?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Designed to increase safety of people and property during their different stages of life (storage, transportation, handling), low-vulnerability ammunitions (LOVA) can lead to ignition issues during their use. Ignition and combustion characteristics of two LOVA RDX-based gun propellants are experimentally investigated in this paper. The ignition is performed by a laser diode, and combustion is studied in a closed-volume reactor for different initial pressures (10–70 bar) and laser powers (1.39 to 10.13 W). The present study especially focuses on the influence of atmosphere nature: new results under synthetic air are compared to results previously obtained under argon and nitrogen. Air appears to have a different effect on combustion properties following the nature of the propellant. For P1, composed of RDX and HTPB, the highest overpressures and propagation rates are obtained under air. On the contrary, similar performances have been encountered under air and argon for the RDX/NC propellant P2. Ignition delays and probabilities, as for them, seem to be more sensitive to laser power than to atmosphere nature.
Introduction
Gun propellants are energetic materials especially employed for weapon propulsion. Historically, nitrocellulose (NC) was widely used for the formulation of solid smokeless gun propellants like single or double-base gun propellants. Nevertheless, the quest for safer gun propellants led to the decrease of the nitrocellulose content for more stable ingredients. For this purpose, RDX represents an excellent candidate which also enables high specific impulse along with less smoke, toxicity, and corrosion. The combination of these two ingredients perfectly embodies the general concept of the low vulnerability ammunitions (LOVA), designed to be both safer and no less effective than before. However, the appearance of this new ammunition kind, more difficult to ignite, also requires the development of new igniters. With its numerous advantages, among repeatability, reliability, and precise spatial and temporal control, the laser ignition represents a serious alternative. Since the early 1960s, it has been the subject of numerous studies about the ignition or initiation of energetic materials (Ahmad and Cartwright Citation2014). Our work is focused on the laser ignition and combustion of insensitive gun propellants. Few data are available on the ignition behavior and the combustion characteristics of this kind of propellant. On the contrary, studies seem rather inclined toward more sensitive materials by incorporation of nano-additives (Song et al. Citation2021; Uhlenhake et al. Citation2022). Fang, Stone, and Stennett (Citation2020) recently demonstrated that incorporation of gold nanoparticles in RDX crystals could reduce thresholds by an order of three compared with pure RDX. Aduev et al. (Citation2020) reported similar results with RDX containing ultrafine aluminum particles. Shi et al. (Citation2020), as for them, found that the use of carbon nanotubes in a NC–NG–RDX propellant could reduce ignition delays, due to the improvement of optical absorption and decrease in reflectivity. The use of nanoparticles is explained by providing better characteristics than microparticles. Indeed, nano-aluminum tends to absorb rather than scatter radiations, whereas nanoboron allows higher reactivity, as boron combustion is a surface reaction (Song et al. Citation2021; Uhlenhake et al. Citation2022).
Combustion of solid gun propellants is a complex multiphase phenomenon. It involves numerous processes such as thermal decomposition along with subsequent reactions coupled with molecular diffusion, convection, conduction, and radiation. Thus to improve the comprehension of these processes, numerous studies were performed, especially for RDX and nitrocellulose, components of our materials.
The modeling of RDX combustion has considerably flourished at the end of the last century. With the development of computing resources, detailed kinetics chemistry including thermal decomposition for the gas phase could be implemented. In 1990, Melius (Citation1990) formulated a detailed scheme for RDX reaction mechanism including 38 species and 158 reactions. Yetter et al. (Citation1995) then improved this scheme which was used by Prasad, Yetter, and Smooke (Citation1997) for the modeling of steady-state combustion of RDX. These schemes have been the basis of ensuing research work. Liau, Kim, and Yang (Citation2001) first accounted for the presence of a foam layer, made of liquid and gas bubbles, in which a global decomposition mechanism derived from Brill et al. (Citation1995) was considered. The foam layer was mathematically represented by a void fraction in the condensed-phase equations. This formalism was further extended to the gas phase by considering the presence of condensed particles. It was detailed by Beckstead et al. (Citation2007) in their review of modeling solid propellants combustion and ignition in 2007. More recently, Patidar and Thynell (Citation2017) developed a detailed mechanism for the liquid-phase decomposition of RDX and subsequent reactions. They proposed new decomposition pathways which were included in the Chakraborty’s scheme for the gas phase (Khichar Citation2021).
