ABSTRACT
The harmful potential of the Colorado potato beetle (Leptinotarsa decemlineata (Say)), and its resistance to many insecticides in current use have drawn the attention of specialists across the world. Starting from the progress made in the last three decades in the field of insect biotechnology, the current paper evaluates the cytogenetic effects of the ethanol extracts obtained from larvae and adults of L. decemlineata (Say) using the Allium test, as well as the catalase activity in the root meristematic cells of Allium cepa L. using the titrimetric method, with a view to extrapolate the utility of this species. According to the results of our study, the cytogenetic effects exerted by the ethanol extracts from larvae and adults of L. decemlineata (Say) were dependent not only on the concentration but also on the insect development stage. The oxidative stress exerted by the ethanol and also by the extracts of L. decemlineata (Say) was suggested by a more intense catalase activity.
Introduction
Leptinotarsa decemlineata (Say 1824) (Coleoptera: Chrysomelidae) is the most important defoliator of potato crops in the northern hemisphere, covering a surface of 16 million km2 in North America, Europe and Asia (Alyokhin et al. Citation2008; Weber Citation2008). Due to the presence of large populations of the species on Solanum rostratum and S. tuberosum in Colorado in 1867, the popular name of the species is the Colorado potato beetle (CPB) (Alyokhin Citation2009; Jacques and Fasulo Citation2015). It is considered that the species originates from central Mexico, where it lives on wild solanaceous species, such as S. rostratum and S. angustifolium, as many of the species of the genus Leptinotarsa are endemic to this region (Alyokhin et al. Citation2008; Jacques Citation1988; Alyokhin Citation2009). In this context, the successful expansion of this subtropical native species into vast temperate areas is remarkable, given the fact that it even managed to reach China in only a century and a half (Izzo et al. Citation2014; Guo et al. Citation2017). Its adaptability to the various environmental conditions and the entry into diapause ensured its success even in the cold northern regions; at present, the species has permanent populations in Estonia (Hiiesaar et al. Citation2006) and Siberia, up to the border with the Krasnoyarsk Territory (Popova Citation2014).
The harmful potential of this species and its ability to develop resistance to 56 insecticides in the last seven decades have led to the pesticide industry’s efforts towards a more efficient control of the pest, which resulted in increasing pressure on the environment (Alyokhin et al. Citation2008; Whalon et al. Citation2008). Today it is obvious that responsible control of the pest populations requires an integrated management approach, with a balance between chemical and environmentally friendly methods, including alternative agricultural practices, non-toxic insecticides, biological control, and physical methods (Rifai et al. Citation2004; Alyokhin et al. Citation2015; Guo et al. Citation2017; Gabaston et al. Citation2018). In this regard, it is imperative to increase the number of research studies in the field of genetics and molecular biology, as well as to improve collaboration between specialists from different scientific disciplines (Cingel et al. Citation2016; Kaplanoglu et al. Citation2017). Kumar et al. (Citation2014) emphasizes the importance of this species in the field of applied biology, including invasive biology, insect phenology and pest management. The recent interest of specialists in insect biotechnology (Ejiofor Citation2016), together with the extraordinary reproductive capacity of CPB, has led to studies into the beneficial effects of insect extracts with antimicrobial, insecticidal, and medicinal activity against this pest species (Luo et al. Citation2012; Seabrooks and Hu Citation2017).
The current research focuses on the study of the antioxidant activity and the cytogenetic effects of ethanol extracts from larvae and adults of L. decemlineata (Say) exerted on the root meristematic cells of Allium cepa L. and on the general mitotic index and the different division stages. Its aim is to expand the utility of the species L. decemlineata (Say).
Materials and methods
Preparation of ethanol extracts ofL. decemlineata (Say)
Specimens of CPB were collected in May 2017, from aerial potato organs from a private garden, located in the southern sub-Carpathian region of Romania. The GPS coordinates are N 45°08ʹ13.2″E, 024°47ʹ24.6″N, 452 m asl. The potato crop occupying a surface of 640 m2 had not been treated with pesticides up to the moment when we collected the insects. The larvae and adults of L. decemlineata (Say) were separated in the laboratory based on their morphological characteristics and they were preserved in the freezer until the preparation of extracts. Adult and larval specimen were kept at −18°C in the Invertebrate Zoology laboratory of the Department of Natural Sciences, University of Piteşti.
For the preparation of the ethanol extracts, 5 g of insects for each development stage of the CPB were ground and transferred into 100 ml absolute ethanol to macerate for 48 h. The extracts filtered through Whatman filter paper no. 1 were used to evaluate the catalase activity and the cytogenetic potential in the root meristematic cells of A. cepa L.
