. Hydrolysis was carried out with concentrated HCl solution at 60°C for 13 min. Root tips were then washed with distilled water. Root tips were then stained with freshly prepared Feulgen for 2 h and squashed with 2% aceto orcein to obtain metaphase chromosomes.
Finally, the best metaphase plates were selected and frozen in liquid nitrogen to make them permanent using Entellan. Permanent slides were photographed 10 × 100.
For each species, at least five plates with metaphase chromosomes were measured on the basis of small and long arm length and arm ratio. Homolog chromosome pairs were identified and arranged.
Chromosome pairs were classified using the nomenclature of Levan et al. (Citation1964) and Stebbins (Citation1971). Romero Zarco (Citation1986) was followed for the karyotype asymmetry parameters like the intrachromosomic asymmetric index (A1), and interchromosomic asymmetric index (A2).
Results
This study was carried out to analyze the karyotypes of five oak species belonging to section Quercus and Cerris in Turkey. Acorns as plant materials for each studied species were collected from different locations. Plant samples, species sections and sampling locations are given in .
Somatic metaphase chromosomes of the investigated Quercus species were very small and similar with a diploid chromosome number 2n = 24 (, and ). Chromosome analyses of 16 Quercus species existing in Turkey has been therefore completed and it is confirmed that the 2n = 24 chromosome number is typical of the genus () (Aykut et al. Citation2008, Citation2011; Yilmaz Citation2017). These results are in agreement with previous studies based on chromosome number of the genus Quercus from different species and different parts of the world (Ohri and Ahuja Citation1990; D’emerico et al. Citation1995, D’emerico et al. Citation2000; Zoldos et al. Citation1998; Kurokawa and Yonezawa Citation2004).
Table 2. Species, somatic chromosome numbers, karyotypic descriptions and other morphometric parameters of studied Quercus taxa.
Table 3. Species, somatic chromosome numbers, karyotypic descriptions and other morphometric parameters of previous analyses on the Turkish Quercus taxa.
Figure 1. Somatic chromosomes of (a) Q. hartwissiana; (b) Q. frainetto; (c) Q. macranthera subsp. syspirensis; (d) Q. virgiliana; (e) Q. trojana.
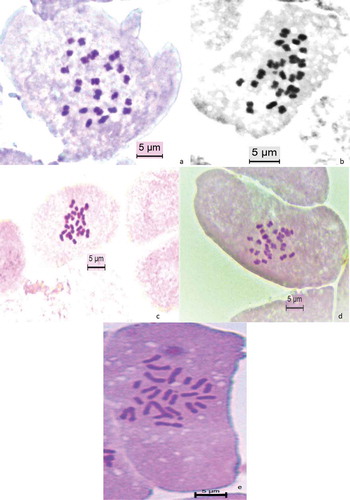
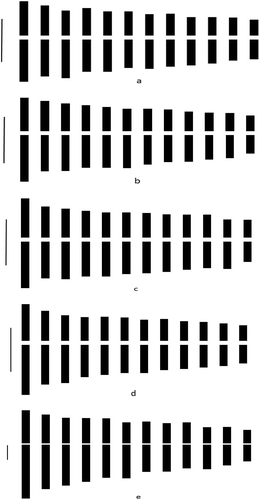
Figure 2. Idiograms of (a) Q. hartwissiana; (b) Q. frainetto; (c) Q. macranthera subsp. syspirensis; (d) Q. virgiliana; (e) Q. trojana. (Bar : 1 µm).
Cytological results obtained from our study are as follows.
Quercus hartwissiana
Plant samples for Q. hartwissiana were collected from Bursa-Yalova location (northwestern region of Turkey) (). The total chromosome number confirmed the consistency of the 2n = 24 chromosome number among Quercus species. Most chromosomes were metacentric (22m + 2sm) (). Q. hartwissiana has the second smallest haploid complement with 15.22 µm and chromosomes set between 0.85–1.83 µm (). Among the studied taxa, the lowest intrachromosomal asymmetry index value (A1) was observed in Q. hartwissiana together with Q. macranthera subsp. syspirensis and Q. virgiliana (0.22). The value obtained for the interchromosomal asymmetry index (A2) of 0.23 was the second lowest after Q. macranthera subsp. syspirensis (). Morphometric parameters of Q. hartwissiana including haploid complement, chromosomal length range, A1 and A2 value were quite small among the studied oak species.
