ABSTRACT
This paper presents a cyto-palynological study of four species of Convolvulaceae from the northern Western Ghats of India. Ipomoea clarkei and Operculina tansaensis are endemic to the region, whereas I. diversifolia and O. turpethum show wide distribution. A new cytotype with 2n = 28 was reported for I. diversifolia. The chromosome number 2n = 30 in O. turpethum was in conformity with earlier reports. Chromosome numbers for I. clarkei and O. tansaensis (both with 2n = 30) were reported for the first time. Meiosis was found to be normal. At diakinesis 14 bivalents were observed for I. diversifolia while I. clarkei and O. tansaensis showed 15 bivalents. In all the species, chromosomes were small with median region centromeres. The largest chromosome was recorded in I. clarkei (2.15 ± 0.25 µm) and the shortest for I. diversifolia (1.62 ± 0.19 µm). The karyotypes were symmetrical and exhibited Stebbins’s 4a category. Pollens were pantoporate-echinate in both the Ipomoea species and tricolpate-smooth in Operculina.
Abbreviations: THL, total haploid chromosome length; CVCL, coefficient of variation of chromosome length; MCA, mean centromeric asymmetry; L, length of the largest chromosome; MCL, mean chromosome length; r, mean arm ratio; R, ratio between the largest and the smallest chromosome of the complement; St, type of asymmetry.
Introduction
Western Ghats of India is a biodiversity hotspot comprising 7402 species of flowering plants, of which 2253 species are endemic to India and 1273 species are exclusive to the Western Ghats (Nayar et al. Citation2014). Biological investigation of endemics has always been a key issue for botanists, and particularly for taxonomists. To study endemics requires precise knowledge of their location and reproductive biology. Lack of comprehensive taxonomic documentation of endemics from the Western Ghats obstructs botanists from taking up studies on cytogenetics, ecology and reproductive biology.
Ipomoea L. is a large and complex genus of Convolvulaceae Juss. containing over 500 species of vines and shrubs that are widely distributed throughout the tropical and subtropical regions of the world (Rane et al. Citation2012). In India, Ipomoea is represented by 51 taxa (48 species, two subspecies and one variety) () (modified after Santapau and Henry Citation1973). Western Ghats comprises 37 species, three subspecies and two varieties (42 taxa) (modified after Nayar et al. Citation2014). Operculina Silva Manso on the other hand comprises 15 species (The Plant List Citation2013). Four species (O. petaloidea (Choisy) Ooststr., O. tansaensis Santapau & V.Patel, O. turpethum (L.) Silva Manso and O. ventricosa (Bertero) Peter) are recorded from India. Two species of Ipomoea (I. clarkei Hook.f. and I. salsettensis Santapau & V.Patel) and one of Operculina, i.e. O. tansaensis, are endemic to the Western Ghats and restricted to the state of Maharashtra. Ipomoea clarkei, I. salsettensis and O. tansaensis are restricted to the state of Maharashtra, India. I. clarkei and I. salsettensis were categorized (following IUCN criteria) as endangered whereas O. tansaensis is considered critically endangered (Mishra and Singh Citation2001). The distribution of I. diversifolia R.Br. ranges from India to north-east Australia and Philippines (Luzon Island, province of Ilocos Norte) (Staples Citation2012) while O. turpethum grows naturally in warm temperate and tropical Asia, and is naturalized, possibly a long time ago, in parts of Africa, Australia and the Pacific islands (Prasad Citation2013).
Table 1. Indian taxa of Ipomoea and Operculina and their diploid chromosome counts (2n).
The ornamental (many Ipomoea species are cultivated as climbers) and medicinal use (some species of Ipomoea and Argyreia Lour. species possess ergoline alkaloids that have hallucinogenic properties) attributed to many Convolvulaceae members has been one of the main drivers of cytogenetical examination in this family. In India, most of the cytological studies on Convolvulaceae were focused on somatic chromosomes (Vij et al. Citation1974; Bir and Sidhu Citation1975; Bir et al. Citation1978; Roy Citation1979; Sampathkumar Citation1979; Rao and Mwasumbi Citation1981; Sinha and Sharma Citation1992; Rane et al. Citation2012). All these studies report chromosome counts for many species of Ipomoea and only a single species of Operculina (i.e. O. turpethum showing 2n = 30) has been investigated cytogenetically. The most common diploid chromosome number reported in Ipomoea is 2n = 30, although species with 2n = 28 and 2n = 32 have also been reported. So far, no instances of polyploidy have been reported except for I. batatas (L.) Lam. where hexaploidy (2n = 6x = 90) was reported by Sinha and Sharma (Citation1992).
