ABSTRACT
The present study covers chromosome counts, male meiosis and pollen fertility in 164 species of dicots from the Parvati Valley, Kullu district, Himachal Pradesh. Gathered cytological information generates first ever chromosome counts, new diploid/polyploid cytotypes and varied chromosome counts. As many as 41 species (25%) showed intraspecific euploid cytotypes. Role of aneuploidy causing chromosomal variation is apparent from the existence of 71 species (43.29%) depicting aneuploid/dysploid cytotypes at diploid and/or polyploid levels and 114 genera showing dibasic/polybasic nature. The existence of abnormalities in several species resulted in the production of genetically imbalanced and sterile pollen grains. The viable gametes with variable chromosome numbers might have played a part in the origin of aneuploids, polyploids and species complexes. Also the non-viable gametes produced as a consequence of meiotic irregularities might have resulted in reduction of reproductive success of species through seeds. Consequently, the plants have adopted alternate means of propagation through asexual and vegetative modes which is quite prevalent in the plants of area.
1. Introduction
Parvati Valley, situated in Kullu district (Himachal Pradesh) along the River Parvati between 31°20ʹ21ʺ–32°25ʹ00ʺ N longitude and 76°56ʹ30ʺ–77°52ʹ20ʺ E latitude, is approximately 130 km long and 1.5 km wide. The altitude ranges between 1100 m at Bhuntar town, a confluence point of Parvati and Beas rivers, to Manatalai Lake at 4100 m. The climate varies from hot and sub-tropical in the southern lower tracts (600–900 m) to warm temperate (900–1800 m), cool temperate (1900–2400 m) and cold alpine to glacial (2400–4800 m) in the northern and eastern mountain ranges. The valley experiences high rainfall (800–900 m) in July–September and heavy snowfall (8–9 m) during winter months of January–March. The valley, which is known for its rich vegetation, supports dense forests of pines, deodar, oaks, spruce, fir, alder and rhododendron. The geographic location, varied climate, topography, altitudinal range and high precipitation in the form of rain and snow have contributed significantly toward the richness in plant diversity and dense forests. The valley has attracted a number of plant collectors and taxonomists. While compiling the flora of Kullu district, Dhaliwal and Sharma (Citation1999) have included a large number of plants from the Parvati Valley. On the basis of extensive and intensive cytomorphological surveys carried out during the period between 2010 and 2015, some interesting results have been reported in the form of the first ever chromosome counts, varied chromosome records, intraspecific euploid and dysploid cytotypes, imbalanced polyploids, structural hybrids and aberrant genotypes (Gupta et al. Citation2010, Citation2017; Himshikha et al. Citation2010, Citation2017; Rana et al. Citation2012; Singhal et al. Citation2012, Citation2014, Citation2017; Kumar et al. Citation2014a, Citation2014b, Citation2015; Kaur et al. Citation2017). The present communication includes cytological information on 164 species. The aims of study were to (i) record the exact chromosome number in the meiocytes at different stages; (ii) analyze the detailed male meiotic course including chromosomal pairing and segregation to determine the base number, ploidy level and type of polyploidy, and to detect structural changes that a species might have undergone during evolution; (iii) estimate pollen fertility and pollen size variability if any; and (iv) unravel the extent of morphological and cytological variability in the flowering plants of the valley.
2. Materials and methods
Wild accessions of the presently analyzed species were collected from the various localities at different altitudes in the Parvati Valley during the months of April-September. For meiotic and pollen grain studies, floral buds were fixed in a freshly prepared Carnoy’s fixative (mixture of alcohol, chloroform and acetic acid in a volume ratio 6:3:1) for 24 h and preserved in 70% ethanol in a refrigerator. Meiocytes preparations were made by squashing the anthers in 1% acetocarmine and observed at different stages of prophase-1, metaphase-1, anaphase-1/II, telophases-1/II and sporads. Pollen fertility was estimated through stainability tests by smearing the mature anthers in glycerol-acetocarmine (1:1) mixture. Well-filled pollen grains with stained nuclei were scored as apparently fertile, while shriveled and flaccid pollen grains with unstained or poorly stained cytoplasm as sterile. Cytologically analyzed plants were identified by consulting the regional floras. The plants were re-validated by comparing the specimens in the Herbarium, Northern Circle, Botanical Survey of India, Dehra Dun. Duly identified specimens were deposited in the Herbarium, Department of Botany, Punjabi University, Patiala. Photomicrographs of meiocytes, sporads and pollen grains were taken from temporary preparations using Leica Microsystems, Wetzlar, Germany and Nikon 80i Eclipse microscope, Melville, USA. The information on 164 species (397 accessions) falling under 114 genera and 37 families of dicotyledons covering sites of collection, vouchers (PUN), gametic chromosome number, meiotic course, ploidy level, pollen fertility and previous chromosome counts is provided in .
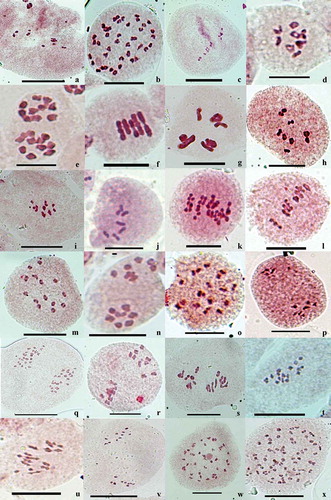
Figure 1. (a-x ) Gametic chromosome counts . 1 (a) Herpetospermum pedunculosum, PMC showing 10 bivalents at M- I. (b) Bilderdykia pterocarpa, PMC showing 40 bivalents at diakinesis . (c) Incarvillea arguta, PMC showing 11 bivalents at M- I. (d) Phagnalon niveum, PMC showing nine bivalents at M- I. (e) two Androsace lanuginosa, PMCs showing 10 bivalents at M- I. (f) Crepis foetida, PMC showing five bivalents at M- I. (g) Delphinium pyramidale, PMC showing eight bivalents at M- I. (h) Impatiens micranthemum, PMC showing nine bivalents at M- I. (i) I. spirifer, PMC showing seven bivalents at M- I. (j) Potentilla leuconata, PMC showing seven bivalents at M- I. (k) Senecio pedunculatus, PMC showing 20 bivalents at M- I. (l) Trigonella pubescens, PMC showing eight bivalents at M- I. (m) Geranium lucidum, PMC showing 14 bivalents at M- I. (n) Gnaphalium affine, PMC showing 7:7 chromsomes at M-II. (o) Nepeta floccosa PMC showing 18 bivalents at diakinesis . (p) Corydalis thyrsiflora, PMC showing 7:7 chromosomes at A- I. (q) Gerbera lanuginosa, PMC showing 24:24 chromosomes at A- I. (r) Gentiana argentea, PMC showing 9:9 chromosomes at A- I. (s) Inula grandiflora, PMC showing 16 bivalents at M-I . (t) Lepidium sativum, PMC showing 16 bivalents at M- I. (u) Potentilla desertorum, PMC showing 14 bivalents at M- I. (v) Roylea cincerea PMC showing 10:10 chromosomes at A- I. (w) Rumex nepalensis, PMC showing 40 bivalents at diakinesis. (x) A PMC showing 60 bivalents at diakinesis. Scale bars = 10 μm.
