ABSTRACT
Relationship between cell cycle progression and different ways of cell and genome reproduction (regular mitotic cycle, polyploidizing mitoses, endoreduplication, depolyploidization) has been studied in the course of development of invasive trabecular trophoblast cell population in the silver fox placenta (Vulpes fulvus Desm.) Cell cycle progression was estimated using Ki-67 immunolabeling. In the region adjoining the endometrium the trophoblast cells keep their mitotic activity, giving rise to diploid or low-polyploid cells via polyploidizing (reduced) mitoses. In the depth of the spongy zone where some trophoblast cells migrate inside the fetal part of placenta, some cells switch to endocycles – polytenization and endomitosis. Judging by the Ki-67 labeling patterns, polytenization correlates with the persistence of cell cycles for a long time and takes part in retention of potential of cell/genome reproduction as well as achievement of the highest ploidy level (up to 256c). Meanwhile, other endoreduplicated cells leave the cell cycle with subsequent cell degradation. Classical endomitosis may be a mode of genome multiplication and correlate with both the persistence and attenuation of cell cycles. Depolytenization and depolyploidization often correlate with attenuation of Ki-67 expression followed by cell degradation. However, in a minor trophoblast cell population, non-mitotic genome segregation most probably takes part in retention of cell reproductive potential.
Introduction
In previous studies we have found that the trophoblast and, partly, uterine epithelium in the developing placenta of the silver fox are mainly characterized by 4–64-fold multiplication of their genome, with some trophoblast cell nuclei reaching 128–256c (Zybina et al. Citation2001). Like in other mammals (Zybina and Zybina Citation1996, Citation2005; Klisch et al. Citation2004), the fox trophoblast demonstrates polyploidizing mitoses and endoreduplication as the main modes of initial genome multiplication. Recently polyploidization has been found in the trophoblast that invades endometrial glands and gradually replaces their epithelium, thereby forming trabeculae of the junctional or a “spongy” zone of placenta (Zybina et al. Citation2001, Citation2016). The peculiarity of the fox placenta compared to other mammals was a high variability of the DNA content, which indicates a high degree of aneuploidy in the trophoblast cells. Such a phenomenon as well as several waves of increase and decrease of trophoblast cell ploidy found in the course of fox placenta development (Zybina et al. Citation2001) are unexplained as yet. In our previous papers we concluded that the trophoblast cells invading endometrium undergo active proliferation and endoreduplication followed by degradation, with cell debris likely to be a source of embryo nutrition through the lamellar zone; the latter constitutes a placental barrier in the carnivore placenta. We have also observed that the trabecular trophoblast has a high level of Ki-67 immunolabeling that decreases gradually in the differentiated trophoblast cells near the zone of their degradation; this tendency is enhanced at the later late stages of embryonic development (Zybina et al. Citation2015).
The aim of this work was to characterize different ways of cell reproduction depending on the maintenance or extinction of the progression of cell cycles in the course of growth, differentiation and degradation of the trabecular trophoblast cells of the silver fox placenta.
Material and methods
Materials
Breeding of silver foxes was carried out at the Experimental Fur Farm of the Institute of Cytology and Genetics SB RAS (Novosibirsk, Russia). The wild-type silver fox females mated with males, then the females were kept in the open cages in natural photoperiod conditions (about 9 h of daily light in February when mating took place). Sacrifice of the pregnant females by electric current (380 V) was carried out in accordance with the rules developed by the Commission for Bioethics of the State Scientific Center of Russia, Institute of Biomedical Problems, Moscow. Implantation sites on the 19th−22nd day of gestation (5–10 embryos at each developmental stages) were fixed with a mixture of ethanol and glacial acetic acid (3:1). The material was embedded in paraffin using standard procedure.
Methods
The paraffin-embedded material was sectioned into 3 μm thick sections. The latter underwent immunohistochemical reactions using primary antibodies the Ki-67 (DAKO, cat. N IS626) according to the standard procedure of the streptavidin-peroxidase test (Mühlhauser et al. Citation1993). Endogenous peroxidase activity was quenched by 15 min incubation with 3% hydrogen peroxide. Non-specific antibody binding was blocked by incubation for 30 min in rabbit serum diluted 1:20 in 0.6% Tris buffer, 1.5% bovine serum albumin (BSA), pH 7.6. The sections were counterstained with hematoxylin. The slides were examined using the inverted microscope Axiovert 200M with objective lenses 10×/0.30, 20×/0.5 and 40×/0.75. The photos were taken with a color CCD camera Leica DFC 420 (Manheim, Germany) format 2592 × 1944.
Results
Both glandular uterine epithelium and trophoblast cells invading the uterine glands show intensive Ki-67 immunostaining in the sites of their mutual contacts at the period of silver fox placenta formation. The cells of the uterine epithelium in the glands invaded by trophoblast have an overwhelming majority of Ki-67-positive cells at the 19–21 gestation day (gd), i.e. 91.1 ± 1.4% at the 19th gd, 76.7 ± 2.4% at the 20th gd, 74.1 ± 2.7% at the 21st gd. At the 22nd gd the percentage of Ki-67-labeled epithelial cells falls to 21.3%. The data are similar to the incidence of Ki-67 labeling of the invasive trophoblast cells. Thus, at the 19–21 gd the percentage of Ki-67-positive cells exceeded 90% whereas at the 22nd gd it fell to 58.4% (Zybina et al. Citation2015). It may be concluded that the uterine epithelium responds the trophoblast invasion by the high proliferative activity. It progressively decreases but remains at a high level at the period when placenta is already well formed. Simultaneously, the trophoblast cells invading uterine glands also show high level of proliferation.