Evaluation of cytogenetic effects
In order to evaluate the cytogenetic effects of the extracts of L. decemlineata (Say) we used bulbs of A. cepa L. (a local variety) with a diameter of approximately 5 cm, harvested in the spring of the same year.
The experimental samples were defined by the concentration of the ethanol extracts obtained from the larvae of L. decemlineata (Say) as Extract Larvae Leptinotarsa (ELL) 100 ml l−1, ELL 200 ml l−1, ELL 300 ml l−1, and from adults of L. decemlineata (Say) as Extract Adult Leptinotarsa (EAL) 100 ml l−1, EAL 200 ml l−1, EAL 300 ml l−1, respectively, which were compared with the control sample and with the equivalent concentrations of alcohol. For all the experimental samples, the onion bulbs were kept in hydroponic culture in tap water, in the dark, for 48 h. The roots that formed were transferred into the ethanol extracts from larvae and adults. After 24 h of exposure to the action of the alcohol extracts of L. decemlineata (Say), the roots ranging between 5 and 10 mm in length were detached from the bulbs and fixed in a mixture of absolute ethanol and glacial acetic acid 3:1 for 24 h. The roots that were not used immediately for microscopic preparations were preserved in 70° ethanol.
Five to seven roots of the fixed biological material were rinsed with distilled water and then transferred to watch glasses containing 10–15 ml of HCl (1 N), previously heated at 60°C. The hydrolysis of the plant material was performed at 60°C, in the thermostat, for 15 min. The elective chromosome colouring was done through immersing the hydrolysed plant material into 2% orcein acetic solution for 15 min, at 60°C. In order to enhance the colour contrast between the chromosomes and the cytoplasm, the coloured roots were subjected to brief rinsing with 45% acetic water.
The root meristematic tip was placed on the object slide in a drop of 45% acetic water, covered with a slide and displayed through the squash technique. The photomicrographs were captured through an Olympus CX-31 microscope (Olympus Corporation, Tokyo, Japan, made in Philippines), at 400× magnification. The microscopic analysis consisted in determining the number of cells at different mitotic phases, the frequency of chromosomal aberrations and nuclear anomalies reported to a number of approximately 3000 cells for each experimental sample. The mitotic index was calculated as the percentage ratio of the number of cells in mitosis and the total number of cells analysed (Tedesco and Laughinghouse Citation2012). The percentage ratios of the cells in prophase, metaphase, anaphase and telophase were calculated based on the total number of cells in mitosis. The frequency of chromosomal aberrations and nuclear anomalies was determined by reference to the adequate phase of the cell cycle and mitosis.
Evaluation of catalase activity
In order to evaluate the catalase activity, we applied the titrimetric method, through which we determined the amount of H2O2 decomposed by the catalase, by measuring the remaining peroxide. Therefore, 1 g of roots was ground together with 0.5 g of CaCO3 and then homogenized in distilled water to a volume of 100 ml. After 24 h in the refrigerator, the mixture was filtered through cotton wool. For the control sample, a volume of 5 ml of the obtained filtrate was kept for 15 minutes on the water bath to boil, and thus to inactivate the catalase. Immediately after cooling, 5 ml of diluted H2O2 and then 2 ml of H2SO4 were added to stop the reaction. The active sample was that containing 5 ml of filtrate, to which we added 5 ml of diluted H2O2, and after 15 min, 2 ml of H2SO4 to stop the reaction. The excess of H2SO4 was titrated with KMnO4 (0.1 N) until it became light pink, but the colour did not last more than 30 s.
The catalase activity was expressed in ml of KMnO4 0.1 N/g of fresh substance. We calculated the difference between the quantity of KMnO4 used to titrate the control sample (C) and that used to titrate the active sample (Ac), as follows:
Statistical interpretation of the results
The values of the general mitotic index (GMI) were determined as percentage ratios of the total number of cells in any of the mitotic phases to the total number of cells examined in the microscopic preparation. The values of the index for each of the phases of mitotic division were calculated as percentage ratios of the total number of cells in a certain mitotic phase (prophase, metaphase, anaphase, telophase) to the total number of cells examined in the microscopic preparation. The relative division ratio (RDR) was calculated according to Equation (1) proposed by Hoda et al. (Citation1991):
The frequency of chromosomal and nuclear aberrations was calculated as percentage ratios of each type of modification to the total number of cells in the corresponding phase of mitotic division, and the total number of cells in the interphase of the cell cycle, respectively.