3. Results and discussion
3.1. Chromosome counts
Apart from the first ever chromosome counts at generic and species level and new intraspecific dysploid/polyploid cytotypes, the rest of the records substantiate the earlier reports. Four genera namely, Herpetospermum Wall. (H. pedunculosum (Ser.) C.B. Clarke (n = 10; )). Bilderdykia Dumort. (B. pterocarpa (Wall. ex Meisn.) Greene, (n = 40; )), Incarvillea Juss. (I. arguta Royle, (n = 11; )) and Phagnalon Cass. (P. niveum Edgew.) (n = 9; )), and 10 species, Androsace lanuginosa Wall. (n = 10; )), Crepis foetida L. (n = 5; )), Delphinium pyramidale Royle (n = 8; )), Impatiens micranthemum Edgew. (n = 9; )), I. spirifer Hook. f. & Thoms. (n = 7; )), Lonicera hypoleuca Decne. (n = 18), Potentilla leuconata D. Don (n = 7; )), Senecio pedunculatus Edgew. (n = 20; )) and Trigonella pubescens Baker (n = 8; )) are worked out chromosomally for the first time on a worldwide basis. The chromosome counts are new for Indian taxa of Geranium lucidum L. (n = 14; )), Gnaphalium affine D. Don (n = 7; )), and Nepeta floccosa Benth. (n = 18; )). New intraspecific diploid/polyploid cytotypes and varied chromosome counts in comparison to the earlier findings have been reported in Corydalis thyrsiflora Prain (n = 7), Gentiana argentea (Royle ex D. Don) DC. (n = 9), Geranium lucidum (n = 14), Gerbera lanuginosa (Wall. ex DC.) Benth. (n = 24), Gynura cusimbua (D. Don) Moore (n = 8), Impatiens thomsonii Hook. f. (n = 9), Lespedeza juncea (L.f.) Pers. (n = 11), Roylea cinerea (D. Don) Baill. (n = 10) and Salvia lanata Roxb. (n = 9).
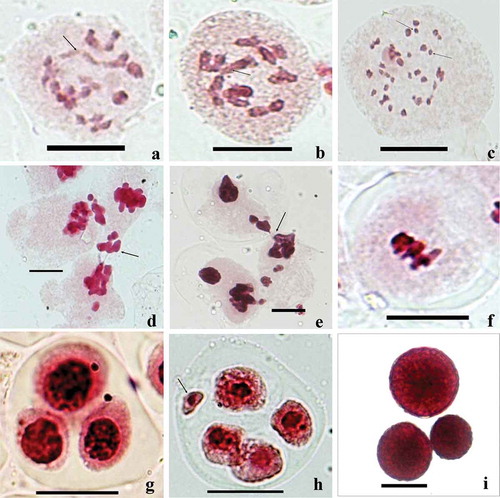
Figure 2. (a-i), Meiotic abnormalities . (a) Deutzia staminea PMC showing chain-shaped quadrivalent at diakinesis (arrowed) . (b) Fagopyrum dibotrys PMC showing chain-shaped quadrivalent at metaphase-I (arrowed). (c–i) araxacum officinale: (c) univalents at diakinesis stage (arrowed ); (d , e) chromatin transfer between neighboring PMCs (arrowed ); (f) a PMC showing chromatin stickiness; (g) a triad ; (h) a tetrad with a micronucleus (arrowed ); (i) variable sized fertile/stained pollen grains. Scale bars = 10 μm.
The analyzed accessions of Corydalis thyrsiflora showed the gametic chromosome count of n = 7 which was confirmed from the presence of 7:7 equal chromosome distribution ()). In spite of regular meiotic course and normal sporads, heterogeneous sized pollen grains were noticed. On the basis of size, pollen grains were categorized as small (19.34–20.48 × 19.27–19.59 µm, 17.05%), typical (24. 56–24.77 × 22.98–23.42 µm, 45.28%) and large-sized (32.56–32.80 × 27.82–31.33 µm, 37.67%). Present analysis adds a new dysploid cytotype (2n = 14) against the earlier reports of 2n = 16 by Kumar and Singhal (Citation2011, Citation2012) from Lahaul-Spiti and by Rani (Citation2012) from Kangra district of Northwest Himalayas in India.
The gametic chromosome count of n = 24 ()) for Gerbera lanuginosa from the valley adds a new tetraploid cytotype to the already existing diploid cytotype (n = 12) reported by Gupta and Gill (Citation1989) and Gupta et al. (Citation1989) from Shiwalik hills. The species is also known to have other chromosome counts of 2n = 36 reported by Bala and Gupta (Citation2013) from Kangra district, Himachal Pradesh and 2n = 46 by Mehra et al. (Citation1965) and Mehra and Remanandan (Citation1976) from other parts of Himalayas in India. It thus indicates that the species depicts a considerable amount of chromosomal diversity involving intraspecific euploid (2x, 3x, 4x) and dysploid (2n = 46, 48) cytotypes.
Gynura cusimbua, which grows as a tall perennial herb, showed a varied diploid chromosome count of n = 8 against the earlier report of 2n = 18 by Mehra and Sidhu (Citation1960) from North India. Mehra et al. (Citation1965) also detected the existence of intraspecific diploid (2n = 20) and tetraploid (2n = 40) cytotypes based on x = 10. It seems that the species has exploited both dysploidy (2n = 18, 20) and euploidy (2x, 4x) during the course of evolution.
Gentiana argentea, an important medicinal herb referred locally as “Karru,” is analyzed meiotically on the basis of three accessions. All the accessions uniformly shared the same diploid chromosome number, 2n = 18, as confirmed on the basis of 9:9 chromosomes distribution at A-I ()). Meiotic course was noticed to be regular, resulting in equal sized 100% fertile pollen grains. The present analysis adds a new intraspecific dysploid cytotype (2n = 18) against the previous reports of 2n = 20 (Mehra and Vasudevan Citation1972) and Bala (Citation2012) from other regions of Northwest Himalayas in India.
Geranium lucidum has been worked out for chromosome number for the first time from India. Present analysis on the basis of accession collected from the valley adds a new intraspecific cytotype at 2x level with 2n = 28 to the already existing chromosome counts of 2n = 20, 40, 42, 60 (Warburg Citation1938; Uhrikova and Májovsky Citation1980; Strid and Franzén Citation1981; Aryavand Citation1983; Galland Citation1988; Luque and Diaz Lifante Citation1991; Hollingsworth et al. Citation1992; Dempsey et al. Citation1994; Albers and Pröbsting Citation1998; Petrova and Stanimirova Citation2003).
We have reported for the first time the existence of 2x, 4x individuals in Inula grandiflora Willd. (2n = 32; )) from Malana Village. In comparison to 2x, the 4x plants possessed larger size leaves and capitula. The 4x plants also differ from the 2x in possessing comparatively larger sized stomata, trichomes and pollen grains (). Previous surveys from India and outside of India have reported the presence of only diploid individuals with a chromosome count of 2n = 16 (Sokolovskaya and Strelkova Citation1940; Shetty Citation1967; Gvinianidze and Avazneli Citation1982; Gadnidze et al. Citation1998).