In the trabecular trophoblast cells at the 19–22 gd a wide range of patterns of Ki-67 immunolocalization is observed ( and ), with the common feature being the predominant staining of the nucleoli. The majority of cells contain dark-colored nucleoli and a lighter colored karyoplasm. At the 19–21 gd in the part of trabeculae adjacent to endometrium Ki-67 immunostaining is most frequently observed in the dark-colored nucleoli as well as in the chromatin net formed from fine strands and small clump structures ( and ()). However, in this zone there are some weakly immunostained nuclei. In the trophoblast cells of this zone a high amount of Ki-67-labeled mitotic figures are also encountered; by their size and shape they belong to both regular and polyploidizing mitoses described previously in the silver fox placenta (Zybina et al. Citation2001). In the depth of the fetal part of placenta, where some differentiated trophoblast cells detach from trabeculae and acquire the migratory phenotype (Zybina et al. Citation2016), variability of Ki-67 localization patterns increases. In this zone cells also varied in ploidy due to a switch to the endoreduplication cycle (Zybina et al. Citation2001, Citation2015; Zybina and Zybina Citation2014). In the majority of cells, nuclear structures progressively lose their Ki-67 labeling. At the same time nucleoli retain their immunostaining for a longer period while chromatin structures lose Ki-67 immunopositivity (() and (, , , )). Finally, some nuclei lose Ki-67 immunostaining completely ((, )). Thus, in the course of the differentiation of the cells capable of migration, progressive attenuation of cell cycle takes place. Nevertheless, there is a subpopulation of trophoblast cells which retains a dark Ki-67 immunostaining of all nuclear structures. This is especially true for the giant, probably migratory, cells of trabecular trophoblast, though Ki-67 negative giant nuclei are also observed here. The largest nuclei very often show Ki-67 immunostaining of large nucleoli as well as chromatin strands bearing small chromomere-like clumps (). Not infrequently these Ki-67-positive chromatin strands (chromonemes) run from the nuclear envelope to nucleolus, as in so-called Rabl orientation (, ()). In some cases, nuclei having especially large sizes show attributes of non-classic polytene chromosomes observed in the rodent giant trophoblast cells (Zybina and Zybina Citation1996, Citation2005), i.e. chromosome bundles attached to the nuclear envelope or nucleoli (). It is noteworthy that the polytene chromosome bundles show, as a rule, an intensive Ki-67 immunostaining.
Figure 1. Ki-67 immunostaining of the silver fox placenta at the 21st day of pregnancy. Glandular uterine epithelium (ge) shows high level of Ki-67 immunostaining whereas trabecular trophoblast (tr) shows a variety of Ki-67 immunostaining that depends, in particular, on the area and size of nuclei. Decrease of immunostaining is observed at the zone of cell detachment of trabeculae (arrows) and peaks in the zone of trophoblast cell destruction (double arrows), although some populations of intensively Ki-67-immunolabelled cells are found in this zone. At the bottom small nuclei of the developing labyrinth (lab) show high Ki-67 immunolabeling.
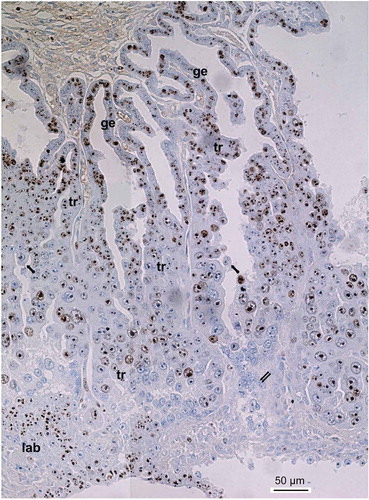
Figure 2. Immunolocalization of the Ki-67 protein in the trophoblast cells of the spongy zone of the silver fox at the 20th gd. (A) intensive Ki-67 immunostaining in the nuclei and karyoplasm of the low-ploid trophoblast cells close to the border with endometrium; (B–D) Ki-67 localization in the chromosome structures in the highly polyploid trophoblast cells; (C, D) Ki-67-positive chromatin strands (chromonemes) run from the nuclear envelope to nucleolus, as in Rabl orientation; the Ki-67-positive chromosome strands running in parallel into the nucleolus (arrowhead) prove some features of non-classic polytene chromosomes; (E–G) endomitotic (em) chromosomes show intensive Ki-67 immunostaining; (H–J) attenuation of Ki-67 immunolabeling in the region of trophoblast cell detachment from trabeculae: nucleoli longer retain the Ki-67 immunostaining, meanwhile endomitotic chromosomes often retain Ki-67 immunostaining longer than disappearing nucleoli; (K–N) subdivision of highly polyploid nucleoli into two or more ones via nuclear fragmentation (*); nucleoli and chromatin lose Ki-67 immunostaining. Magnification (Figure 2(k)) is the same for all photos.