Q. hartwissiana is distributed in northern regions of Turkey and locally around Erzurum (Yaltirik Citation1984). Detailed chromosomal parameters were reported for the first time in Turkey.
Quercus frainetto
The chromosome number of Q. frainetto was found to be 2n = 24. Chromosome analyses showed that karyotypic description is 22m + 2sm. The smallest chromosome set was observed in Q. frainetto according to haploid complement (14.50 µm) and length range (0.76–1.80 µm) ().
Q. frainetto is distributed in northwestern regions (especially Marmara Region) of Turkey (Yaltirik Citation1984). Plant samples of Q. frainetto and Q. hartwissiana was collected from same location. Among the studied species, the karyotype of Q. frainetto in comparison to Q. hartwissiana shows close similarity in terms of chromosome number (2n = 24), chromosome morphology (22 m + 2sm), chromosomal lengths range and small haploid complement with 14.50–15.22, respectively.
Previously detailed karyotype analyses of Q. frainetto was reported by D’emerico et al. (Citation1995). D’emerico et al. (Citation1995) stated that chromosome number of Q. frainetto is 2n = 24 and karyotypic description of this taxon was 14m + 2mSC + 6sm + 2smSC. Chromosome number examined in this study showed consistency with D’emerico et al. (Citation1995). However, comparison of other parameters studied by D’emerico et al. (Citation1995) shows differences in terms of chromosome morphology, haploid complement, A1 and A2 value. Lower parametric values were observed in this study. These differences can be caused by oak species living in different geographical regions, hybridization and gene flow between oak species.
Quercus macranthera subsp. syspirensis
Q. macranthera subsp. syspirensis chromosome number was found as 2n = 24 with 22 metacentric and two submetacentric chromosomes (22m + 2sm). The haploid complement value of 16.04 µm was the second highest among the all studied taxa. Chromosomal asymmetry index; A1 and A2 have the lowest value compared to the other studied taxa ().
Q. macranthera subsp. syspirensis is an endemic taxon for Turkey. This taxon generally occurs in northern parts of Anatolia, in provinces of Bolu, Kastamonu, Yozgat, Çorum, Amasya, Sivas, Gümüşhane, Erzurum, Zonguldak, Bartın, Erzincan, Bayburt and Kars (Hedge and Yaltırık Citation1982; Kargioglu et al. Citation2011). Plant materials belonging to Q. macranthera subsp. syspirensis were collected from Bolu province (). Detailed karyotype analyses of this taxa are reported for the first time.
Quercus virgiliana
Chromosome number of Q. virgiliana was found to be 2n = 24. All chromosomes of this taxa were metacentric. Haploid complement was 15.84 µm and chromosome lengths ranged from 0.85 to 2.16 µm. A1 value was the lowest together with Q. hartwissiana and Q. macranthera subsp. syspirensis.
Chromosome count and detailed measurements of Q. virgiliana has been performed previously (D’emerico et al. Citation1995). Comparisons of these karyotypes show similarity in terms of chromosome number (2n = 24) but chromosome morphologies differentiate according to total lengths of chromosomes and karyotypic description. D’emerico et al. (Citation1995) stated that karyotypic description and A1 value of Q. virgiliana were 10m + 4mSC + 8sm + 2smSC and 0.35, respectively. In this study Q. virgiliana has the smallest chromosome set, all metacentric chromosomes and lowest parametric value for A1.