Table 1. Data on locality with altitude, vouchers (PUN*), gametic chromosome number (n), ploidy level, pollen fertility (%), and previous reports of the presently investigated species.
Table 2. Comparison of macro- and microscopic characters in the two cytotypes of Inula grandiflora.
Impatiens thomsonii, a widely distributed species in Northwest Himalayas, has been investigated chromosomally by several cytologists. As per the previous reports of 2n = 12 (Sharma and Ghosh Citation1976), 2n = 14 (Koul and Gohil Citation1973), 2n = 16 (Sharma and Ghosh Citation1976) and 2n = 20 (Khoshoo Citation1955, Citation1966), the species depicts considerable chromosomal variability involving dysploidy (2n = 12, 14, 16, 20). The presently analyzed accession from the area adds a new chromosome count of 2n = 18.
Lepidium sativum L., seeds, which are used by locals for curing indigestion and constipation, showed a tetraploid chromosome count of n = 16 ()), compared to the earlier diploid reports of 2n = 16 by workers from outside of India (Jaretzky Citation1929; Reese Citation1950, Citation1952; Lan and Cheo Citation1989). The species is also known to have intraspecific 2x (2n = 12) and 4x (2n = 24) cytotypes based on x = 6 (Fedorov Citation1969).
The presently analyzed wild accession of Lespedeza juncea showed a variable diploid chromosome count of 2n = 22 against the earlier reports of 2n = 18 by Cooper (Citation1936), Pierce (Citation1939) from outside of India and 2n = 20 by Pierce (Citation1939), Sokolovskaya et al. (Citation1989) from outside of India and by Kumar and Singhal (Citation2011) from Lahaul-Spiti region of Northwest Himalayas in India.
Nepeta floccosa Benth., which has been counted chromosomally for the first time from India, adds a tetraploid gametic chromosome count of n = 18 (based on n = 9) against the earlier diploid record of n = 9 reported by Khatoon and Ali (Citation1993) from Pakistan.
The gametic chromosome count in Potentilla desertorum Bunge (n = 14, )) from the valley adds a new tetraploid cytotype (based on x = 7) against the earlier report of diploid cytotype (n = 7) by Rani et al. (Citation2012) from Kangra district, Himachal Pradesh.
The studied accession of Roylea cinerea with a gametic chromosome count of n = 10 ()) adds a new intraspecific diploid cytotype against the earlier recorded polyploid cytotype of 2n = 34 by Mehra and Gill (Citation1968), Gill (Citation1970), and Saggoo and Bir (Citation1983) from other regions of Himalayas in India.
Rumex nepalensis Spreng., a highly polymorphic species, is distributed in a wide range of habitats in Northwest Himalayas. The present study based on four accessions revealed the existence of intraspecific 8x (n = 40, )) and 12x (n = 60, )) cytotypes based on x = 10. The individuals of the 12x cytotype grow much taller than those of 8x. We here report for the first time the existence of the 8x cytotype, while the 12x cytotype has already been reported by Mehra and Dhawan (Citation1966) from other region of Northwest Himalayas in India.
The wild accessions of Salvia lanata Roxb. analyzed meiotically showed a diploid chromosome number of 2n = 20 against the already reported chromosome counts of 2n = 22 (Mehra and Gill Citation1968; Gill Citation1971, Citation1984; Sarkar et al. Citation1975) and 2n = 24 (Saggoo and Bir Citation1983) from other parts of India. This thus indicates that Indian taxa of the species depict the existence of intraspecific dysploid cytotypes at diploid level (2n = 20, 22, 24).
3.2. Meiotic behavior
Of the 173 taxa analyzed for male meiotic course, meiocytes in 72 taxa showed various abnormalities which included multiple associations of chromosomes in diploids (Artemisia parviflora Buch.-Ham. ex Roxb., n = 9; Deutzia staminea R.Br. ex Wall., n = 13; Fagopyrum acutatum (D. Don) Hara, n = 8), multivalents and univalents in polyploids (Erigeron annuus (L.) Pers., 2n = 3x = 27; Eupatorium adenophorum Spreng, 2n = 3x = 51; Taraxacum officinale Wigg., 2n = 3x = 24; Chrysanthemum leucanthemum L., 2n = 36; Trichodesma indicum (L.) R. Br., 2n = 4x = 44); a phenomenon of cytomixis involving chromatin transfer (35 species), chromatin stickiness resulting in delayed segregation of chromosomes and laggards in Androsace lanuginosa (n = 10), A. rotundifolia Hardw., (n = 10), Artemisia maritima L., (n = 9), Astragalus chlorostachys Lindl., (n = 8), Bupleurum hamiltonii Balakr. (n = 8), Clematis connata DC. (n = 8), C grata Wall. (n = 8), Conyza stricta Willd. (n = 9), Deutzia staminea (n = 13), Impatiens amphorata Edgew. (n = 7), Pedicularis hoffmeisteri Klotz. (n = 8), P. pectinata Wall. ex Benth. (n = 8), Plantago depressa Willd. (n = 6), P. lanceolata L. (n = 6), Ranunculus laetus Wall. (n = 14), Salvia nubicola Wall. ex Sweet (n = 8), Senecio graciliflorus DC. (n = 20), and Solanum verbascifolium L. (n = 12). In some cases, due to severe stickiness in chromatin material, chromosomes even failed to separate during anaphases/telophases, resulting in the formation of restitution nuclei and abnormal sporads such as monads and dyads and consequently pollen grains of variable sizes. Singhal and Kumar (Citation2008) and recently Kumar et al. (Citation2016) in Meconpsis aculeata Royle and Dewitte et al. (Citation2010) in Begonia have suggested that chromatin stickiness has played an important role in nuclear restitution and ultimately failure of cytokinesis.
Other meiotic irregularities which have been noticed in the meiocytes in the plants of the valley included non-synchronous (early and late) disjunction of bivalents (39 species) inter-bivalent/inter-chromosomal connections, impaired synapsis (I. scabrida DC.), syncyte meiocytes resulting due to whole migration of chromatin during cytomixis and cell fusion (Ranunculus laetus), abnormal spindle activity (Chrysanthemum leucanthemum, Plectranthus coetsa Buch.-Ham. ex D. Don, Potentilla gerardiana Lindl. ex Lehm., Taraxacum officinale, Withania somnifera (L.) Dunal) and meiocytes with supernumerary nucleoli (Aconitum heterophyllum Wall., Clematis connata DC., Impatiens scabrida, Plantago lanceolata L., Potentilla gerardiana, Trichodesma indicum).
3.3. Intraspecific euploid and dysploid cytotypes
Intensive accession based analysis from the valley added new euploid cytotypes at world level in nine species, Lepidium sativum (n = 16, 4x), Potentilla desertorum (n = 14, 4x), Cotula anthemoides L. (n = 20, 4x), Gerbera lanuginosa (n = 24, 4x), Inula grandiflora (n = 16, 4x), Solidago canadensis L. (n = 18, 4x), Nepeta floccossa (n = 18, 4x) Roylea cinerea (n = 10, 2x), and Rumex nepalensis (n = 40, 8x). Such intraspecific diploid/polyploid cytotypes are recorded for the first time in Indian taxa of Fagopyrum dibotrys (n = 8, 2x), Phagnalon niveum (n = 9, 2x), Potentilla desertorum (n = 14, 4x) and Solidago canadensis (n = 18, 4x).