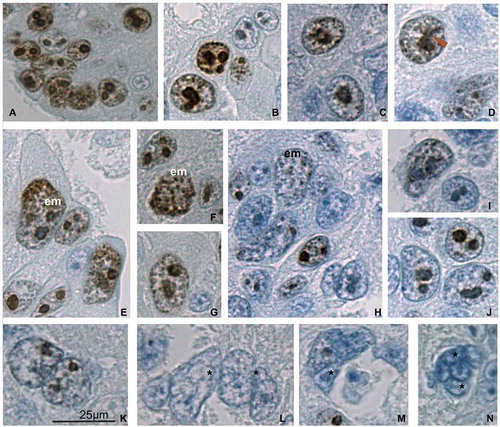
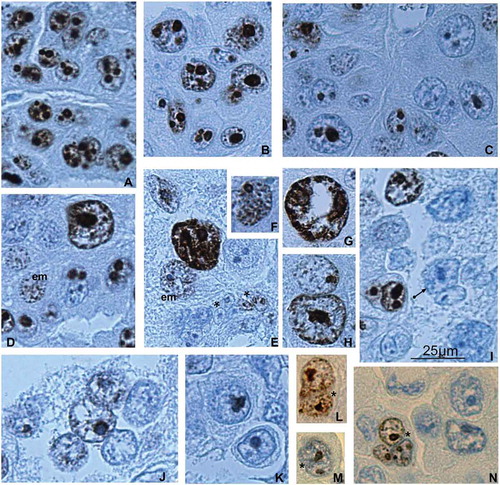
Figure 3. Ki-67 immunostaining of the trophoblast cells of spongy zone of silver fox at the 21st (A–K) and 22nd (L–N) gd. (A, B) Intensive Ki-67 immunostaining in the chromatin and nucleoli of the trabecular trophoblast cells close to the border with the endometrium (A) and in the middle of the spongy zone (B); (С) attenuation of Ki-67 immunostaining in the region detachment of the trophoblast cells from trabeculae; many nucleoli retain the dark immunostaining; (D, E, G, H) highly polyploid nuclei show Ki-67 immunopositivity in the non-classic polytene chromosomes (arrowhead); (D, E) heterogeneity of the trabecular trophoblast cell population: Ki-67-positive polytene nucleus, in endomitosis (em) attenuation of Ki-67 labeling with its relocalization onto endochromosomes, as well as nuclear fragmentation (*) of Ki-67 immunonegative nuclei and nuclei with residual labeling; (F) Ki-67 labeling of endochromosomes and nucleolus in endomitosis; (I–K) the trophoblast cells detaching from the trabeculae and undergoing cytoplasm destruction are mainly Ki-67 immunonegative, many nuclei undergo fragmentation (I, arrow); (L, M) at the 22nd gd many trabecular trophoblast cell nuclei undergo fragmentation (*), not infrequently they show Ki-67 immunopositivity in endomitosis (L, N).
These Ki-67-positive giant trophoblast cells are often observed in the middle of the spongy zone as well as in the zone of destruction of the trabecular trophoblast () where, however, the majority of trophoblast cells lose Ki-67 immunostaining (Zybina et al. Citation2015) and undergo cytoplasm degradation (Zybina et al. Citation2016). The intensive Ki-67 immunolabeling of nuclei with signs of polytenization suggests that in the depth of the spongy zone of silver fox placenta not only attenuation of cell cycle takes place; some amount of cells retain a significant reproductive potential at the account of switch to the endoreduplication cycles.
In some cases, in the middle of the spongy zone, nuclei in the differentiated trabecular trophoblast cells may be filled with short and dense chromosome-like structures ( and ()) similar to endochromosomes (Nagl Citation1978; Zybina and Zybina Citation1996; Zybina and Zybina Citation2005, Citation2011), the degree of their condensation being comparable with the metaphase ones. These pictures are reminiscent of endometaphase or endoanaphase of the classic endomitosis (Geitler Citation1953; Nagl Citation1978, Citation1995; Anisimov Citation2005). The endomitotic figures were both Ki-67 immunopositive and immunonegative. It is noteworthy that, distinct from the interphase and polytene nuclei, Ki-67 immunolabeling may prevail just in endochromosomes, whereas nucleoli often show a weaker immunostaining with the exception of their periphery (). According to classic endomitosis conception, chromosomes undergo successive modifications similar to mitosis but without nuclear envelope disappearance, metaphase plate arrangement and poleward movement of chromosomes in anaphase (Geitler Citation1953; Nagl Citation1978). The data obtained here give an example of Ki-67 immunolabeling of endomitotic nuclei.