In order to process the experimental results, we utilized the statistical analysis program SPSS for Windows (Statistical Package for Social Science, Armonk, NY: IBM Corp.), version 20.0 (2010), applying the one-way ANOVA model and the Duncan test (for multiple comparison), respectively. The significance of the differences between the effects of the experimental factors or their interactions, for which the calculated F had significant values at a 95% confidence level, was noted using lower-case letters.
Results
Mitotic activity in the root meristematic cells ofA. cepa L.
shows the statistical interpretation of the results on the variation of MI in the meristematic cells of A. cepa L., dependent on the insect development stage and the extract concentration, compared with the control sample and the equivalent ethanol concentrations, as well as the RDR values.
Figure 1. Variation of the mitotic index and relative division rate in the meristematic cells of A. cepa L. exposed to the treatment with ethanol extracts from larvae and adults of L. decemlineata (Say). The data are the mean values ± SE of three replicates; a, b, c – interpretation of statistical significance and significant differences according to the Duncan test, p < 0.05.
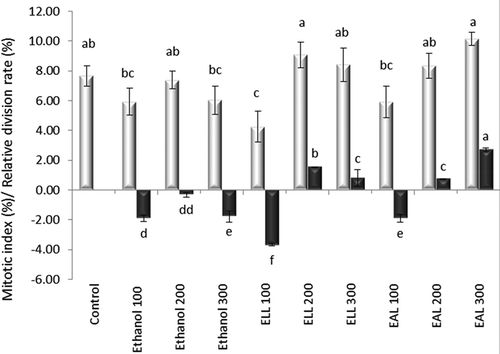
In the control, a percentage of 7.66% of the root meristematic cells was represented by those in different phases of cell division. By incubating the roots in the control solvent with a concentration equivalent to those of the extracts, the proportion of the cells in interphase increased insignificantly, for p < 0.05. The ethanol extracts (100 ml l−1) from larvae of L. decemlineata (Say) had a significant mitoinhibitory effect compared with the control sample and an insignificant one compared with the ethanol extracts from adults. The increase in the concentrations of the extracts, both of those obtained from larvae and of those obtained from adults of L. decemlineata (Say), induced an insignificant increase of the MI compared with the control sample. An increase dependent on the dosage was determined only for the ethanol extracts from adults.
Except for the EAL (300 ml l−1) experimental sample, which was noted for its significantly higher value of the MI (10.14%), the extract concentrations tested did not determine significant changes of the MI compared with the equivalent concentrations of the solvent used. The mitostimulatory effect was obvious with the increase in the extract concentration and more pronounced for the extracts from adults. The incubation in EAL (300 ml l−1) induced an over 32% increase of the mitotic activity compared with the control and over 68% compared with the equivalent solvent concentration. The negative values of RDR were calculated for the experimental samples defined by ethanol – 100 ml l−1 (−1.88%), 200 ml l−1 (−0.32%) and 300 ml l−1(−1.77%), as well as for the concentration of 100 ml l−1 of the extracts from larvae (−3.69%) and adults (−1.91%).
Distribution of the phases of mitotic division
In the control roots the distribution of the mitotic phases was balanced; the values of the indices were 53.95% for the prophase index, 15.23% for the metaphase index, 15% for the anaphase index and 15.79% for the telophase index (). The immersion of the roots into ethanol extracts from larvae and adults of L. decemlineata (Say) for 24 h led to an increase in the percentage of prophases in those samples characterized by a low mitotic index, such as the EAL (100 ml l−1) and ELL (100 ml l−1). The high prophase index was associated with a low metaphase index in the EAL 100 ml l−1 experimental sample. The metaphase index increased to 31.47%, 20.53%, 18.54% in ethanol at 100, 200 and 300 ml l−1, respectively. A balanced distribution of the phases of mitotic division in the root meristematic tips of the onion, similar to that observed for the control sample, was also determined for the EAL (200 ml l−1) and EAL (300 ml l−1).
Chromosomal and nuclear aberrations in the root meristematic cells ofA. cepa L.