Additional dysploid chromosome numbers have also been reported in nine species, Corydalis thyrsiflora (2n = 14, 16), Geranium lucidum (2n = 20, 28, 40, 42, 60), Impatiens thomsonii (2n = 12, 14, 16, 18, 20), Gentiana argentea (2n = 18, 20), Gerbera lanuginosa (2n = 46, 48) Gynura cusimbua (2n = 16, 18, 20), Lespedeza juncea (2n = 18, 20, 22), Roylea cinerea (2n = 20, 34) and Salvia lanata (2n = 20, 22, 24). The dysploid cytotypes have also been recorded in the analyzed accessions of Impatiens amphorata (2n = 14, 18) and Swertia ciliata (2n = 18, 26) from the valley. Also, intraspecific euploid cytotypes are recorded in six species analyzed from different localities of the valley, Silene vulgaris L. (2x, 4x), Agrimonia eupatoria L. (4x, 6x), Inula grandiflora (2x, 4x), Taraxacum officinale (2x, 3x, 4x), Solanum nigrum L. (2x, 4x) and Rumex nepalensis (8x, 12x). Among these, the 4x cytotype in Inula grandiflora (2n = 32) and 8x cytotype in Rumex nepalensis (2n = 80) are the first records on a worldwide basis.
3.4. Pollen fertility
On the basis of stainability tests, it has been noticed that a large number of taxa showed a varying amount of pollen sterility (). Pollen grains of heterogeneous sizes have also been noticed in some taxa. In majority of the cases, pollen malformation is attributed to the consequence of meiotic irregularities.
3.5. Multivalents in diploids
Multiple associations of chromosomes (ring or chain type) reported in the wild accessions of Deutzia staminea (2n = 26; )), Artemisia parviflora (2n = 18) and Fagopyrum dibotrys (2n = 16; )) seem to be the result of structural heterozygosity for reciprocal translocations. Varying amount of pollen sterility in such structural heterozygotes has resulted as a consequence of adjacent type orientation of multivalents during M-I as suggested earlier (Burnham Citation1956; Sharma and Gohil Citation2003; Ghaffari et al. Citation2009; Gupta et al. Citation2010; Talukdar Citation2010; Rana et al. Citation2012; Kumar and Singhal Citation2013; Singhal et al. Citation2014, Citation2016; Kumar et al. Citation2015).
3.6. Imbalanced polyploids
Of the 64 polyploid taxa covered presently, imbalanced polyploids were detected in Erigeron annuus (2n = 3x = 27), Eupatorium adenophorum (2n = 3x = 51) and Taraxacum officinale (2n = 3x = 24). Meiosis in these triploid taxa was characterized by the high frequency of univalents ()), scattered and unequal distribution of chromosomes during anaphases, abnormal sporads and high frequency of sterile pollen grains. Owing to good amount of viable seed production through apomixis, these polyploids seem to have established well in the Himalayas. The tetraploid taxa of Chrysanthemum leucanthemum (2n = 36) and Trichodesma indicum (2n = 44) showed the presence of some multivalents besides bivalents. In the remaining polyploid taxa, the meiocytes depicted complete bivalent formation which might indicates to their allopolyploid nature.
3.7. Cytomixis and associated meiotic irregularities
Cytomixis involving chromatin transfer among proximate meiocytes has been observed in 35 species (, 2(e)), i.e. Andrachne cordifolia (Decne) Müell. Arg. (n = 12), Androsace lanuginosa (n = 10), A. rotundifolia (n = 10), Anemone rivularis Buch.-Ham. ex DC. (n = 8), Artemisia maritima (n = 9), Aster peduncularis Wall. ex Nees (n = 27), Astragalus graveolens Benth. (n = 8), Bupleurum hamiltonii (n = 8), Clematis connata DC. (n = 8), C. grata (n = 8), Crepis foetida (n = 5), Cynoglossum lanceolatum Forssk. (n = 12), Delphinium pyramidale (n = 8), Deutzia staminea (n = 13), Erigeron annuus (2n = 27), Inula grandiflora (n = 8), Lactuca dolicophylla Kitam. (n = 8), Lamium album L. (n = 9), Lotus corniculatus L. (n = 6), Pedicularis hoffemeistri (n = 8), P. pectinata (n = 8), Plantago depressa (n = 6), P. lanceolata (n = 6), Plectranthus coetsa (n = 12), Potentilla argyrophylla Wall. ex Lehm. (n = 14), P. gerardiana (n = 42), Prenanthes brunoniana Wall. ex DC. (n = 8), Ranunculus hirtellus Royle (n = 16), R. laetus (n = 14), Salvia nubicola (n = 8), Senecio graciliflorous (n = 20), S. rufinervis DC. (n = 20), Solanum viarum Dunal (n = 12), Thymus linearis Benth. (n = 13), and Trifolium repens L. (n = 16).
The chromatin migration during cytomixis is either partial or complete, resulting in hypoploid, hyperploid or enucleated meiocytes. The products of such meiocytes led to the formation of pollen grains/male gametes with variable genetic constitution and sterile pollen grains. Meiocytes depicting chromatin transfer also showed pycnotic chromatin material, extra chromatin masses, agglutinated chromatin, inter-bivalent/inter-chromosomal connections, abnormal spindle activity, abnormal sporads (, 2(h)) and laggards and chromatin bridges. The products of such aberrant meiocytes resulted in considerable amounts of pollen sterility and pollen grains of variable sizes ()). Similar effects of cytomixis in inducing meiotic irregularities and pollen malformation have been reported in Chlorophytum comosum (Lattoo et al. Citation2006), Meconopsis aculeata (Singhal and Kumar Citation2008), Hippophae rhamnoides (Singhal et al. Citation2008), Salvia miltiorrhiza (Song and Li Citation2009), Artemisia absinthium (Malik et al. Citation2010), Clematis orientalis (Kumar et al. Citation2010), Plantago lanceolata (Himshikha et al. Citation2010), Nicotiana tabacum (Mursalimov and Deineko Citation2011), Ranunculus laetus (Kumar et al. Citation2011), Thalictrum foetidum (Singhal et al. Citation2011), Dianthus angulatus (Kumar et al. Citation2012), Houttuynia cordata (Guan et al. Citation2012), Pinellia ternata (Liu et al. Citation2012), Nepeta govaniana (Kaur and Singhal Citation2014), Ophrys lutea (Feijo and Paris Citation1989), Silene vulgaris (Kumar et al. Citation2014b) and Anemone rivularis (Kumar et al. Citation2015). Bedi (Citation1990) had also reported that cytomixis is mainly responsible for the reduced fertility of gametes in several forest tree species.
3.8. Chromosomal evolution
The present study also suggests that plants of this phytogeographically isolated valley with ecologically disturbed habitats are quite active in evolution, as heterogeneity in chromosome numbers is shown, involving polyploidy and aneuploidy.