As it has been shown in a previous paper, passing from the border with endometrium to the deeper regions of trabeculae, there occurs attenuation of Ki-67 protein expression, and this process is enhanced at the later developmental stages – 21–22 gd (Zybina et al. Citation2015). Meanwhile, Ki-67 first disappears in the chromatin and chromosome structures, and then in nucleoli. The data of the present paper show that the weakening of Ki-67 immunostaining in the chromosome structures and nucleoli affects both polytene () and endomitotic nuclei (). At the same time, it is noteworthy that in the endomitotic figures nucleoli do not show the longest retaining of Ki-67 labeling whereas endochromosomes show a tendency to preserve the immunostaining until it completely disappears (()).
At the 20–22 gd, especially at the later developmental stages, in some highly polyploid trabecular trophoblast cells there were pictures similar to nuclear fragmentation described in the secondary giant trophoblast cells of the rat and field vole (Zybina and Zybina Citation2005). That is to say, there may be observed some lobulations or fissures that subdivide a nucleus into separate areas; arrangement of chromatin and nucleoli in these areas is similar to individual nuclei (). In other cases, a number of attached nuclei are seen, and the contours of agglomeration look like a border of a united nucleus (). Finally, often a cell membrane surrounds a couple or a cluster of tightly attached nuclei ( and ).
The nuclear fragmentation was described as a way of non-mitotic division of a highly endopolyploid nucleus that gives rise to a multinucleate cell with a number of near-euploid (mostly 2c–8c) nuclei; this process is characteristic of endopolyploid trophoblast cells (Zybina et al., Citation2005, Zybina and Zybina Citation2005, Citation2014). The electron microscopic observations proved that the nuclei of giant trophoblast cells in rats fall into multinucleate cells, the nuclear envelope and its derivatives playing an active role in this process (Zybina and Zybina Citation2008). According to the present data, the probable subdivision of trabecular trophoblast cell nuclei in the fox placenta into fragments takes place, as a rule, mostly in the Ki-67-negative nuclei. However, a few Ki-67-positive nuclei undergoing fragmentation are observed ()). These nuclei often show endomitotic figures. The figures mostly show signs of attenuation of Ki-67 expression but not infrequently they have dark-colored immunostaining both in endochromosomes and in nucleoli (). Interestingly, at the 22nd gd when the overwhelming majority of trabecular trophoblast cells already leave the cell cycle (Zybina et al. Citation2015), the Ki-67-positive nuclei are represented mostly by the figures of endomitosis and/or nuclear fragmentation ().
As has been shown previously, nuclei devoid of cytoplasm scattered among the extracellular matrix are found in the region of destruction of trabecular trophoblast lying nearby the region of labyrinth formation in the deepest part of the spongy zone (Zybina et al. Citation2016). It is noteworthy that nuclei devoid of cytoplasm are, most often, closely adjacent to each other, thereby forming agglomerates. Judging by the shapes and size, these agglomerates may have been formed by fragmentation of highly polyploid nuclei ( and ()). In these agglomerates Ki-67 immunolabeling is not observed, although in some nuclei residual labeling may be found ()).
The nuclear fragmentation was described as a final step of depolytenization and, finally, depolyploidization in the giant trophoblast cells in the rodent placenta (Zybina et al. Citation1979; Zybina and Zybina Citation1996, Citation2005, Citation2008, Citation2014). This process was accompanied by the cessation of DNA replication and polyploidization as well as attenuation of RNA synthesis at the late stages of pregnancy, so that depolyploidization represent the final stage of differentiation and cell lifespan of the rodent giant trophoblast cells at the late stages of pregnancy (Zybina and Zybina Citation1996).
In the trabecular trophoblast of the fox placenta, the pictures resembling the nuclear fragmentation are also in most cases Ki-67-negative and are often characteristic of cells with signs of degradation. Therefore, in most cases, like in the rodent placenta, they probably represent the terminal step of their differentiation. Meanwhile, in some cases ((–)), Ki-67 labelling points that this process is connected with retention of cell reproduction capacity of trophoblast cell population that persists in the spongy zone for a long time.
Discussion
The patterns of intranuclear Ki-67 immunolocalization observed here prove the cell cycle progression in the fox trophoblast cells that undergo genome multiplication. The data give some details of chromosome transformation in these cells in the course of polytenization and endomitosis.
In general, Ki-67 immunolocalization peculiarities are known to be bound with phases of cell cycle. As shown in the dermal fibroblast culture, at the early G1-phase Ki-67 is detected mainly in numerous foci of centromere and telomere DNA localization scattered throughout the nucleoplasm (Bridger et al. Citation1998). At the later G1, S and G2-phase Ki-67 is found mainly inside the well-formed nucleolus as well as in the foci of condensed chromatin (Verheijen et al. Citation1989a; Kill Citation1996); and at the transition to mitosis the protein associates mitotic chromosome arms (Verheijen et al. Citation1989b; Bridger et al. Citation1998; Suurmejer and Boon, Citation1999; Booth et al. Citation2014). In a shortened cell cycle of culture of embryonic stem cells, in late S-phase Ki-67 is restricted by nucleolar localization (Becker et al. Citation2006). In the regenerating liver, similar localization of Ki-67 has been observed. Meanwhile, in this case, at the G2-phase Ki-67-positive nucleoli form a united bright discontinuous structure in which prophase chromosome-like entities may be seen (Vorotelyak et al., Citation2014).