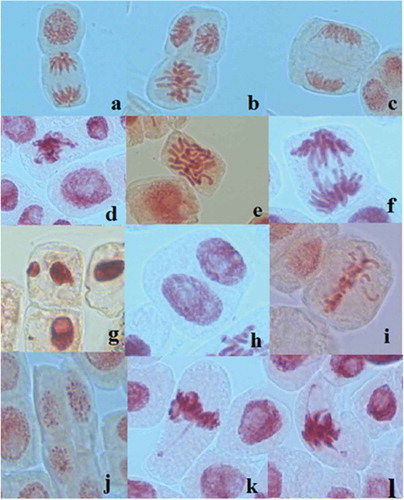
presents the types of chromosomal aberrations and their frequency in the meristematic tips of A. cepa L. incubated in ethanol extracts from larvae and adults of L. decemlineata (Say). The microscopic analysis of the preparations and the statistical interpretation of the results pointed to the significantly higher frequency of chromosomal aberrations and nuclear anomalies in the root meristematic cells of onion incubated in ethanol, compared with those treated with extracts of L. decemlineata (Say), regardless of their concentration, thus suggesting the genoprotective effect of the extracts from larvae and adults of CPB. This significantly higher frequency of chromosomal and nuclear aberrations was also accompanied by a higher diversity, such as sticky chromosomes, C-mitosis, binucleated cells, micronuclei, anaphase bridges, as well as other modifications that were observed sporadically in the microscopic preparations (). Significantly lower percentages of chromosomal aberrations were calculated for the experimental samples ELL (100 ml l−1) and EAL (300 ml l−1) and the highest frequency was determined for ethanol 100 ml l−1. The analysis of the microscopic preparations highlights the reduction in the frequency of micronuclei in the ELL (100 ml l−1) experimental sample and their absence in the root meristematic cells exposed to the treatment with ELL (300 ml l−1). Micronuclei were observed with a low frequency (maximum 0.1% in EAL 100 ml l−1).
Table 1. Frequency of chromosomal and nuclear aberrations in the meristematic cells of A. cepa L. incubated in extracts of L. decemlineata (Say).
Figure 2. Distribution of the phases of mitotic division (prophase, metaphase, anaphase, telophase) in the root meristematic tips of A. cepa L. exposed to the treatment with ethanol extracts from larvae and adults of L. decemlineata (Say). The data are the mean values ± SE of three replicates; a, b, c, d, e, f, g, h, i, j, k – interpretation of statistical significance and significant differences according to the Duncan test, p < 0.05.
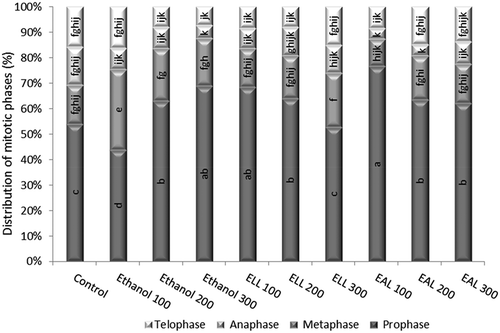
Figure 3. Chromosomal aberrations and nuclear anomalies observed in the root meristematic cells of A. cepa L. incubated in ethanol extracts from larvae and adults of L. decemlineata (Say): (a) prophase and anaphase (ethanol 200 ml l−1); (b) metaphase (ethanol 200 ml l−1); (c) telophase (ethanol 200 ml l−1); (d) sticky chromosomes (control 100 ml l−1); (e) C-mitosis (EAL 200 ml l−1); (f) anaphase bridge (ethanol 200 ml l−1); (g) micronucleus (ELL100 ml l−1); (h) binucleated cell (EAL 300 ml l−1); (i) vagrant chromosomes (ethanol 200 ml l−1); (j) apoptotic bodies (ethanol 200 ml l−1); (k, l) despiralization of the distal part of the chromosome arms (ethanol 100 ml l−1).
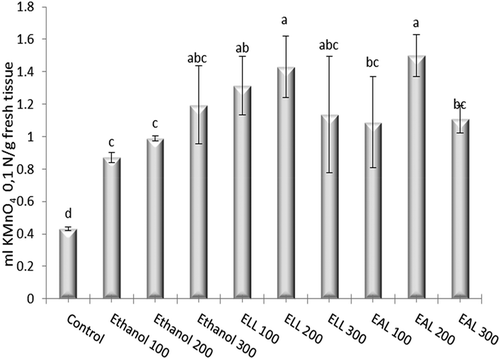
The results of the catalase activity in the onion roots exposed to different treatments, as presented above, are shown in . The catalase activity was significantly more intense in the cells incubated in ELL 200 ml l−1 and EAL 200 ml l−1, compared with the control and the equivalent solvent concentrations (p < 0.05). At the same time, the lowest antioxidant activity was recorded for the control sample (0.43 ml KMnO4 0.1 N/g fresh tissue), which reflects the oxidative stress exerted both by ethanol, and especially by the extracts of L. decemlineata (Say).