3.8.1. Polyploidy
Perusal of the existing literature reveals that as many as 41 cytologically investigated species (25%) exist at different ploidy levels with the highest level of 12x (Rumex nepalensis). The role of polyploidy becomes more evident as 64 out of the 164 presently analyzed species possess intraspecific euploid cytotypes. The number of such species with intraspecific cytotypes is likely to increase when more data become available from other unexplored and isolated regions, particularly the higher hills of the valley. This is apparent from the first ever reports of diploid/polyploid cytotypes recorded presently in Lepidium sativum (n = 16, 4x), Potentilla desertorum (n = 14, 4x), Gerbera lanuginosa (n = 24, 4x), Inula grandiflora (n = 16, 4x), Solidago canadensis (n = 18, 4x), Nepeta floccossa (n = 18, 4x) Roylea cinerea (n = 10, 2x), and Rumex nepalensis (n = 40, 8x). Gustafasson (Citation1948), Schranz et al. (Citation2005) and Lo et al. (Citation2009) opined that vegetative and apomictic modes of reproduction and polyploidy are two important processes of evolution which are closely associated with each other. Taxa having high incidence of polyploidy often reproduce vegetatively or are apomicts. Vegetative modes of propagation, which have been considered as a pre-condition for polyploidy (Lewis and John Citation1963), have been observed presently in polyploid taxa of Agrimonia eupatoria (4x, 6x), Erigeron annuus (3x) and Taraxacum officinale (3x, 4x).
3.8.2. Aneuploidy
Aneuploidy, which causes chromosomal variation in the plants of the valley, is equally significant as is evident from the existence of dysploid cytotypes in 71 species at diploid and/or polyploid levels (see ). The role of aneuploidy in evolution is also evident from the existence of several dibasic and polybasic genera which seem to have originated via increase or decrease in chromosome numbers through aneuploidy, as suggested by a number of workers (Raven and Kyhos Citation1965; Stebbins Citation1966; Khosla Citation1975; Kaur et al. Citation2014; Rani et al. Citation2014).
3.8.3. Dibasic
Andrachne L. (x = 11, 12), Androsace L. (x = 9, 10), Anemone L. (x = 7, 8), Boenninghausenia Rehb. ex Meisn. (x = 9, 10), Chenopodium L. (x = 8, 9), Emblica L. (x = 7, 13), Heracleum L. (x = 10, 11), Lathyrus L. (x = 6, 7), Melissa L. (x = 16, 17), Ranunculus L. (x = 7, 8), Tridax L. (x = 9, 10), Trifolium L. (x = 7, 8), Wulfenia Jacq. (x = 8, 9) and Youngia Cass. (x = 8, 9).
3.8.4. Polybasic
Blumea DC. (x = 8, 9, 10, 11), Corydalis DC. (x = 5, 6, 7, 8), Filipendula Mill. (x = 7, 8, 9), Gnaphalium L. (x = 7, 8, 9), Lepidium L. (x = 6, 7, 8), Lespedeza Michx. (x = 9, 10, 11), Lotus L. (x = 5, 6, 7), Pimpinella L. (x = 8, 9, 10, 11), Silene L. (x = 9, 10, 11, 12, 13, 14), Stellaria L. (x = 9, 10, 11, 12, 13, 14), Sonchus L. (x = 7, 8, 9, 10), Swertia L. (x = 7, 8, 9, 10, 11, 12, 13), Thalictrum L. (x = 6, 7, 8), Verbena L. (x = 5, 6, 7), Vicia L. (x = 5, 6, 7), and Zanthoxyllum L. (x = 16, 17, 18) are the genera where the base numbers are present in regular dysploid series. Such base numbers seems to have originated through dysploid increase or decrease in chromosome numbers.
In the remaining genera, i.e. Aconitum L. (x = 8, 10, 12, 13, 17), Ajuga L. (x = 8, 14, 15), Artemisia L. (x = 7, 8, 9, 10, 17), Aster L. (x = 4, 5, 7, 8, 9, 13), Astragalus L. (x = 6, 7, 8, 11, 12, 13), Bidens L. (x = 10, 11, 12, 18), Bupleurum L. (x = 4, 6, 7, 8, 11), Campanula L. (x = 7, 8, 10, 12, 14, 17), Chaerophyllum Lindl. (x = 6, 7, 11), Cotula L. (x = 9, 10, 13), Crepis L. (x = 3, 4, 5, 6, 7, 8, 11), Epilobium L. (x = 9, 10, 12, 13, 16), Eupatorium L. (x = 10, 17), Gentiana L. (x = 5, 6, 7, 8, 9, 10, 12, 13, 19), Gerbera L. (x = 9, 12, 23, 25), Geranium L. (x = 9, 10, 11, 12, 13, 14, 15, 16, 17, 23), Gynura Cass. (x = 5, 8, 9, 10, 17), Hypericum L. (x = 7, 8, 9, 10, 12), Impatiens L. (x = 3, 4, 5, 6, 7, 8, 9, 10, 12, 13), Lactuca L. (x = 5, 8, 9, 17), Leucas R.Br.(x = 8, 9, 11, 13, 14, 15), Mazus Lour. (x = 8, 10, 19), Nepeta L. (x = 7, 8, 9, 13, 17), Plantago L. (x = 4, 5, 6, 7, 9), Plectranthus L’ Hér. (x = 7, 11, 12, 13, 15, 17), Roylea Wall. ex Benth. (x = 10, 17), Salvia L. (x = 6, 7, 8, 9, 10, 11, 13), Scrophularia L. (x = 9, 10, 12, 13, 29), Senecio L. (x = 5, 9, 10, 11, 12, 13, 14, 16, 19, 23), Spiraea L. (x = 5, 7, 8, 9, 17), Strobilanthes Blume (x = 7, 8, 9, 10, 11, 13, 14, 15, 16, 21), Thlaspi L. (x = 7, 9, 12, 13), Torilis Adans. (x = 6, 8, 11), Trichodesma R.Br. (x = 7, 10, 11, 12), Trigonella L. (x = 7, 8, 9, 11), Thymus L. (x = 12, 13, 14, 15, 27, 29), Verbascum L. (x = 12, 13, 14, 15, 16, 17, 18, 22, 23), Viola L. (x = 5, 6, 7, 8, 9, 11, 13, 17) and Youngia Cass. (x = 5, 8, 10). The dibasic and polybasic nature might have originated through polyploidy followed by aneuploidy or dibasic polyploidy.
Aneuploidy is often correlated in flowering plants with the asexual mode of reproduction. Nassar (Citation2003) on the basis of cytological and embryological studies in the apomictic clones of cassava (Manihot esculenta) correlated the occurrence of apomixis with aneuploidy. Levin (Citation2002) also discussed the role of aneuploidy with asexual mode of reproduction. Anemone rivularis, Delphinium pyramidale, Lotus corniculatus, Potentilla argyrophylla, Ranunculus hirtellus, R. laetus, Trifolium pratense and T. repens, where aneuploid chromosomal variations are quite common, are observed to reproduce vegetatively through rhizomes and stolons. As such, aneuploidy seems to have played a fairly active role in the evolution of these species.