Recently it has been shown that the possible function of Ki-67 is to regulate relocalization of the majority of nucleolus-specific proteins onto chromosome arms during mitosis (Booth et al. Citation2014).
The data obtained here prove that polytene, i.e. highly endopolyploid, nuclei in the trophoblast cells may be Ki-67-immunolabeled, indicating persistence of their cell cycle progression. In addition, Ki-67 immunolabeling of the chromosome strands constituting the non-classic polytene chromosomes prove that Ki-67 is localized both on their chromosome arms and in the nucleoli. As the degree of condensation of polytene chromosomes with their chromomere structure match prophase chromosomes (Muller Citation1935; Nagl Citation1978, Citation1995), Ki-67 immunostaining of the chromomere-like structures may be due to a prophase-like degree of condensation, i.e. most probably, G-phase of the endoreduplication cycle (Edgar and Orr-Weaver Citation2001). It is quite possible that at this stage the Ki-67, by analogy with mitotic events, takes part in formation of the perichromosomal layer (PCL) described on the mitotic chromosomes (Sheval and Polyakov Citation2008; Booth et al. Citation2014; Takagi et al. Citation2016; Matheson and Kaufman Citation2017).
We were able to show Ki-67 immunolabeling in the classic endomitotic figures of the trophoblast cells. According to the classical conception of endomitosis, chromosomes undergo successive modifications similar to mitosis but without nuclear envelope disappearance, metaphase plate arrangement and poleward movement of chromosomes in anaphase (Geitler Citation1953; Nagl Citation1978). Classical endomitosis seems to represent a specific cell cycle that retains cycles of DNA replication, chromosome condensation and transcriptional activity; however, this is a shortened cell cycle in which several key events of mitosis that result in chromosome distribution into daughter cells are omitted (Anisimov Citation1997a, Citation1997b, Citation1997c; Zybina and Zybina Citation2011, Citation2014). Endochromosomes stay visible throughout this cycle, but their degree of condensation varies at different phases of the endomitotic cycle (Sarto et al. Citation1982; Therman et al. Citation1983, Citation1986). As for transcriptional activity, it proves to be highest at the endointerphase whereas at endometaphase and endoanaphase it falls to a minimum (Anisimov Citation1997c). In other words, rather condensed endochromosomes and their transcriptional activity persist throughout the whole endomitotic cycle but undergo transformation in the course of this cycle. The latter confirms the conception of the endomitosis as a special kind of the transcriptionally active nucleus (Brodsky and Uryvaeva Citation1985). The data obtained here give an example of Ki-67 immunolabeling of endomitotic nuclei that prove the possibility of progression of cell cycle in the fox trophoblast cells. It is noteworthy that, distinct from the interphase and polytene nuclei, Ki-67 immunolabeling may prevail just in endochromosomes whereas nucleoli often show a weaker immunostaining with the exception of their periphery (). As the degree of condensation of endochromosomes is comparable with mitotic ones, it seems possible that in the endomitotic nuclei, Ki-67 relocalizes from nucleoli and heterochromatin and takes part in formation of the PCL by analogy to the mitotic PCL (Sheval and Polyakov Citation2008; Booth et al. Citation2014; Takagi et al. Citation2016; Matheson and Kaufman Citation2017). According to the recent data, Ki-67 supports mitotic chromosome architecture (Takagi et al. Citation2014, Citation2016). Recently it has been shown that Ki-67, as a component of the mitotic chromosome periphery, plays the role of a biological surfactant, thereby preventing chromosomes from collapsing into a single chromatin mass after nuclear envelope disassembly, thus enabling independent chromosome motility and efficient interactions with the mitotic spindle (Cuylen et al. Citation2016). By analogy to mitosis, Ki-67 probably plays a role in the formation of endochromosomes which can facilitate the transition of the trophoblast to the endomitotic cycle. It, in turn, may enable cell progression through endoprophase, endometaphase, endoanaphase and telophase.
On the other hand, in endointerphase Ki-67 plays a key role in the chromosome association with the nucleolus (Matheson and Kaufman Citation2017). A clear-cut association of the Ki-67-labeled non-classic polytene chromosomes with nucleoli was observed in the highly endopolyploid trophoblast cells of the silver fox. Thus, in the polytene cells the Ki-67 seems to promote further progression of genome replication cycles. Therefore, the present data on Ki-67 immunolabeling of polytene and endomitotic nuclei suggests that the classic endomitosis along with polytenization may represent the cell cycle modifications that ensure progression of genome reproduction in the course of differentiation of trabecular trophoblast cells in the silver fox placenta.
The nuclear fragmentation was described as a final step of depolytenization and, finally, depolyploidization in the giant trophoblast cells in the rodent placenta (Zybina and Zybina Citation1996). In these cells, the non-classic polytene chromosomes disintegrated into olygotene chromosome strands and then into endochromosomes, the nuclei subsequently undergoing fragmentation.
In the trabecular trophoblast of the fox placenta, pictures of nuclear fragmentation that results in depolyploidization also mostly correlates with cell cycle attenuation and accompanies cell degradation. In most cases, like in rodent placenta, it likely represents the terminal step of their differentiation. Meanwhile, in some cases this process is connected with preservation of a trophoblast cell population capable of cell/genome reproduction over a long time.