Figure 4. Catalase activity in the meristematic cells of A. cepa L. incubated in extracts of L. decemlineata (Say). The data are the mean values ± SE of three replicates; a, b, c, d – interpretation of the statistical significance and of the significant differences according to the Duncan test, p < 0.05.
Discussion
The Allium test is largely applied in the evaluation of cytotoxicity for different substances (Pesnya Citation2013; Firbas and Amon Citation2014; cited in Ślusarczyk et al. Citation2014), as it ensures an easy analysis of the cytologic parameters as indicators of the mitostimulatory/mitoinhibitory activity and of the clastogenic/aneugenic/turbagenic effects, as well as through the correspondence with other bioindicators (Fiskesjö Citation1985). Anti-proliferative, genotoxic and anti-genotoxic effects of some medicinal plants were evaluated using the A. cepa L. assay (Oyeyemi and Bakare Citation2013; Ös and Çelik Citation2014; Coelho et al. Citation2017; Kundu and Ray Citation2017; Pesnya et al. Citation2017).
The studies on the chemical composition of some insects underlined the presence of glycerol and amines as major components of the alcoholic extracts, α-carotenes, xanthophylls, chlorophyll in the period of active feeding (Li et al. Citation2003), hydrocarbons, fatty acids, alcohols, diols, esters, ethers, ketones, aldehydes and oxoaldehydes (Golębiowski et al. Citation2011) and different toxic substances. The hemolymph of the CPB contains the protein leptinotarsin, with toxicity similar to that of bee venom, organic phosphates and cholinergic drugs (Hsiao and Fraenkel Citation1969). Leptinotarsin-D is lethal for insects and vertebrates (Hsiao and Fraenkel Citation1969); in mammals it acts as a neurotoxin (Miljanich et al. Citation1988), paralysing the cardiac muscle (Bortels Citation1994, cited in Sablon et al. Citation2013).
In our study, the increase of mitotic activity in the onion root meristems may be due to the potentially existing amines from the extracts of L. decemlineata (Say). Some studies revealed the genotoxic and proliferative potential of amines on animal cells (Romen and Bannasch Citation1972; Grant et al. Citation1990; Das et al. Citation1994). The low number of cells in division identified in the meristematic tips subjected to the treatment with extracts from larvae and adults with a concentration of 100 ml l−1 suggests their incapacity to combat the mitoinhibitory effect of the solvent. At the same time, the increase in the MI associated with the higher concentrations of the tested extracts suggests their cytotoxicity and effectiveness in minimizing the mitoinhibitory effect of the ethanol. The positive values of the RDR determined only for high extract concentrations confirm their mitostimulatory effect.
The decrease of the mitotic index may be generated by inhibiting the DNA synthesis (Schneiderman et al. Citation1971) or by blocking the cell cycle in the G2 phase (Van’t Hof Citation1968). According to Leme and Marin-Morales (Citation2009), the significant increase of the MI compared with the control may lead to a disordered cell proliferation and even to the formation of tumours. In our study, the MI increase determined by a concentration of 300 ml l−1 was not significantly higher, but further studies targeting the testing of other concentrations, as well as the extracts obtained using other solvents should be conducted. The increase of the prophase and metaphase indices and the decrease of the anaphase and telophase indices are due to the malfunctioning of the mitotic spindle under the action of the solvent and of the low concentration extracts. In agreement with Prokhorova et al. (Citation2008) the increase in the frequency of prophases is due to the disruption of the supramolecular structure of the chromosomes.
The C-mitoses observed in the root meristematic cells incubated in ethanol are an indicator of its genotoxicity exerted through partial or complete inactivation of the mitotic spindle. In its absence, the equatorial plate does not form and diploid cells are generated (Fiskesjö Citation1985; Leme and Marin-Morales Citation2009; cited in Ventura-Camargo and Marin-Morales Citation2016). The sticky chromosomes are considered to be an indicator of toxic effects, leading to irreversible cell damage and cell death (Fiskesjö Citation1997). It is believed that the formation of sticky chromosomes is the result of a more intense contraction and condensation of the chromatin fibres or of DNA depolymerization and partial dissolving of nucleoproteins (Kaufmann et al. Citation1955; Klasterska et al. Citation1976), or of the inter-chromosomal links of the sub-chromatid strands associated with an excess formation of nucleoproteins and an incorrect interproteic interaction (Nefic et al. Citation2013). In the absence of migration, the chromosomes remain in a condensation process (Fernandes et al. Citation2009). The despiralization of the distal part of the metaphase chromosomes could lead to the incorrect segregation of chromatids and formation of micronuclei.