Although thermal decomposition of nitrocellulose has been of great interest, its understanding is still limited which makes its modeling difficult. Authors seem to agree on the existence of two major mechanisms implicating homolysis of a RO–NO2 bond which leads to the formation of NO2 and RO• radical: thermolysis (2–3 reactions) and acid hydrolysis (5–6 reactions) (Chin et al. Citation2007; Trache and Tarchoun Citation2018). Other processes have been formulated for more specific decomposition conditions. The one of Katoh et al. (Citation2005) addresses the isotherm storage at 120°C under different atmospheres involving a reaction chain with O2 named autoxidation. Kinetics of nitrocellulose decomposition is mainly studied in a global way and appears to be consistent with two simultaneous phenomena corresponding to the slow bond breaking of ONO2 and a fast autocatalytic process (Brill and Gongwer Citation1997). Our state-of-the-art has not enabled to find a detailed kinetic mechanism for the nitrocellulose combustion including its decomposition reactions. However, note that Miller and Anderson (Citation2004) have attempted to avoid the decomposition issue by assuming a condensed phase process with a set of decomposition species. These products, emerging from the combustion surface, are then introduced in a detailed mechanism for the gas phase (59 species, 365 reactions).
The huge variety of energetic materials would require an infinity of experiments to be precisely characterized. From this perspective, modeling enables reduction in time consumption, cost, and improvement of safety. However, it is not yet completely predictive and experimental work is still necessary. The aim of this work is to study the influence of atmosphere on ignition and combustion properties of two low-vulnerability RDX-based gun propellants ignited by a laser diode. Previous studies employing these materials under argon and nitrogen were published (Ehrhardt et al. Citation2020; Gillard et al. Citation2018). Courty et al. (Citation2021) also studied them, emphasizing the effect of graphite at their surface. Here, experiments were conducted under synthetic air. The next section details the experimental aspects of the study. Section 3 is dedicated to results and discussions.
Experimental details
RDX-based propellants
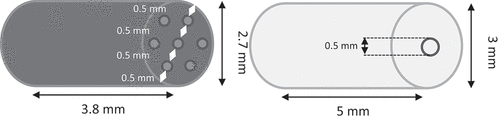
P1 is a black low-vulnerability gun propellant composed, in weight, of 84% of RDX and 16% of a binder, mainly HTPB. These pellets are commercial propellants provided by ArianeGroup. The average mass, diameter, and length are, respectively, 26 mg, 2.7 mm, and 3.8 mm.
The second propellant P2, is a white laboratory propellant composed, in weight, of RDX (89.7%), nitrocellulose (10%) and centralit I (0.3%). It is produced at the French-German Research Institute of Saint-Louis and funded by the Direction Générale de l’Armement (DGA). It weighs 40 mg, has a 3 mm diameter, and is 5 mm long.
Propellants do not suffer any treatment except heating during 24 h at 50°C to remove discrepancies due to humidity before tests. As presented in , pellets are perforated cylinders. P1 contains seven holes and P2 only one.
Experimental setup
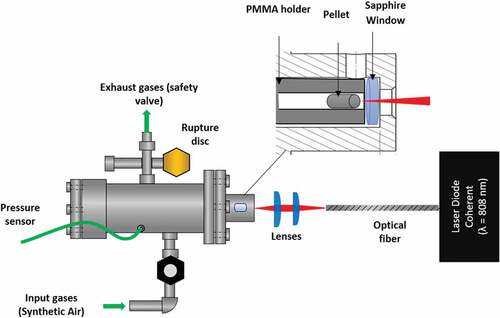
Pellets are ignited in a stainless steel cylindrical closed-volume vessel of 55 cm3 by means of a laser diode (Coherent FAP System, λ = 808 nm) coupled to an optical system and a pressure sensor. An overview of the setup is presented . At the end of the laser diode, an optical fiber brings the laser beam to the optical system composed of two plano-convex spherical lenses with focal lengths of 16 and 25 mm. The latter enables to obtain a spot diameter of 1.25 mm on the sample. A pressure sensor (Kistler 603 B) is connected to a digital oscilloscope (Tektronix DPO2014B). An experiment is conducted as follows: a pellet is placed in a PMMA holder placed itself in the combustion chamber and closed by a sapphire window. Samples are placed against the window. Reactor is then pressurized with argon, nitrogen, or synthetic air (20% O2 + 80% N2) at a pressure ranging from 10 to 70 bar (±5 bar). These gases are provided by Air Liquide so as to have acontrolled and reproducible atmosphere. Finally, laser energy is brought and pressure signal is recorded if ignition occurs. Laser pulse and power can be varied, and laser temperature is fixed at 20°C. Laser current intensities between 10 and 22 A were studied. A calibration relates them to laser powers on the sample. According to the position of the optical system, these powers can slightly vary between the different experimental campaigns. Finally, matching laser powers range from 1.39 to 10.13 W (1.13 to 8.25 MW/m2). For each power, the delivery of energy is controlled by the duration of the laser pulse (tpulse).