This paper is a part of continuous program on karyological investigation of endemics of northern Western Ghats (Gosavi et al. Citation2011; Lekhak et al. Citation2011; Lekhak and Yadav Citation2011; Bagane et al. Citation2014; Gavade et al. Citation2015; Joshi et al. Citation2016) and aims to generate new cytogenetical data. As there is no information on record for karyotype of I. clarkei and O. tansaensis, we focus on the cytogenetics of these endemic species from Western Ghats. Since I. diversifolia and O. turpethum are widespread species, cytogenetical variation, if any, was also assessed. This resulted in the reporting of a new cytotype for I. diversifolia while the chromosome count of O. turpethum was confirmed. Additionally, palynological details, as observed under scanning electron microscope, for all the four species have been provided.
Materials and methods
The plant materials for the present investigations were collected in the field in northern Western Ghats, and for each species a herbarium specimen was prepared and deposited in the Herbarium of Department of Botany, Shivaji University, Kolhapur (acronym SUK) (). Mitotic preparations were made from the root-tips of germinated seeds. Seed surface was sterilized with 0.1% (w/v) HgCl2 for 4 min, nicked at the distal end by a sharp razor and germinated on wet filter paper in Petri dish. The well grown root-tips (6‒10 mm long) were excised and pre-treated with saturated solution of para-dichlorobenzene (pDB) for 4–5 h at 9 ± 3°C. Further, the root-tips were hydrolysed in 1N HCl and squashed in 2% propionic-orcein. For meiotic studies, appropriate sized flower buds were fixed in Carnoy’s fixative (3:1 absolute ethanol and acetic acid) and smears of floral buds were stained using 2% propionic-orcein. Suitable somatic and meiotic plates from freshly prepared slides were photographed with a camera mounted on a fluorescence microscope (Leica DM2000, Wetzlar, Germany) at 1000× magnification. Ten plates with well-separated somatic chromosomes were selected for karyotype analysis by adopting the method of Levan et al. (Citation1964). The degree of karyotype asymmetry was determined using the categories of Stebbins (Citation1971) and the CVCL (coefficient of variation of chromosome length) and MCA (mean centromeric asymmetry) as proposed by Peruzzi and Eroğlu (Citation2013).
Table 2. Geographical distribution and voucher specimen details of I. clarkei, I. diversifolia, O. tansaensis and O. turpethum.
Pollen grains fixed in glacial acetic acid were acetolysed (in freshly prepared 9:1 acetic anhydride: concentrated sulphuric acid) following the technique of Erdtman (Citation1960) and mounted on a double sided sticky carbon tape bound to an aluminium stub. Pollen grains were then coated with gold/palladium for 75 s on a Quorum SC7620 sputter coater and examined using a TESCAN VEGA3 scanning electron microscope at 10 and 15 kV. The measurements were made from semi-permanent preparation of acetolysed pollen grains mounted in glycerine jelly. Measurements of at least 20 pollen grains for each species were taken and expressed as mean ± standard deviation. The values of P (polar axis length) and E (equatorial diameter) were calculated to find out P/E ratio.