Cytophotometrical measurement of DNA content performed in our previous paper showed fluctuations of ploidy of silver fox trophoblast cells at the 19–23 day of gestation. The progressive polyploidization up to 16c–64c is observed at 19–20 gd (Zybina et al. Citation2001). Then, at 21 gd, a percentage of nuclei of lower ploidy increases. At the 22nd gd, the changes of ploidy move in two opposite directions: an increase in percentage of low-ploid (2–4c) nuclei is observed and, in parallel, highly polyploid (128c and 256c) nuclei arise. The two processes – progressive polyploidization and depolyploidization – may take place here simultaneously due to polytenization and depolytenization, with endomitosis possibly an intermediate stage.
Interestingly, some pathological processes including carcinogenesis imply the possibility of polyploidization cycles followed by depolyploidization that may result in the formation of actively proliferating cell clones. Thus, in experiments of irradiation and treatment with spindle inhibitor SK&F in the p53-mutated Burkitt lymphoma cells, a reserve mechanism was revealed that allows cells to overcome the impossibility of cell division (Erenpreisa et al. Citation2002). The cells became giant, then underwent lobulation, segmentation and budding with subsequent production of numerous micronuclei and subnuclei. Electron microscopy showed many similarities between the processes of budding of lymphoma cells (Erenpreisa et al. Citation2002) and fragmentation of the secondary giant trophoblast cells in the rat placenta (Zybina and Zybina Citation1996, Citation2008). Thus, in carcinogenesis, distinct from rodent giant trophoblast cells, depolyploidization is not the final step of the cell lifespan but can be a way to continue cell proliferation. Moreover, the polyploidization–depolyploidization cycle makes altered DNA repair possible (Erenpreisa et al. Citation2005, Citation2008) using meiosis-like mechanism(s) (Kalejs et al. Citation2006; Ianzini et al. Citation2009) as well as propagation of proliferative cell populations in which expression of stem cell markers has been shown (Erenpreisa, Cragg Citation2010).
The data obtained on the fox trophoblast cells showed that in normal development, and not only in carcinogenesis, there may be minor cell populations that may include depolyploidization as a mechanism of regular cell/genome reproduction.
illustrates the hypothetical transition ways between different states of the trabecular trophoblast cell nuclei characterized by different Ki-67 expression. In the region adjoining the endometrium the trophoblast cells keep their mitotic activity, giving rise to diploid or low-polyploid cells; the latter are achieved via polyploidizing (reduced) mitoses (Zybina et al. Citation2001; Zybina and Zybina Citation2005, Citation2014). This contributes to widening of the zone of trabecular trophoblast cells that subsequently differentiate and move into the depth of the fetal part of placenta. In the depth of spongy zone, a significant number of cells continue genome reproduction by switch to endocycles – polytenization and endomitosis. Polytenization, judging by the Ki-67 labeling patterns, promotes persistence of cell cycles for a long time, which makes it a way to preserve the reproductive potential of cells or genome and achievement of the highest ploidy levels (up to 256c). Meanwhile, other endopolyploid cell trophoblast cell populations leave a cell cycle that is, most probably, connected with their placenta-specific functions and degradation.
Figure 4. The hypothetical scheme of different modes of cell cycle modifications in the trabecular trophoblast cells in the course of their differentiation followed by degradation. In the region adjoining the endometrium the trophoblast cells actively proliferate and polyploidize via polyploidizing (reduced) mitoses, showing a high level of cell cycle progression; then some of the cells leave the cell cycle. The differentiated trabecular trophoblast cells continue genome reproduction cycles switching to endocycles – polytenization and endomitosis, thereby reaching high ploidy levels of 8–256c. The endomitotic nuclei may also result from depolytenization. In turn, it may lead to genome segregation. These processes are mostly accompanied by cell cycle attenuation with the exception of a minor cell population capable of cell cycle persistence. Ki-67-immunostained structures are shown by black color, Ki-67 immunonegative are gray.
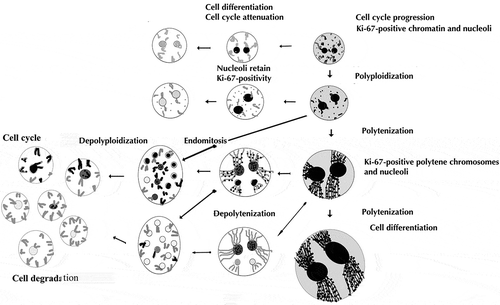
The endomitotic nuclei may arise in several ways, e.g. (1) low-ploid cells switching to endomitotic cycle; and (2) depolytenization like in the giant trophoblast cells of rodent placenta (Zybina and Zybina Citation1996). In this case, transformation of polytene nucleus implies dissociation of polytene chromosomes into separate chromatids, then forming endochromosomes. Such a transformation may promote easier chromosome redistribution in the course of genome segregation.