Catalase is an enzyme with a moderate antioxidant activity, which decomposes hydrogen peroxide into water and oxygen (Loewen et al. Citation1985). When often subjected to the stress produced by the environmental factors that generate reactive oxygen species and implicitly to oxidative stress (Elstner et al. Citation1988; Baisak et al. Citation1994; Shah et al. Citation2001), plants develop different resistance mechanisms in order to tolerate stressful periods, such as modifications of the lipid compounds, isoenzymes and enzyme activity, of the content of amino acids and sugars, soluble proteins and expression of genes. These adaptations involve some metabolic modifications that may ensure competitive advantages or may affect the survival capacity in plants (Schützendübel and Polle Citation2002). In the current study the increase of the catalase activity in the experimental samples ELL (200 ml l−1) and EAL (200 ml l−1) may be the consequence of an adaptation of the antioxidant enzyme system to the ROS production. According to Li et al. (Citation2003), the carotenoids, which are compounds with multiple unsaturated bonds that can store oxygen, may have an antioxidant activity, but in our study, the catalase exhibited a more intense activity compared with the control. Additionally, just as in tumour cells, the increase of oxidative stress supports cell proliferation as a result of aerobic glycolysis, fermentative reactions and of the generated secondary metabolites (Schieber and Chandel Citation2014; Mittler Citation2017).
Conclusion
The increase in the values of the general mitotic index was associated with the increase of the concentration of the extracts from larvae and adults of L. decemlineata (Say). The extracts from larvae showed cytostatic properties and low toxicity. The frequency of chromosomal aberrations and nuclear anomalies, as well as their diversity, was lower in the root meristematic cells exposed to the treatment with alcoholic extracts, thus suggesting their genoprotective effect. The oxidative stress exerted both by the ethanol and especially by the extracts of L. decemlineata (Say) was manifested through a more intense catalase activity. The results of the current study are important as they emphasize the antigenotoxic effect of the extracts of L. decemlineata (Say), both at the larval and adult stages. However, further studies are needed (using in vitro and in vivo test systems) in order to provide clear conclusions on this topic.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alyokhin A. 2009. Colorado potato beetle management on potatoes: current challenges and future prospects. Fruit Veg Cereal Sci Biotech. 3(1):10–19. http://works.bepress.com/andrei_alyokhin/5/
- Alyokhin A, Baker M, Mota-Sanchez D, Dively G, Grafius E. 2008. Colorado potato beetle resistance to insecticides. Am J Potato Res. 85(6):395–413.
- Alyokhin A, Mota-Sanchez D, Baker M, Snyder WE, Menasha S, Whalon M, Dively G, Moarsi WF. 2015. The red queen in a potato field: integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag Sci. 71(3):343–356.
- Baisak R, Rana D, Acharya PBB, Kar M. 1994. Alteration in the activities of active oxygen scavenging enzymes of wheat leaves subjected to water stress. Plant Cell Physiol. 35:489–495.
- Bortels AA. 1994. Cation selective channel induced by two protein fractions from the larval hemolymph of the Colorado potato beetle, Leptinotarsa decemlineata (Say) an electrophysiological characterization on Guinea-pig ventricular myocytes and on artificial membranes. Leuven (Belgium): K.U.Leuven.
- Cingel A, Savić J, Lazarević J, Ćosić T, Raspor M, Smigocki A, Ninković S. 2016. Extraordinary adaptive plasticity of Colorado potato beetle: “Ten-Striped Spearman” in the era of biotechnological warfare. Int J Mol Sci. 17(9):1538.
- Coelho APD, Laughinghouse HD IV, Kuhn AW, Boligon AA, do Canto-Dorow TS, da Silva ACF, Tedesco SB. 2017. Genotoxic and antiptoliferative potential of extracts of Echinodorus grandiflorus and Sagittaria montevidensis (Alismataceae). Caryologia. 70(1):82–91.
- Das L, Das SK, Hooberman BH, Chu EHY, Sinsheimer JE. 1994. Chromosomal aberrations in mouse lymphocytes exposed in vitro and in vivo to benzidine and 5 related aromatic amines. Mutat Res. 320(1–2):69–74.
- Ejiofor AO. 2016. Insect Biotechnology. In: Raman C, Goldsmith MR, Agunbiade TA, editors. Short views on insect genomics and proteomics. Entomology in focus, Vol. 4. Switzerland: Springer International Publishing; p. 185–210.
- Elstner EF, Wagner GA, Schutz W. 1988. Activated oxygen in green plants in relation to stress situation. Curr Top Plant Biochem Physiol. 7:159–187.