Results
Cytogenetics
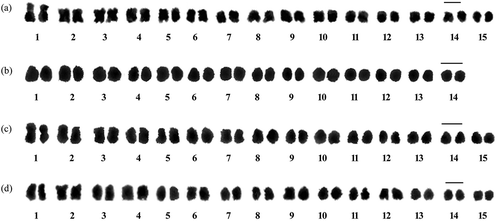
I. diversifolia exhibited 2n = 2x = 28 chromosomes while the rest of the species had 2n = 2x = 30 chromosomes (, ), ), )). Comparative karyotypes of all the species investigated are provided in whereas in the karyograms of the species are represented. All the studied species had only one type of chromosomes, i.e. chromosomes with median region centromere, and hence the karyotype formula 14m (I. diversifolia) or 15m (I. clarkei, O. tansaensis and O. turpethum). Karyotypes of all the species were symmetrical and occupied Stebbins’s 4a category of karyotype asymmetry. Mean chromosome length (MCL) was recorded to be the highest (1.62 ± 0.26 µm) in the case of I. clarkei and lowest (1.29 ± 0.17 µm) in I. diversifolia (). Total haploid chromosome length (THL) ranged from 18.07 ± 0.17 µm (I. diversifolia) to 24.37 ± 0.26 µm (I. clarkei). I. clarkei showed maximum value (1.82) for R (ratio of largest and the smallest chromosome of the complement) while the minimum (1.59) was recorded for O. turpethum ().
Table 3. Comparative karyotypes of I. clarkei, I. diversifolia, O. tansaensis and O. turpethum.
Figure 1. Somatic chromosomes and meiotic behaviour in Ipomoea: (a–f) I. clarkei (a) mitotic metaphase (2n = 30); (b) PMC at diakinesis (n = 15); (c) PMC at metaphase-I; (d) PMC at early anaphase-I; (e) PMC at metaphase-II; (f) PMC at anaphase-II. (g-o) I. diversifolia (g) mitotic metaphase (2n = 28); (h) PMC at diakinesis (n = 14); (i-k) PMCs at metaphase-I showing precocious separation of chromosomes; (l) PMC at anaphase-I; (m) PMC at metaphase-II; (n) PMC at anaphase-II; (o) decussate tetrads. Bars: 5 μm.
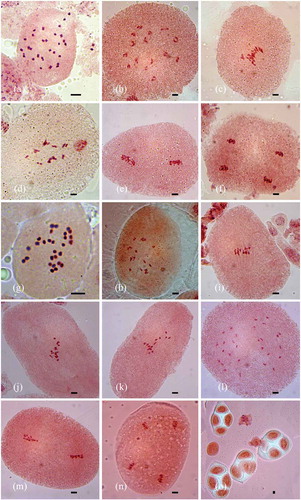
Figure 2. Somatic chromosomes and meiotic behaviour in Operculina: (a) mitotic metaphase of O. tansaensis (2n = 30); (b) PMC at diakinesis (n = 15); (c) PMC at metaphase-I; (d, e) PMCs at early anaphase-I; (f) mitotic metaphase of O. turpethum (2n = 30). Bars: 5 μm.
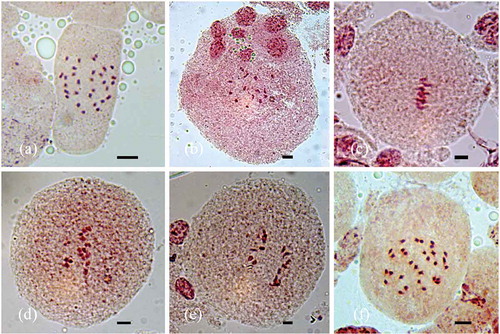
Figure 3. Karyograms of the studied species: (a) I. clarkei; (b) I. diversifolia; (c) O. tansaensis; (d) O. turpethum. Bars: 2 μm.
Meiotic studies were carried out in three species (I. clarkei, I. diversifolia and O. tansaensis). Meiotic course was found to be normal with no anomalous behaviour of chromosomes. At diakinesis, pollen mother cells (PMCs) of I. diversifolia showed 14 bivalents (n = 14) ()) while 15 bivalents (n = 15) were noted in I. clarkei and O. tansaensis ( and (b)). Different stages such as metaphase-I and anaphase-II were also observed (-), ) and )). Microspore tetrads were tetrahedral for I. clarkei and O. tansaensis whereas decussate tetrads were seen in I. diversifolia ()).