The meiotic abnormalities in the presently analyzed species from the valley have affected the genetic constitution and viability of male gametes. The viable gametes with variable genetic constitution have resulted in the origin of aneuploids, polyploids and species complexes. Non-viable gametes, resulting due to meiotic disturbances, might have been responsible for reduced reproductive success in several species through seeds. Consequently, the plants have adopted alternate means of propagation through vegetative modes, which are quite common in the plants of the valley.
Acknowledgments
The authors are grateful to the head, Department of Botany, Punjabi University, Patiala and Dr P. Singh, Director, Botanical Survey of India, Headquarters Kolkata and Head of- Office, NRC, Dehra Dun, for the necessary herbarium (PUN, BSD) and library facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albers F, Pröbsting W. 1998. In: Wisskirchen R, Haeupler H. Standard list of fern and flowering plants in Germany. Stuttgart: Federal Office for Nature Conservation & Publishing Eugen Ulmer. (In German).
- Aryavand A. 1983. IOPB Chromosome numbers reports LXXIX. Taxon. 32:321–322.
- Bala S. 2012. Exploration of cytomorpholgical diversity in Gamopetalae from district Kangra (H.P.) [ Ph.D. Thesis], Patiala: Punjabi University.
- Bala S, Gupta RC. 2013. Male meiosis and chromosome number in Asteraceae family from district Kangra of H.P. (Western Himalayas). Int J Bot Res. 3:43–58.
- Bedi YS. 1990. Cytomixis in woody species. Proc Ind Natl Sci Acad. 100:233–238.
- Bolkovskikh Z, Grif V, Matvejeva T, Zakharyeva, O. 1969. Chromosome numbers of flowering plants. Fedorov AnA, editor; Bolkovskikh, Z. et al. compilers. Leningrad: Izdatellstvo Nauka; p. 926.
- Burnham CR. 1956. Chromosomal interchanges in plants. Bot Rev. 22:419–552.
- Cooper DC. 1936. Chromosome numbers in the Leguminosae. Am J Bot. 23:231–233.
- Darlington CD, Wylie AP. 1955. Chromosome altas of flowering plants. London: George Allen and Unwin Ltd.
- Dempsey RE, Gornall RJ, Bailey JP. 1994. Contributions to a cytological catalogue of the British and Irish flora, 4. Watsonia. 20:63–66.
- Dewitte A, Eeckhaut T, Van Huylenbroeck J, Van Bockstaele E. 2010. Meiotic aberrations during 2n pollen formation in Begonia. Heredity. 104:215–223.
- Dhaliwal DS, Sharma M. 1999. Flora of Kullu District (Himachal Pradesh). Dehra Dun: Bishen Singh Mahendera Pal Singh.
- Fedorov AA. 1969. Chromosome numbers of flowering plants. Academy of sciences of the USSR. Leningrad: Komarov Botanical Institute. (Reprint 1974).
- Feijo JA, Paris MSS. 1989. Cytomixis in meiosis during the microsporogenesis of Ophrys lutea: an ultra structural study. Cytologia. 69:7–11.
- Gadnidze RI, Gviniashvii TN, Danelia IM, Churadze MV. 1998. Chromosome numbers of the species of Georgian flora. Bot Zhurn (Moscow Leningard). 83:143–147.
- Galland N. 1988. Research on the origin of the orophilous flora of Morocco: caryological and cytogéographic study. Trav Inst Scientifique Rabat Sér Bot. 35:1–168. (In French).
- Ghaffari SM, Karimzadeh G, Najafi AA. 2009. Occurrence of reciprocal translocations in Lathyrus boissieri Sirj (Fabaceae) from Iran. Cytologia. 74:195–199.
- Gill LS. 1970. Cytological observations on West-Himalayan Labiatae. Tribe Stachydeae. Phyton. (Buenos Aires). 27:177–184.
- Gill LS. 1971. Cytology of West Himalayan Labiatae. Tribe Saturieneae. Caryologia. 24:203–207.
- Gill LS. 1984. The incidence of polyploidy in the West-Himalayan Labiatae. Rev Cytol Biol Vég Bot. 7:5–16.
- Goldblatt P. 1981. Index to plant chromosome numbers 1975–1978. Monogr. Syst. Bot. Missouri Bot. Gard. 6:1–553.
- Goldblatt P. 1984. Index to plant chromosome numbers 1979–1981. Monogr. Syst. Bot. Missouri Bot. Gard. 8:1–427.
- Goldblatt P. 1985. Index to plant chromosome numbers 1982–1983. Monogr. Syst. Bot. Missouri Bot. Gard. 13:1–224.
- Goldblatt P. 1987. Index to plant chromosome numbers 1984–1985. Monogr. Syst. Bot. Missouri Bot. Gard. 23:1–264.
- Goldblatt P, Johnson DE. 1990. Index to plant chromosome numbers 1986–1987. Monogr. Syst. Bot. Missouri Bot. Gard. 30:1–243.
- Goldblatt P, Johnson DE. 1991. Index to plant chromosome numbers 1988–1989. Monogr Syst Bot Missouri Bot Gard. 40:1–238.
- Goldblatt P, Johnson DE. 1994. Index to plant chromosome numbers 1990–1991. Monogr Syst Bot Missouri Bot Gard. 51:1–267.
- Goldblatt P, Johnson DE. 1996. Index to plant chromosome numbers 1992–1993. Monogr Syst Bot Missouri Bot Gard. 58:1–276.
- Goldblatt P, Johnson DE. 1998. Index to plant chromosome numbers 1994–1995. Monogr Syst Bot Missouri Bot Gard. 69:1–208.
- Goldblatt P, Johnson DE. 2000. Index to plant chromosome numbers 1996–1997. Monogr Syst Bot Missouri Bot Gard. 81:1–188.
- Goldblatt P, Johnson DE. 2003. Index to plant chromosome numbers 1998–2000. Monogr Syst Bot Missouri Bot Gard. 94:1–297.
- Goldblatt P, Johnson DE. 2006. Index to plant chromosome numbers 2001–2003. Monogr Syst Bot Missouri Bot Gard. 106:1–242.
- Guan J-Z, Wang J-J, Cheng Z-H, Liu Y, Z-Y L. 2012. Cytomixis and meiotic abnormalities during microsporogenesis are responsible for male sterility and chromosome variations in Houttuynia cordata. Genet Mol Res. 11:121–130.
- Gupta H, Gupta RC, Kumar R, Singhal VK. 2017. A profile of chromosome counts, male meiosis and pollen fertility in forty-five species of Asteraceae from Parvati Valley in Kullu district, Himachal Pradesh. Caryologia. 70:128–140.
- Gupta RC, Gill BS. 1989. Cytopalynology of north and central indian compositae. J Cytol Genet. 24:96–105.
- Gupta RC, Gill BS, Garg RK. 1989. Chromosomal conspectus of Himalayan Compositae. In: Trivedi, ML, Gill BS, Saini SS, editors. Plant science research in India: present status and future challenges. New Delhi: Today and Tomorrow’s Printers and Publishers; p. 427–437.
- Gupta RC, Himshikha RPK, Kumar P, Singhal VK. 2010. First report of structural heterozygosity in Artemisia parviflora from Parvati Valley, Kullu district (H.P.). Bot Serbica. 34:63–66.
- Gustafasson A. 1948. Polyploidy, life-form and vegetative reproduction. Hereditas. 34:1–22.