Judging by the present data, depolytenization and subdivision of nuclei of lower ploidy mostly results in formation of non-proliferative cell populations; it partly is followed by cell degradation. This degradation can be included in the developmental program of the fox placenta because, as we stated in the previous paper, the material that results from disintegration of trabecular trophoblast may be used to supply the embryo with nutrition (holocrine secretion, Zybina et al. Citation2016). Meanwhile, in a minor population of trabecular trophoblast cells, depolyploidization via non-mitotic genome segregation may take part in retention of cell reproductive potential.
The presence of trophoblast cell populations capable of self-renewal may be significant for maintenance of the barrier between semiallogenic fetal and maternal tissues during the lengthier (60 days) pregnancy as compared to rodent placenta. A diversity of cell reproduction patterns – regular and polyploidizing mitoses, endoreduplication, depolyploidization – may be important for maintaining a range of trophoblast cell populations with different functions.
Acknowledgments
The authors are grateful to Prof. Kazimir M. Pozharisski for providing the antibodies and other reagents and help in immunohistochemistry.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anisimov AP. 1997a. A study of genome multiplication mechanisms in the development of albumen gland cells of Succinea lauta (Gastropoda, Pulmonata). V. Polyploidizing mitosis and endomitosis. Tsitologiya. 39:218–228. Russian.
- Anisimov AP. 1997b. A study of genome multiplication mechanisms in the development of albumen gland cells of Succinea lauta (Gastropoda, Pulmonata). VI. Ultrastructural research of the endomitotic cycle. Tsitologiya. 39:229–236. Russian.
- Anisimov AP. 1997c. A study of genome multiplication mechanisms in the development of albumen gland cells of Succinea lauta (Gastropoda, Pulmonata). VII. Transcriptive activity of the nuclei in the endomitotic cycle. Tsitologiya. 39:237–243. Russian.
- Anisimov AP. 2005. Endopolyploidy as a morphogenic factor of development. Cell Biol Int. 29:993–1004.
- Becker K, Chule PN, Therrien JA, Lian JB, Stein J, van Wijnen AJ, Stein GS. 2006. Renewal of human embryonic stem cells is supported by shortened G1 cell cycle phase. J Cell Physiol. 209:883–893.
- Booth DG, Takagi M, Sanchez-Pulido L, Petfalski E, Varglu G, Samejima K, Imamoto N, Ponting CP, Tollerly D, Earnshaw WC, et al. 2014. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife. 3:e01641.
- Bridger JM, Kill IR, Lichter P. 1998. Association of pKi-67 with satellite DNA of the human genome in early G1 cells. Chromosome Res. 6:13–64.
- Brodsky V, Uryvaeva IV. 1985. Genome multiplication in growth and development. Cambridge: Univ. Press.
- Cuylen S, Blaukopf C, Politi AZ, Müller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA, Gerlich DW. 2016. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 535(7611):308–312.
- Edgar BA, Orr-Weaver TL. 2001. Endoreduplication cell cycle: more for less. Cell. 105:297–306.
- Erenpreisa J, Cragg MS. 2010. MOS, aneuploidy and the ploidy cycle of cancer cells. Oncogene. 29:1–5.
- Erenpreisa J, Ivanov A, Cragg MS, Selivanova G, Illidge TM. 2002. Nuclear envelope-limited chromatin sheets are parts of mitotic death. Histochem Cell Biol. 117:243–255.
- Erenpreisa J, Ivanov A, Wheatley SP, Kosmacek EA, Ianzini F, Anisimov AP, Mackey MA, Davis PJ, Plakhins G, Illidge TM. 2008. Endopolyploidy in irradiated p53-deficient tumour cell lines: persistence of cell division activity in giant cells expressing Aurora-B kinase. Cell Biol Int. 32:1044–1056.
- Erenpreisa J, Kalejs M, Ianzini F, Kosmacek EA, Mackey MA, Emzinsh D, Cragg MS, Ivanov A, Illidge TM. 2005. Segregation of genomes in polyploidy tumour cells following mitotic catastrophe. Cell Biol Int. 29:1005–1011.
- Geitler L. 1953. Endomitose and endomitotische Polyploidizierung. Protoplasmatologia. 60:1–89.
- Ianzini F, Kosmacek EA, Nelson ES, Napoli E, Erenpreisa J, Kalejs M, Mackey MA. 2009. Activation of meiosis-specific genes is associated with depolyploidization of human tumor cells following radiation-induced mitotic catastrophe. Cancer Res. 6:2296–2304.
- Kalejs M, Ivanov A, Plakhins G, Cragg MS, Emzinsh D, Illidge TM, Erenpreisa J. 2006. Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC Cancer. 6:6.
- Kill JR. 1996. Localization of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J Cell Sci. 109:1253–1263.
- Klisch K, Thomsen PD, Dantzer V, Leiser R. 2004. Genome multiplication is a generalised phenomenon in placentomal and interplacentomal trophoblast giant cells in cattle. Reprod Fertil Dev. 16:301–306.
- Matheson TD, Kaufman PD. 2017. The p150N domain of chromatin assembly factor-1 regulates Ki-67 accumulation on the mitotic perichromosomal layer. Mol Cell Biol. 28(1):21–29.
- Mühlhauser J, Crescimanno C, Kaufmann P, Hofler H, Zaccheo D, Castellucci M. 1993. Differentiation and proliferation patterns in human trophoblast revealed by c-erb-2 oncogene product and EGF-R. J Histochem Cytochem. 41:165–173.