Palynology
Investigation of the pollen grains led to recognition of two types, viz. pantoporate-echinate (I. clarkei and I. diversifolia) and tricolpate-smooth (O. tansaensis and O. turpethum) (-h)). Pollen grains of I. clarkei were spheroidal, radially symmetrical, 80–98 μm in diameter, exine echinate; spines 8.98 ± 0.83 µm long with blunt tip and bulbous base, compactly arranged, tapering towards apex; microreticulum only on the basal cushion of the spines. I. diversifolia had spheroidal, pantoporate pollen grains, radially symmetrical, 78–98 μm in diameter, exine echinate; microreticulum spread throughout the exine; pores elliptic, 6.33 ± 1.44 μm in diameter; spines 6.7 ± 0.52 μm long with blunt tip and bulbous base, laxly arranged, non-tapering towards apex.
Figure 4. SEM micrographs showing whole and enlarged pollen: (a, b) I. clarkei; (c, d) I. diversifolia; (e, f) O. tansaensis; (g, h) O. turpethum. Bars: 5 μm.
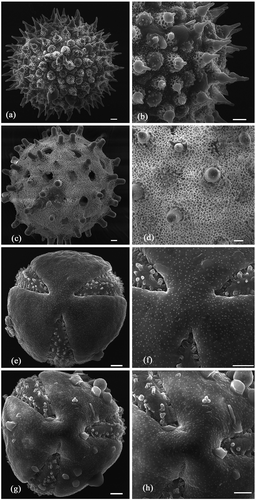
Operculina tansaensis had oblate to spheroidal pollen grains with P/E ratio 0.92 ± 0.04 tricolpate; colpus with microgranulate membrane, 12.39 ± 2.37 µm wide, usually acute at the ends mesocolpium psilate, minutely dotted rarely with few spheroidal deposits. In O. turpethum pollen grains were oblate to spheroidal with P/E ratio 0.89 ± 0.06, tricolpate; colpus with microgranulate membrane, 12.15 ± 0.86 µm wide, usually acute at the ends, mesocolpium minutely dotted with gemma-like depositions.
Discussion
Cytogenetics
The basic chromosome number for the genus Ipomoea is x = 15 (Darlington and Wylie Citation1955). According to Löve and Löve (in Sinha and Sharma Citation1992) x = 15 is a secondarily derived number while x = 5 is the primary basic number of Convolvulaceae. Of the 51 taxa occurring in India, 33.3%, i.e. 17 are yet to be examined cytogenetically (). About 68.6% (35 taxa) of the Indian species are diploid with 2n = 30 chromosomes. This further corroborates the basic chromosome number x = 15 for Ipomoea. Two species (I. purpurea and I. staphylina) have 2n = 32 chromosomes, three species (I. coptica, I. diversifolia and I. pes-tigridis) possess 2n = 28 chromosome and a single species (I. triloba) has been reported with 2n = 38 (). The cytotypes 2n = 32 and 2n = 38 have not previously been confirmed by any other worker and hence need to be reinvestigated. The unusual chromosome number may be attributed to polyploidy, aneuploidy or dysploidy or because of occurrence of polysomaty (cells with different ploidy levels in the same organ or tissue) in the root-tip cells. Polyploidy has only been reported in case of I. batatas (2n = 6x = 90), a cultivated tuber crop (Sinha and Sharma Citation1992). Polyploidy in cultivated crops is of common occurrence and is on account of the fact that polyploids have bigger size and vigour as compared to their diploid counterparts.