Results and discussions
Maximum overpressures (ΔPmax), ignition delays (ti) and maximum propagation rates (rp,max) are derived from the pressure signal. ΔPmax represents the maximum value of the overpressure signal. In graphs, it is divided by the mass of the pellet (m) to make results independent of this parameter. ti is defined as the time between the beginning of the laser pulse and the beginning of the increase in pressure. More precisely, the pressure rise is defined as the intersection between the horizontal and astraight line. This line is an approximation of the slope of the pressure signal: points between 5% of ΔPand 90% of ΔPare considered to make alinear regression which provides an approximation of the slope. Regarding rp,max, it is computed as the maximum value of the derivative of the pressure signal . It is also divided by the mass of the sample. For these measures, error bars, when they are drawn up, represent standard deviations calculated for at least three repetitions in the same conditions.
Ignition energies (E50) are determined by the Langlie method. For a given atmosphere, initial pressure (P0) and laser power (Plaser), E50 represent energies giving 50% of probability of ignition. Langlie method is a statistical method based on dichotomy principle which assumes a repartition of ignition threshold following a standard normal distribution (Gillard and Opdebeck Citation2007). For this measure, error bars represent the square root of the obtained normal law variance. Results on ΔPmax, ti, rp,max, and E50 are presented for different atmospheres (synthetic air, argon, nitrogen) as functions of laser power at P0 = 50 bar and as functions of initial pressure at Ilaser = 12 A (2.80 W for air, 2.86 W for others) and 22 A (9.62 W for air, 10.13 W for others).
Influence of atmosphere on combustion properties
Maximum overpressures as functions of initial pressure
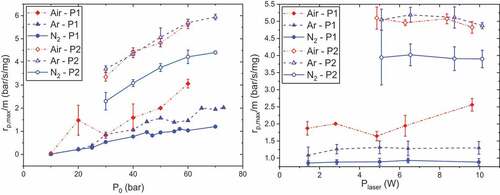
presents maximum overpressures for P1 and P2 as functions of initial pressure for the three atmospheres at Ilaser = 12 and 22 A. As already noted, maximum overpressures are globally increasing with initial pressure. However, there are some critical pressures for which this trend is not observed for P1: at 47 bar for nitrogen and 55 bar for argon.
Figure 3. Maximum overpressures as functions of initial pressure and atmosphere, tpulse = 500 ms, propellants P1 (left, Ilaser = 12 A) and P2 (right, Ilaser = 22 A).
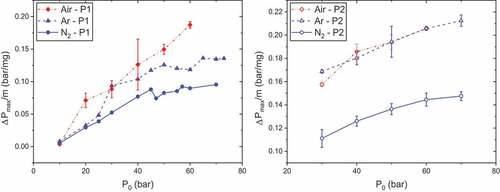
Regarding P1, with reference to atmosphere, maximum overpressures are higher under air except at 10 and 30 bar. This observation could find an explanation in the fact that oxygen balance of RDX is negative: OB = −21.6%. Indeed, oxygen present in RDX molecule is not sufficient to completely oxidize the other components. Under air atmosphere, exothermic reactions such as (1) or (2) could be enhanced, releasing then more heat and leading to greater temperatures and pressures (Hiyoshi and Brill Citation2002):
Hiyoshi and Brill (Citation2002) have reported the great importance of reaction (1) in RDX/air combustion (~70% of CO2 and 0% of CO in products) but do not really enable to conclude on its enhancement compared with argon, since only pyrolysis was noticed. Reaction (2) seems to have a minimal role in the combustion process since little NO and NO2 were detected after ignition. In previous studies, the best combustion properties under argon, compared with nitrogen, were explained by a lower molar heat capacity at constant volume, enabling to reach higher temperatures and pressures (Gillard et al. Citation2018).