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. 2009. Origin of nuclear and chromosomal alterations derived from the action of an aneugenic agent - Trifluralin herbicide. Ecotoxicol Environ Safety. 72:1980–1986.
- Firbas P, Amon T. 2014. Chromosome damage studies in the onion plant Allium cepa L. Caryologia. 67(1):25–35.
- Fiskesjö G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102:99–112.
- Fiskesjö G. 1997. Allium test for screening chemicals; evaluation of cytological parameters. In: Wang W, Gorsuch JW, Hughes JS, editors. Plants for environmental studies. Boca Raton (New York): CRC Lewis Publishers; p. 307–333.
- Gabaston J, El Khawand T, Waffo-Teguo P, Decendit A, Richard T, Mérillon JM, Pavela R. 2018. Stilbenes from grapevine root: a promising natural insecticide against Leptinotarsa decemlineata. J Pest Sci. 1–10. doi:10.1007/s10340-018-0956-2
- Golębiowski M, Boguś MI, Paszkiewicz M, Stepnowski P. 2011. Cuticular lipids of insects as potential biofungicides: methods of lipids composition analysis. Anal Bioanal Chem. 399:3177–3191.
- Grant AL, Thomas JW, Liesman JS. 1990. Effects of dietary amines on small intestinal variables in neonatal pigs fed soy protein isolate. J Anim Sci. 68(2):363–371.
- Guo W, Li C, Ahemaiti T, Jiang W, Li G, Wu J, Fu K. 2017. Colorado potato beetle Leptinotarsa decemlineata (Say). In: Wan F, Jiang M, Zhan A, editors. Biological invasions and its management in China. Invading nature - Springer series in invasion ecology (Vol. 11). Dordrecht: Springer; p. 195–217.
- Hiiesaar K, Metspalu L, Jõudu J, Jõgar K. 2006. Over-wintering of the Colorado potato beetle (Leptinotarsa decemlineata Say) in field conditions and factors affecting its population density in Estonia. Agron Res. 4(1):21–30.
- Hoda Q, Bose S, Sinha SP. 1991. Vitamin C mediated minimization of Malathion and Rogor induced mitoinhibition and clatogeny. Cytologia. 56:389–397.
- Hsiao TH, Fraenkel G. 1969. Properties of leptinotarsin: A toxic hemolymph protein from the Colorado potato beetle. Toxicon. 7(2):119–128.
- Izzo VM, Hawthorne DJ, Chen YH. 2014. Geographic variation in winter hardiness of a common agricultural pest, Leptinotarsa decemlineata, the Colorado potato beetle. Evol Ecol. 28:505–520.
- Jacques RL. 1988. The potato beetles. The genus Leptinotarsa in North America (Coleoptera: chrysomelidae). Flora & Fauna Handbook Series No. 3. Leiden: Brill.
- Jacques RL, Fasulo TR 2015. Colorado potato beetle, Leptinotarsa decemlineata (Say), and false potato beetle, Leptinotarsa juncta (Germar) (Insecta: coleoptera: chrysomelidae). EENY146/IN303 http://edis.ifas.ufl.edu/in303.
- Kaplanoglu E, Chapman P, Scott IM, Donly C. 2017. Overexpression of a cytochrome P450 and a UDP-glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Sci Rep. 7:1762. http//:www.nature.com/scientificreports/
- Kaufmann BP, McDonald MR, Bernstein MH. 1955. Cytochemical studies of changes induced in cellular materials by ionizing radiations. Ann N Y Acad Sci. 59(4):553–566.
- Klasterska I, Natarajan AT, Ramel C. 1976. An interpretation of the origin of subchromatid aberrations and chromosome stickiness as a category of chromatid aberrations. Hereditas. 83(2):153–169.
- Kumar A, Congiu L, Lindström L, Piiroinen S, Vidotto M, Grapputo A. 2014. Sequencing, de novo assembly and annotation of the Colorado potato beetle, Leptinotarsa decemlineata, transcriptome. PLoS ONE. 9(1):e86012.
- Kundu LM, Ray S. 2017. Mitotic abnormalities and micronuclei inducing potential of colchicine and leaf aqueous extracts of Clerodendrum viscosum Vent in Allium cepa root apical meristem cells. Caryologia. 70(1):7–14.
- Leme DM, Marin-Morales MA. 2009. Allium cepa test in environmental monitoring: a review on its application. Mutat Res. 82:71–81.
- Li NG, Osakovskii VL, Ivanova SS. 2003. Chemical composition and cryoprotective activity of ethanol extract from wintering caterpillars of the black-veined white Aporia crataegi L. Biol Bull. 30(5):453–457.