Rane et al. (Citation2012) studied the karyomorphology of 10 species of Ipomoea from Maharashtra and found that the THL value ranges from 29.88 to 58.83 µm. The highest values of MCL (3.39 ± 0.74 µm) and THL (50.83 µm) was recorded for I. carnea and the lowest value of MCL (1.99 ± 0.38 µm) and THL (29.88 µm) for I. aquatica Forssk. The THL values recorded for I. clarkei and I. diversifolia were 24.37 ± 0.26 µm and 18.07 ± 0.17 µm, respectively. The low value of THL is due to smaller chromosomes of I. clarkei and I. diversifolia. The MCL value in the former was 1.62 ± 0.26 µm and for the latter 1.29 ± 0.17 µm. Additionally, I. diversifolia has only 28 somatic chromosomes in its complement which decreases the THL. All the chromosomes in I. clarkei and I. diversifolia possess median region centromeres and hence the karyotype formulae 15m and 14m, respectively (). This makes the karyotype highly symmetrical (4a category). On the other hand, studies by Sampathkumar (Citation1979) and Rane et al. (Citation2012) reported chromosomes with median to submedian centromere in Ipomoea. Nakajima (Citation1963) could also observe median to terminal centromere in I. lacunosa L. and I. violacea L. and hence reported asymmetrical karyotypes. A highly symmetrical karyotype is due to the prevalence of median or submedian centromeres (Levitzky Citation1931). Consequently, the karyotypes of I. clarkei and I. diversifolia with median region centromeres are highly symmetrical when compared with the karyotypes of species analysed by Nakajima (Citation1963) and Rane et al. (Citation2012). Arm ratio (r) and ratio between the largest and the smallest chromosome of the complement (R) for I. clarkei and I diversifolia showed slight differences. The former was on the higher side for I. diversifolia and the latter for I. clarkei. Low MCL of I. diversifolia explains this while larger chromosomes in I. clarkei account for its high R ratio. The maximum value (16.12) for CVCL (a measure of interchromosomal asymmetry) was observed for I. clarkei and the minimum (13.03) for O. tansaensis. In I. clarkei, the chromosomes ranged from 1.18 ± 0.17 µm to 2.15 ± 0.25 µm while in O. tansaensis the range was 1.22 ± 0.11 µm to 2.00 ± 0.25 µm. The heterogeneity in chromosome sizes (interchromosomal asymmetry) was recorded more in I. clarkei as compared to O. tansaensis. MCA (a measure of intrachromosomal asymmetry) reflects heterogeneity in centromere position. The highest value (11) was recorded for O. turpethum while O. tansaensis had the lowest (7.73). However, on account of small chromosomes it was not possible to observe much difference in the centromere positions in the chromosome complements of both the species. Both I. clarkei and I. diversifolia fall under similar Stebbins’s karyotype asymmetry classes, i.e. 4a. MCA is higher for I. diversifolia and CVCL for I. clarkei. Therefore, it is not possible to assess the overall karyotype symmetry based on MCA and CVCL. Nevertheless, larger chromosomes and higher THL value of I. clarkei indicate that the karyotype is more symmetric than I. diversifolia.
Amongst Operculina species, cytogenetical data are available only for O. turpethum. Karyological parameters of O. tansaensis are comparable to that of O. turpethum () indicating genetic proximity between both the species. Cytological details of O. turpethum obtained in the present study were in good agreement with Sampathkumar (Citation1979). Sampathkumar (Citation1979) reported 2n = 30 chromosomes with median and submedian centromeres, R value of 1.57 and the chromosome range of 1.9–3.0 µm. We have observed that our population of O. turpethum also has 2n = 30 chromosomes with median region centromeres, R value of 1.59 and chromosomes ranging from 1.15 ± 0.22 µm to 1.82 ± 0.23 µm. The differences in chromosome length can mainly be attributed to the tremendous genetic diversity existing in this widespread species. Based on higher values of CVCL (13.37) and MCA (8.86) it can be said that the karyotype of O. turpethum is more asymmetric. It has been noticed that karyotypes of the genera like Hewittia Wight & Arn. (H. sublobata (L.f.) Kuntze), Merremia Dennst. ex Endl. (M. dissecta (Jacq.) Hallier f., M. hederacea (Burm.f.) Hallier f.) and Operculina (O. turpethum) show similarity (Sampathkumar Citation1979). O. tansaensis, however, showed some differences with the karyotypes of Hewittia and Merremia. The chromosomes of H. sublobata were larger (2–4 µm) than O. tansaensis which has chromosomes ranging from 2.00 ± 0.25 µm to 1.22 ± 0.11 µm. Both Hewittia and Merremia possessed chromosomes with median and submedian centromere while only median centromeres were observed for chromosomes of O. tansaensis. The R value of H. sublobata (2.0) was comparable to O. tansaensis (1.63); however, M. dissecta and M. hederacea showed higher values of 2.5 and 3.3, respectively (modified after Sampathkumar Citation1979).