For P2, conclusions are relatively different as very similar results are obtained in air and argon. The main difference between the two gun propellants is the content of P2 in NC in place of HTPB. First, although NC has also a negative OB (~ −34% for a 12.62% nitration rate), HTPB one is much weaker (−323%). This is consistent with the fact that the differences observed between air and argon should be more important for P1 than P2. Hiyoshi and Brill (Citation2002) have noted the low influence of surrounding atmosphere on decomposition products of nitrocellulose. They explain this by the absence of vapor pressure for NC, which indicates a decomposition in condensed phase. Therefore, in the very first moments of combustion, there would be few reactions of the decomposition products with atmosphere. However, this argument alone is not enough since the large amount of O2 in air should favor oxidation of these decomposition products.
Maximum overpressures as functions of laser power
As presented , the influence of laser power on maximum overpressures is almost inexistent. This is rather consistent since overpressures are characteristic of processes occurring after ignition and should be independent of ignition source properties. A recent study on the decomposition products of P2 has highlighted the influence of the temperature probe on the decomposition products (Ehrhardt Citation2020; Ehrhardt et al. Citation2020). This study also pointed out the low effect of temperature rate, and so laser power, on decomposition under argon and nitrogen at low pressures. This could account for the trend observed. Only the combustion of P1 under air seems to constitute an exception: at 9.62 W, maximum overpressure increases by 17% in comparison with the previous value. This fact could be the sign of a threshold from which decomposition and reactions would be emphasized under air.
Figure 4. Maximum overpressures as functions of initial pressure and atmosphere, P0 = 50 bar and tpulse = 500 ms, propellants P1 (left) and P2 (right).
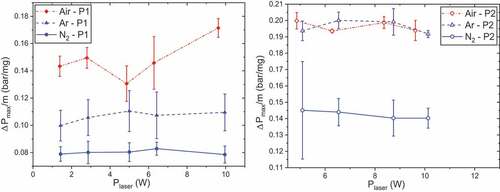
Similar to maximum overpressures, maximum propagation rates are determined thanks to the pressure sensor and have therefore similar trends. They are presented in for sake of comparisons. The combustion is globally faster when the initial pressure increases, while the laser power has a low influence on it. Indeed, an increase in initial pressure has the effect of reducing the flame standoff distance and therefore of intensifying the thermal feedback of gas to condensed phase. Thisdecreases the thin of the melt layer and accelerates the regression of the solid (Beckstead et al. Citation2007). The effect of atmosphere on the maximum propagation rates is different following the propellant studied: the combustion of P1 presents higher rates under air, whereas almost no difference is noticeable for P2 between air and argon.
Influence of atmosphere on ignition properties
Ignition delays as functions of initial pressure
Ignition delays against initial pressure at Ilaser = 12 and 22 A are plotted in . We can immediately notice the very different behaviors of P1 and P2 even if air enables better performances in general. The effect of O2 on laser ignition delays has already been observed on RDX-based materials (Kuo et al. Citation1993) and is consistent with differences noticed concerning decomposition products (Hiyoshi and Brill Citation2002).
Figure 6. Ignition delays as functions of initial pressure and atmosphere, tpulse = 500 ms, propellants P1 (left, Ilaser = 12 A) and P2 (right, Ilaser = 22 A).
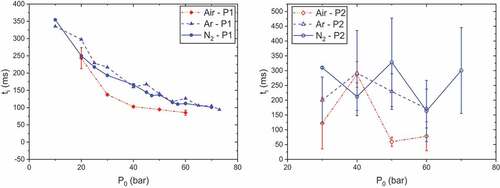
For P1, ignition delays tend to decrease with initial pressure. There is no clear difference between shots under argon and nitrogen. The difference between air/argon and air/nitrogen appears to increase until a maximum at P0 = 40 bar before decreasing. The existence of this maximum difference can seem unusual. Indeed, air and nitrogen have very similar thermal properties, whereas air has a chemical advantage as it contains O2. An increase in air pressure results in a higher amount of O2 which would suggest an increase in probability of reactions with decomposition products. However, an increase in initial pressure leads to a reduction of O2 diffusivity (Kuo Citation2005), which could explain why ignition delay differences between three atmospheres decrease at high pressures. Regarding P2, ignition delays versus initial pressure do not show a particular trend and appear to oscillate around a mean value.
Ignition delays as functions of laser power
Concerning ignition delays, presented , for P1, there is a clear effect of laser power until Plaser = 5.00 W, then, the ti remains quasi-constant. In aprevious study, Courty et al. (Citation2021) explained that ti is the sum of three physical times: the physical heating time (th), the pyrolysis time (tp) and the chemical time (tc) to ignite decomposition gases. The latter is very short compared to others;thus, ti is limited by th and tp. A rise in Plaser causes an increase in heating rate and so a reduction in th.