Quercus trojana
Q. trojana chromosome number was found to be 2n = 24. Among the studied taxa, the biggest variation was determined in Q. trojana. Chromosome length range and haploid complement of this taxon have the highest value with 2.29–6.65 µm and 49.62 µm respectively, in comparison to other studied taxa. Karyotypic description of this taxon consist of 14 metacentric and 10 submetacentric chromosomes (14m + 10sm). Chromosomal asymmetry index, A1 and A2 have highest value with 0.28 and 0.30, respectively.
This taxon lives in west and southwest Anatolia between 300 and 1800 m above sea level. Q. trojana show approximately equal sum of metacentric and submetacentric chromosomes (14m + 10sm). Q. trojana chromosome count and other detailed measurements were reported by D’emerico et al. (Citation1995). Chromosome types analyzed in this study showed similarity with the results provided from D’emerico et al. (Citation1995) (8m + 4mSC + 10sm + 2smSC).
Discussion
Previous karyotype analyses made on the genus Quercus in Turkey showed that the chromosome number of the genus is stable with 2n = 24 and all chromosomes are metacentric () (Aykut et al. Citation2008, Citation2011; Yilmaz Citation2017). However, in this study, it is observed that Q. hartwissiana, Q. frainetto and Q. macranthera subsp. syspirensis have two submetacentric chromosomes and Q. trojana has the 10 submetacentric chromosomes. In other words, the majority of the examined species in Turkey had karyotypes with a predominance of metacentric chromosomes. Nevertheless, the European oak species analyzed before clearly have higher number of submetacentric chromosomes (D’emerico et al. Citation1995, Citation2000). For example, while it is stated that Q. coccifera, Q. ilex and Q. robur in previous studies performed in Turkey consist of all metacentric chromosomes (Aykut et al. Citation2011; Citation2017), D’emerico et al. (Citation1995) and (Citation2000) stated that these species contain submetacentric chromosome pairs.
Although the results provided from previous studies on Turkish and European oaks support that the chromosome number of the genus is 2n = 24, some exceptions with different chromosome number are reported (Zoldos et al. Citation1998; Chokchaichamnankit et al. Citation2008; Ribeiro et al. Citation2011). However, occasionally ploidy variation may be observed in individual trees such as triploid samples of Q. robur with 2n = 3x = 36 (Butorina Citation1993). Similarly, the presence of endopolyploid cells (2n = 4x = 48) together with diploid cells are reported in an individual tree of Q. frainetto (D’emerico et al. Citation1995).
It can be stated that the haploid chromosome lengths of all Quercus taxa analyzed in Turkey range from 14 µm to 23 µm, except Q. robur and Q. trojana having chromosome lengths 31.78 µm and 49.62 µm, respectively (Aykut et al. Citation2008, Citation2011; Yilmaz Citation2017). If the haploid chromosome lengths are taken into consideration in the evaluation of European and Turkish oaks, it can be seen that European oak species for the same and other oak species clearly have larger chromosome size and parametric values than Turkish oak species.
It is observed that taxa belonging to section Quercus in Turkey are compatible with each other according to length range, haploid complement, and A1 and A2 values, except for Q. vulcanica and Q. robur ( and ). Q. vulcanica is an endemic taxon distributed in restricted areas such as Isparta/Eğirdir and Afyon/Sultan Mountains in Turkey. High variation observed in Q. vulcanica could be caused by its distribution in a restricted area and more isolated habitats in comparison to other oak species. Q. robur is located in the Uşak/Uşak University Campus location. This taxon is present as mixed oak populations with Q. cerris, Q. ithaburensis, Q. robur and Q. coccifera in the same location. Q. trojana from section Cerris was collected from the same location as Q. robur. All taxa analyzed from section Cerris showed similar parametric values except Q. trojana. Q. trojana showed the highest values in all morphometric parameters such as length range, haploid complement, karyotypic description, and A1 and A2 values. Hybridization is mostly observed in restricted zones where the habitats of two or more species overlap (Muller Citation1952; Rushton Citation1993). This situation may be a reason for the high variation for these two taxa. Finally, three species (Q. coccifera, Q. ilex and Q. aucheri) belonging to section Ilex showed high similarity in all chromosomal parameters (). The main reason for the similarity between species belonging to the same section may be the gene flow and genetic similarity.