- Muller HG. 1935. On the dimensions of chromosomes and genes in Dipteran salivary glands. Am Nat. 69:405–411.
- Nagl W. 1978. Endopolyploidy and polyteny in differentiation and evolution. Toward an understanding of quantitative and qualitative variation of nuclear DNA in ontogeny and phylogeny. Amsterdam, NY, Oxford: North Holland Publishing Company.
- Nagl W. 1995. Cdc-2 kinases, cyclins and the switch from proliferation to polyploidization. Protoplasma. 188:143–150.
- Sarto GE, Stubblefield PA, Therman E. 1982. Endomitosis in human trophoblast. Human Genetics. 62:228–232.
- Sheval EV, Polyakov VY. 2008. The peripheral chromosome scaffold, a novel structural component of mitotic chromosomes. Cell Biol Int. 32:708–712.
- Suurmeijer AJH, Boon ME. 1999. Pretreatment in a high-pressure microwave processor for MIB-1 immunostaining of cytological smears and paraffin tissue sections to visualize the various phases of the mitotic cycle. J Histochem Cytochem. 47:1015–1020.
- Takagi M, Natsume T, Kanemaki MT, Imamoto N. 2016. Perichromosomal protein Ki67 supports mitotic chromosome architecture. Genes Cells. 21:1113–1124.
- Takagi M, Nishiyama Y, Taguchi A, Imamoto N. 2014. Ki67 antigen contributes to the timely accumulation of protein phosphatase 1γ on anaphase chromosomes. J Biol Chem. 289:22877–22887.
- Therman E, Sarto G, Stubbliefield P. 1983. Endomitosis: a reappraisal. Hum Genet. 63:13–18.
- Therman E, Sarto GE, Kuhn EM. 1986. Cancer Genet Cytogenet. 19:301–310.
- Verheijen R, Kuijpers HJ, Schlingemann RO, Boehmer AL, van Driel R, Brakenhoff GJ, Ramaekers FC. 1989a. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J Cell Science. 92:123–130.
- Verheijen R, Kuijpers HJ, van Driel R, Beck JL, van Dierendonck JH, Brakenhoff GJ, Ramaekers FC. 1989b. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. localization in mitotic cells and association with chromosomes. J Cell Science. 92:531–540.
- Vorotelyak EA, Delone GV, Rippa AL, Uryvaeva IV. 2014. Immunohistochemical detection of the proliferation-associated nuclear pKi-67 in the hepatocyte cell cycle. Tsitologiya. 56:648–649. Russian.
- Zybina EV, Kudryavtseva MV, Kudryavtsev BN. 1979. The distribution of chromosome material during giant nucleus division by fragmentation in the trophoblast of rodents. Morphological and cytophotometrical study. Tsitologiya. 21:12–20. Russian.
- Zybina EV, Zybina TG. 1996. Polytene chromosomes in mammalian cells. Int Rev Cytol. 165:53–119.
- Zybina EV, Zybina TG. 2005. Cell reproduction and genome multiplication in the proliferative and invasive trophoblast cell populations of mammalian placenta. Cell Biol Int. 29:1071–1083.
- Zybina EV, Zybina TG. 2008. Modifications of nuclear envelope during differentiation and depolyploidization of rat trophoblast cells. Micron. 39:593–606.
- Zybina EV, Zybina TG, Bogdanova MS, Stein GI. 2005. Whole-genome chromosome distribution during nuclear fragmentation of giant trophoblast cells of Microtus rossiaemeridionalis studied with the use of gonosomal chromatin arrangement. Cell Biol Int. 29:1066–1070.
- Zybina T, Zybina E. 2011. Cell cycle modification in trophoblast cell population in the course of placenta formation. In: International ed. Kusic-Tisma J, editor. DNA replication and related cellular processes. Rijeka: InTech; p. 227–258. http://www.intechopen.com/articles/show/title/cell-cycle-modification-in-trophoblast-cell-populations-in-the-course-of-placenta-formation
- Zybina TG, Pozharisski KM, Stein GI, Kiknadze II, Zhelezova AI, Zybina EV. 2015. Placenta development and Ki-67 nuclear immunolocalization in placental tissues of the wild type and domesticated silver fox (Vulpes fulvus Desm). Annu Res Rev Biol. 8:1–12.
- Zybina TG, Pozharisski KM, Stein GI, Kiknadze II, Zhelezova AI, Zybina EV. 2016. Development, degradation and possible function of trabecular trophoblast in the course of placentation of silver fox Vulpes fulvus Desm. Annu Res Rev Biol. 10:1–9.
- Zybina TG, Zybina EV. 2014. Genome variation in the trophoblast cell lifespan: diploidy, polyteny, depolytenization, genome segregation. World J Med Genet. 4:77–94.
- Zybina TG, Zybina EV, Kiknadze II, Zhelezova AI. 2001. Polyploidization in the trophoblast and uterine glandular epithelium of the endotheloichorial placenta of silver fox (Vulpes fulvus Desm). Placenta. 22:490–498.