- Gvinianidze ZI, Avazneli AA. 1982. Chromosomal numbers of some representatives of the Caucasian highland floristic complexes. AN GSSR. 106:577–580. (In Russian).
- Himshikha, Gupta RC, Singhal VK, Kumar R. 2017. IAPT/IOPB Chromosome data 25. Taxon. 66:1248–1249.
- Himshikha KP, Gupta RC, Kumari S, Singhal VK. 2010. Impact of chromatin transfer and spindle abnormalities on pollen fertility and pollen size in Plantago lanceolata L. Cytologia. 75:421–426.
- Hollingsworth PM, Gornall RJ, Bailey JP. 1992. Contribution to a cytological catalogue of the British and Irish flora, 2. Watsonia. 19:134–137.
- Holmgren PK, Holmgren NH. 1998. Index herbariorum: a global directory of public herbaria and associated staff. New York (NY): New York Botanical Garden.
- Jaretzky R. 1929. The chromosome number in the genus Matthiola. Ber Deutsch Bot Ges. 47:82–85. (In German).
- Kaur H, Mubarik N, Kumari S, Gupta RC. 2014. Chromosome numbers and basic chromosome numbers in monocotyledonous genera of the Western Himalayas (India). Acta Biol Cracov Ser Bot. 56:9–19.
- Kaur M, Himshikha, Singhal VK. 2017. Occurence of syncytes: a possible mechanism owing to the origin of polyploid cytotype in Achillea millefolium L. within Indian Himalayas. Cytologia. 82:375–384.
- Kaur M, Singhal VK. 2014. First report of cytomixis and meiotic abnormalities in Nepeta govaniana from Solang Valley, Kullu District, Himachal Pradesh. Cytologia. 79:227–233.
- Khatoon S, Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi: Department of Botany, University of Karachi.
- Khoshoo TN. 1955. Cytology of Impatiens. Curr Sci. 24:165–166.
- Khoshoo TN. 1966. Cytology of pollen with particular reference to Impatiens and Allieae. Proc Indian Acad Sci. 63:35–45.
- Khosla PK. 1975. Chromosomal evolution in commercial hardwoods. Nucleus. 18:54–60.
- Koul AK, Gohil RN. 1973. Cytotaxonomical conspectus of the flora of Kashmir (I). Chromosome numbers of some common plants. Phyton. 15:57–66.
- Kumar P, Rana PK, Himshikha, Singhal VK, Gupta RC. 2014a. Chromosome numbers, characterization of chromosomal pairing during meiosis, origin and natural propagation in polyploid cytotypes (4x, 5x and 6x) of Agrimonia eupatoria L. (Rosaceae) in northwest Himalayas (India). Protoplasma. 251:781–795.
- Kumar P, Rana PK, Himshikha, Singhal VK, Gupta RC. 2014b. Cytogeography and phenomenon of cytomixis in Silene vulgaris from cold regions of Northwest Himalayas (India). Plant Syst Evol. 300:831–842.
- Kumar P, Singhal VK. 2011. Chromosome number, male meiosis and pollen fertility in selected angiosperms of the cold deserts of Lahaul-Spiti and adjoining areas (Himachal Pradesh, India). Plant Syst Evol. 297:271–297.
- Kumar P, Singhal VK. 2012. Meiotic aberrations and chromosomal variation in the plants of Lahaul-Spiti and adjoining high hills in Himachal Pradesh. In: Atri NS, Gupta RC, Saggoo MIS, Singhal VK, editors. Biodiversity evaluation- botanical perspective. Dehra Dun: Bishen Singh and Mahendra Pal Singh; p. 147–170.
- Kumar P, Singhal VK. 2013. Reduction in chiasma frequency and pollen fertility due to multiple chromosomal associations and univalents in Saxifraga diversifolia from alpine regions of northwest Himalayas (India). Caryologia. 66:120–127.
- Kumar P, Singhal VK, Kaur D. 2012. Impaired male meiosis due to irregular synapsis and cytomixis in Dianthus angulatus Royle ex Benth. from Indian cold deserts. Folia Geobot. 47:59–68.
- Kumar P, Singhal VK, Kaur D, Kaur S. 2010. Cytomixis and associated meiotic abnormalities affecting pollen fertility in Clematis orientalis. Biol Plantarum. 54:181–184.
- Kumar P, Singhal VK, Rana PK, Kaur S, Kaur D. 2011. Cytology of Ranunculus laetus Wall. ex. Royle from cold desert regions and adjoining hills of North-West Himalayas. Caryologia. 64:25–32.
- Kumar R, Rana PK, Himshikha KD, Kaur D, Kaur M, Singhal VK, Gupta RC, Kumar P. 2015. Structural heterozygosity and cytomixis driven pollen sterility in Anemone rivularis Buch.-Ham. ex DC. from Western Himalayas (India). Caryologia. 68:246–253.
- Kumar R, Rana PK, Singhal VK. 2016. Chromatin stickiness and abnormal spindle resulting into meiotic irregularities and pollen sterility in Meconopsis aculeata Royle from Northwest Himalayas. Cytologia. 81:83–87.
- Kumar V, Subramanian B. 1986. Chromosome atlas of flowering plants of the Indian sub-continent. Vol. I. Dicotyledons. Calcutta: BSI.
- Lan Y-Z, Cheo T-Y. 1989. Cytotaxonomical studies on Chinese species of Cruciferae. In: Hong D, editor. Plant chromosome research. 1987. Beijing, China: Organizing Committee Sino-Japanese Symposium on Plant Chromosomes; p. 283.
- Lattoo SK, Khan S, Bamotra S, Dhar AK. 2006. Cytomixis impairs meiosis and influences reproductive success in Chlorophytum comosum (Thunb.) Jacq. – an additional strategy and possible implications. J Biosci. 31:629–637.
- Levin DA. 2002. The role of chromosomal changes in plant evolution. London: Oxford University Press.
- Lewis KR, John B. 1963. Chromosome marker. London: J.A. Churchill Ltd.
- Liu Y, Hui R-K, Deng R-N, Wang -J-J, Wang M, Li Z-Y. 2012. Abnormal male meiosis explains pollen sterility in the polyploid medicinal plant Pinellia ternata (Araceae). Genet Mol Res. 11:112–120.
- Lo EY, Stefanović S, Dickinson TA. 2009. Population genetic structure of diploid sexual and polyploid apomictic hawthorns (Crataegus; Rosaceae) in the Pacific Northwest. Mol Ecol. 18:1145–1160.
- Löve A, Löve D. 1975. Cytotaxonomical atlas of the arctic flora. Vaduz: J Cramer.
- Luque T, Diaz Lifante Z. 1991. Chromosome numbers of plants collected during Iter Mediterraneum I in the SE of Spain. Bocconea. 1:303–364.
- Malik RA, Gupta RC, Kumari S. 2010. Genetic diversity in different populations of Artemisia absinthium Linn. from Kashmir Himalaya. Cytologia. 75:273–276.
- Mehra PN, Dhawan H. 1966. Cyto-taxonomy of some N. Indian Polygonaceae. Proc Indian Sci Congr. Assoc. 53:277.