- Loewen PC, Switala J, Triggs-Raine BL. 1985. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 243:144–149.
- Luo XH, Wang XZ, Jiang HL, Yang JL, Crews P, Valeriote FA, Wu QX. 2012. The biosynthetic products of chinese insect medicine, Aspongopus chinensis. Fitoterapia. 83(4):754–758.
- Miljanich GP, Zeager RE, Hsiao TH. 1988. Leptinotarsin-D, a neurotoxic protein, evokes neurotransmitter release from, and calcium flux into, isolated electric organ nerve terminals. J Neurobiol. 19(4):373–386.
- Mittler R. 2017. ROS are good. Trends Plant Sci. 22(1):11–19.
- Ös A, Çelik TA. 2014. Protective effect of lycopene on ethyl methane sulfonate induced chromosome cberrations in Allium cepa. Caryologia. 59(3):220–225.
- Nefic H, Musanovic J, Azra M, Kurteshi K. 2013. Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by Alprazolam. Med Arch. 67(6):388–392.
- Oyeyemi IT, Bakare AA. 2013. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia. 66(4):360–367.
- Pesnya DS. 2013. Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia. 66(3):275–281.
- Pesnya DS, Romanovsky AV, Serov DA, Poddubnaya NY. 2017. Genotoxic effects of Heracleum sosnowskyi in the Allium cepa test. Caryologia. 70(1):55–61.
- Popova EN. 2014. The influence of climatic changes on range expansion and phenology of the Colorado potato beetle (Leptinotarsa decemlineata, Coleoptera, Chrysomelidae) in the territory of Russia. Entomol Rev. 94(5):643–653.
- Prokhorova IM, Kovaleva MI, Fomicheva AN, Babanazarova OV. 2008. Spatial and temporal dynamics of mutagenic activity of water in lake Nero. Inland Water Biol. 1(3):1–25.
- Rifai NM, Astatkie T, Lacko-Bartošová M, Otepka P. 2004. Evaluation of thermal, pneumatic and biological methods for controlling Colorado potato beetles (Leptinotarsa decemlineata Say). Potato Res. 47:1. doi:10.1007/BF02731967
- Romen W, Bannasch P. 1972. Karyokinesis and nuclear morphology during hepatocarcinogenesis. I. Mitosis and mitotic abnormalities in hepatocytes and hepatoma cells of the nitrosomorpholine-intoxicated rat liver. Virchows Arch B. 11:24–33.
- Sablon L, Dickens JC, Haubruge É, Verheggen FJ. 2013. Chemical ecology of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: chrysomelidae), and potential for alternative control methods. Insects. 4(1):31–54.
- Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
- Schneiderman MH, Dewey WC, Highfield DP. 1971. Inhibition of DNA synthesis in synchronized Chinese hamster cell treated in G1 with cycloheximide. Exp Cell Res. 67(1):147–155.
- Schützendübel A, Polle A. 2002. Plant response to abiotic stress: heavy metal-induced oxidative stress and protection by mycorrhization. Exp Bot. 53:1351–1365.
- Seabrooks L, Hu L. 2017. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sin B. 7(4):409–426.
- Ślusarczyk J, Dudek M, Wierzbicka M, Suchocki P, Kuraś M. 2014. Antimitotic effect of Selol and sodium selenate (IV) on Allium test cells. Caryologia. 67:250–259.
- Shah K, Kumar RG, Verma S, Dubey RS. 2001. Effect of cadmium on lipid peroxodation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 161:1135–1144.
- Tedesco SB, Laughinghouse HD IV. 2012. Bioindicator of genotoxicity: the Allium cepa test. In: Srivastava JK, editor. Environmental contamination. Rijeka: Tech Publisher; p. 137–156.
- Van’t Hof J. 1968. The action of IAA and kinetin on the mitotic cycle of proliferative and stationary phase excised root meristem. Exp Cell Res. 51(1):167–176.
- Ventura-Camargo BC, Marin-Morales MA. 2016. Micronuclei and chromosome aberrations derived from the action of Atrazine herbicide in Allium cepa meristematic cells. SDRP J Earth Sci Environ Stud. 1(1):1–7.
- Weber DC. 2008. Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: chrysomelidae). In: Capinera JL, editor. Encyclopedia of Entomology. Dordrecht: Springer.
- Whalon ME, Mota-Sanchez D, Hollingworth RM 2008. Arthropod pesticide resistance database. Michigan State University. http://www.pesticide.resistance.org/index.php.