Palynology
The pollen morphology of Convolvulaceae illustrates great diversity and has taxonomic importance (Tellería and Daners Citation2003). Extensive investigation of the pollen morphology of the family was carried out by Sengupta (Citation1972).
In the present investigation two kinds of pollen, namely pantoporate-echinate and tricolpate-smooth, were recognized following Ferguson et al. (Citation1977). The former were observed in I. clarkei and I. diversifolia and the latter type in O. tansaensis and O. turpethum. Echinate, micro-reticulate and pantoporate pollens have been also reported in I. indica, I. nil, I. pubescens Lam, I. purpurea and I. rubriflora O'Donell (Tellería and Daners Citation2003). The pollens of O. tansaensis differ slightly from O. turpethum in the absence of gemma-like depositions on mesocolpium. Similar tricolpate-smooth pollens have also been observed in M. lobata Verdc., M. dissecta and O. macrocarpa (L.) Urb. by Ferguson et al. (Citation1977). The pollens of I. clarkei and I. diversifolia convincingly differentiate each other in terms of the presence of microreticulum on the exine, spine density and its structure and hence are of diagnostic value in taxonomy. This does not hold good for the two Operculina species presently investigated. More palynological data on remaining Operculina species will certainly throw light on the taxonomic utility of pollen character in the genus.
Acknowledgements
The authors wish to express sincere thanks to the Head, Department of Botany, Shivaji University, Kolhapur for providing all necessary facilities. One of the authors (SDP) is also grateful to DBT for financial support under ‘Network Programme for Enrichment and Update of Plant Chromosome Database for Spermatophytes and Archegoniate’ vide sanction letter BT/PR7866/NDB/39/272/2013 dated 23 March 2015.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bagane SA, Kattee AV, Sardesai MM, Lekhak MM, Yadav SR. 2014. Mitotic chromosome studies in Nesphostylis bracteata, an endemic legume from Western Ghats. Cytologia. 79(1):59–62.
- Bir SS, Kumari S, Shoree SP, Sagoo MS. 1978. Cytological studies in certain bicarpellatae from north and central India. J Cytol Genet. 13:99–106.
- Bir SS, Sidhu M. 1975. Weed flora of cultivable lands in Punjab maize fields in Patiala district. Acta Bot Indica. 3:136–141.
- Darlington CD, Wylie AP. 1955. Chromosome atlas of flowering plants. London: George Allen & Unwin.
- Erdtman G. 1960. The acetolysis technique: a revised description. Sv Bot Tidskr. 54(4):561–564.
- Ferguson IK, Verdcourt B, Poole MM. 1977. Pollen morphology in the genera Merremia and Operculina (Convolvulaceae) and its taxonomic significance. Kew Bull. 31(4):763–773.
- Gao CZ, Zou QL. 1995. The karyotype analysis of Cyamopsis tetragonoloba and Calonyction muricatum. Guihaia. 15(2):163–165.
- Gavade SK, Lekhak MM, Yadav SR. 2015. Taxonomy and karyology of Nogra dalzellii (Baker) Merr. (Leguminosae: Papilionoideae), a little-known Indian legume. Webbia. 70(2):313–318.
- Gosavi KVC, Lekhak MM, Chandore AN, Yadav SR. 2011. Karyology of Barleria grandiflora Dalzell (Acanthaceae), a potential ornamental endemic to Northern-Western Ghats of India. Nucleus. 54(3):133–136.
- Joshi HS, Yadav PB, Lekhak MM, Yadav SR. 2016. Cytogenetics of two endemic Barleria species (Acanthaceae) from the northern Western Ghats (India). Caryologia. 69(2):170‒174.
- Khatoon S, Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi: Department of Botany, University of Karachi.
- Lekhak MM, Nandikar MD, Yadav SR. 2011. Karyomorphology of Flemingia nilgheriensis (Baker) Wight ex T. Cooke: an endemic from western ghats. Cytologia. 76(3):243–248.