Unlike Turkish oaks, European oaks showed higher values for many chromosomal parameters. This situation may be caused by gene flow between different oak species because of weak reproductive barriers in different geographical regions.
Quercus is a dominant tree having wide distribution in forests of Turkey. Northwest Turkey in particular has some of the highest species diversity and distribution of oaks. Northwest Turkey contains 13–15 oak species (Uslu and Bakış Citation2012). The main reason why this region is rich in oak variation is due to it being transitional zone between Asia and Europe. Anatolia has served as a migration route facilitating the penetration of Asiatic plant elements into southeast Europe (Davis Citation1971). Turkey has been under the influence of numerous climatic regions and three phytogeographic regions (Euro-Siberian, Irano-Turanian and Mediterranean regions) due to its geomorphologic structure (Uslu and Bakış Citation2012). Another reason for the high species diversity for northwest Turkey is that it is the place where the two different phytogeographical regions (Euro-Siberian and Mediterranean regions) overlaps.
In this study, four species (Q. hartwissiana, Q. frainetto, Q. macranthera subsp. syspirensis, and Q. virgiliana) belonging to section Quercus were collected from northwest Turkey. The fact that many oak species are located in the same region or even at the same location due to the factors mentioned above increases hybridization, especially between species belonging to the same section. Furthermore, as a result of interspecific hybridization in oaks, hybrid individuals often exhibit intermediate morphological features between parent taxa, even sometimes exhibiting high morphological variation, and it is not possible to identify an oak tree to a species (Borazan and Babaç Citation2003). Another important reason that makes it difficult to understand the relationships among the oaks and increases the taxonomic problems in Turkey is the lack of adequate conservation programs for the use of oak trees. Furthermore, all studies on Turkish oak species show that average chromosome lengths of oak species analyzed are below 2 µm. The effects on the chromosome lengths of chemicals used to obtain metaphase chromosomes during cytological studies considerably complicates cytological comparison in species with very small chromosomes, such as oaks.
The study results contribute to understanding the relations among 16 Turkish oak taxa from three sections and relations between Turkish oaks and European oaks. Additionally, the results obtained in this study with previous reports provide useful cytogenetic knowledge of the genus Quercus.
Acknowledgments
The author would like to thank Uşak University Directorate of Scientific Research Projects (BAP) for providing financial support and also special thanks to İbrahim Melih Öztürkmen and Ayhan Yılmaz for helping to collect plant material.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Atalay İ 2005. Kuvarterner’deki İklim Değişmelerinin Türkiye Doğal Ortamı Üzerindeki Etkileri. Türkiye Kuvaterner Sempozyumu. İTÜ Avrasya Yer Bilimleri Enstitüsü. Jun 2–5; İstanbul. pp. 121–127.
- Aykut Y, Uslu E, Babaç MT. 2008. Karyological Studies on Four Quercus L. Species in Turkey. Caryologia. 61(4):397–401.
- Aykut Y, Uslu E, Babaç MT. 2011. Cytogenetic studies on Quercus L. (Fagaceae) species belonging to Ilex and Cerris section in Turkey. Caryologia. 64(3):297–301.
- Bacilieri R, Ducousso A, Petit RJ, Kremer A. 1996. Mating system and asymmetric hybridization in mixed stand of European oaks. Evolution. 50:900–908.
- Borazan A, Babaç MT. 2003. Morphometric leaf variation in oaks (Quercus) of Bolu, Turkey. Ann Bot Fenn. 40:233–242.
- Butorina AK. 1993. Cytogenetic study of diploid and spontaneous triploid oaks, Quercus robur L. Species in Turkey. Annales des Sciences Forestieres. 50:144–150.
- Çıplak B, Demirsoy A, Bozcuk N. 1993. Distribution in Orthoptera in relation to the Anatolian Diagonal in Turkey. Articulata. 8:1–20.