- Mehra PN, Gill BS, Mehta JK, Sidhu SS. 1965. Cytological investigations on the Indian Compositae. I. North Indian Taxa. Caryologia. 18:35–68.
- Mehra PN, Gill LS. 1968. IOPB Chromosome number reports XVI. Taxon. 17:419–422.
- Mehra PN, Remanandan P. 1976. Cytological investigations on Indian Compositae V. Tribes: arctotideae, Cynareae, Calenduleae and Mutiseae. Nucleus. 19:8–12.
- Mehra PN, Sidhu SS. 1960. Cytology of the North-West Indian composiate. Proc Indian Sci Congr Assoc. 47:376–377.
- Mehra PN, Vasudevan KN. 1972. IOPB Chromosome number reports. XXXVI. Taxon. 21:333–346.
- Moore R J. 1970. Index to plant chromosome numbers for 1968. Regnum Veg. 68:1–115.
- Moore R J. 1971. Index to plant chromosome numbers for 1969. Regnum Veg. 77:1–116.
- Moore R J. 1972. Index to plant chromosome numbers for 1970. Regnum Veg. 84:1–134.
- Moore RJ. 1973. Index to plant chromosome numbers 1967–1971. Regnum Veg. 90:1–539.
- Moore RJ. 1974. Index to plant chromosome numbers for 1972. Regnum Veg. 91:1–108.
- Moore RJ. 1977. Index to plant chromosome numbers for 1973/74. Regnum Veg. 96:1–257.
- Mursalimov SR, Deineko EV. 2011. An ultrastructural study of cytomixis in tobacco pollen mother cells. Protoplasma. 248:717–724.
- Nassar NM. 2003. Is apomixis in cassava (Manihot esculenta Crantz) associated with aneuploidy? Gene Conserve. 2:106–110.
- Petrova A, Stanimirova P. 2003. Karyological study of some Geranium (Geraniaceae) species growing in Bulgaria. Bocconea. 16:675–682.
- Pierce WP. 1939. Cytology of the genus Lespedeza. Am J Bot. 26:736–744.
- Rana PK, Himshikha, Kumar P, Singhal VK, Gupta RC. 2012. Impact of reciprocal translocations and non-synchronous disjunction of chromosomes on pollen fertility in Astragalus chlorostachys from North West Himalayas (India). Cytologia. 77:173–179.
- Rani S. 2012. Exploration and evaluation of cytomorpholgical diversity in Polypetalae from Kangra district (Himachal Pradesh) [ Ph.D. Thesis]. Patiala: Punjabi University.
- Rani S, Jeelani SM, Kumar S, Kumari S, Gupta RC. 2014. An overview of chromosome and basic numbers diversity in cytologically investigated polypetalous genera from the Western Himalayas (India). Caryologia. 67:1–24.
- Rani S, Kumar S, Jeelani SM, Kumari S, Gupta RC. 2012. Additions to the cytologically investigated species of Potentilla L. (Rosaceae) from India. Plant Syst Evol. 298:485–497.
- Raven PH, Kyhos DW. 1965. New evidence concerning the original basic chromosome number of angiosperms. Evolution. 19:244–248.
- Reese G. 1950. Contributions to the effect of cochine in the treatment of semen. Planta. 38:324–376. (In German).
- Reese G. 1952. Additional information on chromosome numbers of Mediterranean European vascular plants. I. Ber Deutsch Bot Ges. 64:240–255. (In German).
- Saggoo MIS, Bir SS. 1983. Cytopalynological studies on Indian members of Acanthaceae and Labiatae. J Palynol. 19:223–258.
- Sarkar AK, Datta N, Raychowdhury M, Das S. 1975. IOPB chromosome number reports L. Taxon. 24:671–678.
- Schranz ME, Dobes C, Koch MA, Mitchell-Olds T. 2005. Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae). Am J Bot. 92:1797–1810.
- Sharma AK, Ghosh P. 1976. Cytological studies in Balsaminaceae and Ranunculaceae. Ann Report Cytogen Lab Dept Bot Univ Calcutta Res Bull. 3:39.
- Sharma G, Gohil RN. 2003. Cytology of Allium roylei Stearn. I. Meiosis in a population with complex interchanges. Cytologia. 68:115–119.
- Shetty BV. 1967. IOPB Chromosome number reports XIV. Taxon. 16:552–571.
- Singhal VK, Kaur D, Kaur M, Himshikha, Rana PK, Kumar P, Gupta RC. 2014. Multiple associations due to structural heterozygosity in the wild plants of Achillea millefolium L. from northwest Himalayas (India). Cytologia. 79:151–159.
- Singhal VK, Kaur D, Kumar P. 2008. Effect of cytomixis on the pollen size in ‘Seabuckthorn’ (Hippophae rhamnoides L., Elaeagnaceae). Cytologia. 73:167–172.
- Singhal VK, Kaur M, Himshikha, Kumar P, Gupta RC. 2012. High pollen sterility and 2n pollen grains in an asynaptic 4x cytotype (2n=48) of Solanum nigrum L. Cytologia. 77:333–342.
- Singhal VK, Kumar P. 2008. Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle). J Biosci. 33:371–380.
- Singhal VK, Kumar R, Himshikha, Kumar P, Kaur D, Kaur M, Rana PK, Gupta RC. 2017. A profile of male meiosis, chromosomal variation and chromosomal status in species of Impatiens from North-West Himalayas in India. Caryologia. 70:258–269.
- Singhal VK, Rana PK, Kumar P, Kaur D. 2011. Persistent occurrence of meiotic abnormalities in a new hexaploid cytotype of Thalictrum foetidum L. from Indian cold desert. Biologia. 66:458–464.
- Singhal VK, Tantray YR, Gupta RC. 2016. Structural heterozygosity for reciprocal translocation in Tanacetum artimisioides Sch. Bip. ex Hook. f. from Ladakh division of J. & K. Cytologia. 81:319–322.
- Sokolovskaya AP, Probatova NS, Rudyka EG. 1989. Chromosome numbers in some species of the flora of Soviet Far East from the families of actindiaceae, aristolochiaceae, fabaceae, ranunculaceae, saxifragaceae. Bot Žurn. 74:268–271.
- Sokolovskaya AP, Strelkova OS. 1940. Karyological studies of the alpine flora of the main Caucaus ridge and the problem of geographical distribution of polyploids. Doklady Akad Nauk SSSR. 29:413–416. (In Russian).
- Song Z-Q, Li X-F. 2009. Cytomixis in pollen mother cells of Salvia miltiorrhiza. Caryologia. 62:213–219.
- Stebbins GL. 1966. Chromosomal variation and evolution. Science. 152:1463–1469.
- Strid A, Franzén R. 1981. IOPB Chromosome numbers reports LXXIII. Taxon. 30:829–842.
- Talukdar D. 2010. Reciprocal translocations in grass pea (Lathyrus sativus L.): pattern of transmission, detection of multiple interchanges and their independence. J Hered. 101:169–176.
- Uhríková A, Májovsky J. 1980. IOPB chromosome numbers reports LXIX. Taxon. 29:725–726.
- Warburg EF. 1938. Taxonomy and relationship in the Geraniales in the light of their cytology. New Phytol. 37:130–159.