- Lekhak MM, Yadav SR. 2011. Karyotype studies in two critically endangered and endemic Crinum species (Amaryllidaceae) from Northern-Western Ghats of India. Nucleus. 54(1):25–30.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201‒220.
- Levitzky GA. 1931. The morphology of the chromosome. Bull Appl Bot Pl Breed. 27:220‒240.
- Mishra DK, Singh NP. 2001. Endemic and threatened flowering plants of Maharashtra. Calcutta: Botanical Survey of India; p. 167–171.
- Nakajima G. 1963. Karyotype of genus Ipomoea. Cytologia. 28:351‒359.
- Nayar TS, Rasiya Beegam A, Sibi M. 2014. Flowering plants of the western ghats of India. Vol. 1. Thiruvananthapuram: St. Joseph’s Press; p. 93‒299.
- Ogunwenmo KO. 1999. Evolutionary and taxonomic studies of Ipomoea L. sect. Involucratae Bak. & Randle (Convolvulaceae) in Nigeria. Feddes Repert. 110:499–514.
- Ozias-Akins P, Jarret RL. 1994. Nuclear DNA content and ploidy levels in the genus Ipomoea. J Amer Soc Hort Sci. 119:110–115.
- Peruzzi L, Eroğlu HE. 2013. Karyotype asymmetry: again, how to measure and what to measure? Comp Cytogenet. 7(1):1–9.
- Prasad R. 2013. Operculina turpethum. In: Schmelzer GH, Gurib-Fakim A, editors. Plant resources of tropical Africa 11 (2). Medicinal plants 2. Wageningen (Netherlands/ CTA, Wageningen, Netherlands): PROTA Foundation; p. 185‒188.
- Probatova NS, Rudyka EG, Sokolovskaya AP. 1996. Chromosome numbers in synanthropic plants from the Russian Far East. Bot Zhurn (Moscow & Leningrad). 81(5):98–101.
- Rane VA, Patel BB, George J. 2012. Karyomorphological analysis of ten species of Ipomoea Jacq. Cytologia. 77(2):239‒249.
- Rao PN, Mwasumbi LB. 1981. In Chromosome number reports LXXII. Taxon. 30:701.
- Roy R. 1979. Karyotype of Ipomoea purpurea. Proc Indian Sci Congr Assoc (III, C). 66:84–85.
- Sampathkumar R. 1979. Karyomorphological studies in some south Indian Convolvulaceae. Cytologia. 44:275‒286.
- Sampathkumar R, Ayyangar KR. 1984. On the meiotic characteristics of some species of Ipomoea. Proc Indian Sci Congr Assoc. 71(3–VI):85.
- Santapau H, Henry AN. 1973. A dictionary of the flowering plants in India. New Delhi: Council of Scientific and Industrial Research; p. 83.
- Sengupta S. 1972. On the pollen morphology of Convolvulaceae with special reference to taxonomy. Rev Palaeobot Palynol. 13:157–212.
- Sinha S, Sharma SN. 1992. Taxonomic significance of karyomorphology in Ipomoea spp. Cytologia. 57:289–293.
- Staples G. 2012. Convolvulaceae-the Morning glories and bindweeds. Convolvulaceae Unlimited. Accessed 2017 Mar 31. http://convolvulaceae.myspecies.info/node/9.
- Stebbins G. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- Tellería MC, Daners G. 2003. Pollen types in southern new world Convolvulaceae and their taxonomic significance. Plant Syst Evol. 243:99–118.
- The Plant List. 2013. Version 1.1. Published on the Internet; Accessed 2016 Apr 06. http://www.theplantlist.org/
- Vij SP, Singh S, Sachdeva VP. 1974. In IOPB chromosome number reports XLV. Taxon. 23:619–624.
- Wang JX, Lu SY, Zhou HY, Liu QC. 1998. The second report of studies on overcoming the interspecific incompatibility and hybrid abortion between series A and B in the section Batatas of the genus Ipomoea. Acta Agron Sin. 24(2):139–146.
- Yen DE, Gaffey PM, Coates DJ. 1992. Chromosome numbers of Australian species of Ipomoea L. (Convolvulaceae). Austrobaileya. 3(4):749‒755.