- Chokchaichamnankit P, Anamthawat-Jonsson K, Chulalaksananukul W. 2008. Chromosomal Mapping of 18S-25S and 5S Ribosomal Genes on 15 Species of Fagaceae from Northern Thailand. Silvae Genetica. 57(1):5–13.
- D’emerico S, Bıanco P, Medaglı P, Schırone B. 1995. Karyotype Analysis in Quercus ssp. Silvae Genetica. 44:2–3.
- D’emerico S, Pacıolla C, Tomması F. 2000. Contribution to the karyomorphology of some species of the Genus Quercus. Silvae Genetica. 49:6.
- Davis PH. 1971. Distribution patterns in Anatolia with particular reference to endemism. In: Davis PH, Harper PC, Hedge IC, editors. Plant life of South West Asia. Edinburgh: Botanical Society of Edinburgh; p. 15–27.
- Denk T, Grimm GW. 2010. The oaks of western Eurasia: traditional classifications and evidence from two nuclear markers. Taxon. 59(2):351–366.
- Govaerts R, Frodin DG. 1998. World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodenraceae). Great Britain: Royal Botanic Gardens, Kew.
- Hedge IC, Yaltırık F. 1982. Quercus L. In: Davis PH, edtior. Flora of Turkey and the East Aegean Islands. Vol. 7. Edinburgh: Edinburgh University Press; p. 659–683.
- Kargioglu M, Serteser A, Senkul C, Konuk M. 2011. Bioclimatic Characteristic of oak species Quercus macranthera subsp. syspirensis and Quercus petraea subsp. pinnatiloba in Turkey. J Environ Biol. 32(1):127–131.
- Kurokawa Y, Yonezawa Y. 2004. Karyotype analysis of fifteen species of Quercus L. (Fagaceae) in Japan. Chromosome Sci. 8(4):209.
- Levan A, Fredga K, Sandbdberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Manos PS, Doyle JJ, Nixon KC. 1999. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). Mol Phylogenet Evol. 12:333–349.
- Manos PS, Zhou Z, Cannon CH. 2001. Systematics of Fagaceae: phylogenetic tests of reproductive trait evolution. Int J Plant Sci. 162:1361–1379.
- Muller CH. 1952. Ecological control of hybridization in Quercus: a factor in the mechanism of evolution. Evolution. 6:147–161.
- Oh SH, Manos PS. 2008. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 57:434–451.
- Ohrı D, Ahuja MR. 1990. Giemsa C-Banded Karyotype in Quercus L. (Oak). Silvae Genetica. 39:5–6.
- Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A. 2003a. Hybridization as a mechanism of invasion in oaks. New Phytologist. 161:151–164.
- Ribeiro TLoureiro J, Santos C, Morais-Cecílio L. 2011. Evolution of rDNA FISH patterns in the Fagaceae. Tree Genetics & Genomes. 7: 1113 –1122
- Rushton BS. 1993. Natural hybridization within the genus Quercus L. Annales des Sciences Forestières. 50:18.
- Simeone MC, Piredda R, Papini A, Vessella F, Schirone B. 2013. Application of plastid and nuclear markers to DNA barcoding of Euro-Mediterranean oaks (Quercus, Fagaceae): problems, prospects and phylogenetic implications. Bot J Linnean Soc. 172:478–499.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- Uslu E, Bakış Y. 2012. Geographic distribution of Turkish Oaks. Dendrobiology. 67:41–48.
- Yaltirik F. 1984. Türkiye meşeleri teşhis kılavuzu. İstanbul: Yenilik Basımevi.
- Yilmaz A. 2017. Cytotaxonomic study of Quercus L. species from Section Quercus in Turkey. Caryologia. 70(2):141–146.
- Zarco CR. 1986. A new method for estimating karyotype asymmetry. Taxon. 35(3):526–530.
- Zoldos V, Pape D, Brown SC, Panaud O, Siljak-Yakovlev S. 1998. Genome size and base composition of seven Quercus species: inter- and intra- population variation. Genome. 41:162–168.