Figure 7. Ignition delays as functions of laser power and atmosphere, P0 = 50 bar and tpulse = 500 ms, propellants P1 (left) and P2 (right).
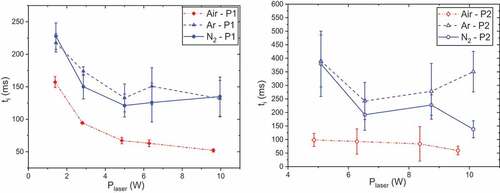
Note that P2 could not be ignited under 5.09 W.This can be explained by the fact that P2 is awhite propellant and its laser absorption is lower than the one of P1. There is avery low effect of laser power on ignition delays under air, similarly to P1 for the same range of Plaser. For nitrogen atmosphere, ignition delays globally decrease while they seem to present a minimum at P = 6.42 W under argon. For both propellants, the lowest ignition delays are obtained under air, whereas results under nitrogen and argon are similar.
Ignition energies vs laser power
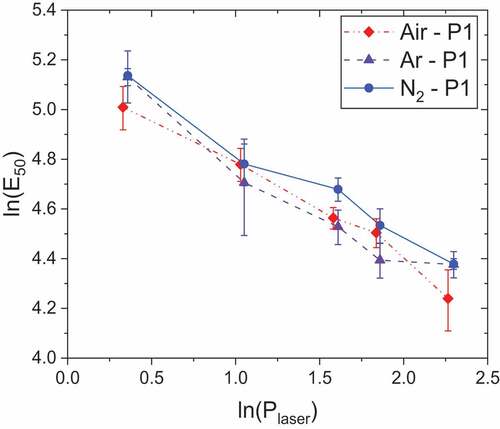
The logarithm of E50 versus logarithm of laser power is plotted . The almost linear trend illustrates the power-law behavior of ignition energies with laser power. Coefficients of the regressions are presented in . Concerning atmospheric parameters, there is no clear effect of atmosphere nature and initial pressure on ignition energies, as shown by Gillard et al. (Citation2018).
Table 1. Regression coefficients of ln(E50) versus ln(Plaser).
Conclusion
Ignition and combustion properties of two insensitive RDX-based gun propellants were experimentally investigated. Results especially focused on the influence of atmosphere on ignition delays, energies giving 50% of probability of ignition, maximum overpressures, and maximum propagation rates. Initial pressure and laser power were also studied as driving parameters. Combustion under synthetic air has given very different results depending on the propellant. For P1, air turned out to provide the best combustion properties, overcoming the negative oxygen balance of RDX and HTPB, compared with argon and nitrogen. On the contrary, combustion of P2 under air and argon appeared to be very similar: the low effect of atmosphere on the decomposition products of NC and the high molar heat capacity of air seem to balance its chemical advantage compared with argon. This experimental study is part of a wider project which aims to model ignition and combustion processes of low-vulnerability gun propellants using detailed kinetics chemistry. The results presented here will thus enable the comparison with overpressures or burning rates provided by the future model.
Acknowledgements
This work was funded by the project Chaire Industrielle ACXEME supported by the Agence Nationale de la Recherche, in France.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aduev, B. P., D. R. Nurmukhametov, I. Y. Liskov, A. V. Tupitsyn, and G. M. Belokurov. 2020. Laser pulse initiation of RDX-Al and PETN-Al composites explosion. Combust. Flame 216:468. doi:10.1016/j.combustflame.2019.10.037.
- Ahmad, S., and M. Cartwright. 2014. Review of Laser Initiation. Laser ignition of energetic materials. 17–33. Chichester: John Wiley & Sons, doi:10.1002/9781118683521.ch2.
- Beckstead, W. B., K. Puduppakkam, P. Thakre, and V. Yang. 2007. Modeling of combustion and ignition of solid-propellant ingredients. Prog. Energy Combust. Sci. 33 (6):497. doi:10.1016/j.pecs.2007.02.003.
- Brill, T. B., H. Arisawa, P. J. Brush, P. E. Gongwer, and G. K. Williams. 1995. Surface chemistry of burning explosives and propellants. J Phys Chem 99 (5):1384. doi:10.1021/j100005a005.
- Brill, T. B., and P. E. Gongwer. 1997. Thermal decomposition of energetic materials 69. analysis of the kinetics of nitrocellulose at 50 °C-500 °C. Propellants Explos. Pyrotech 22 (1):38. doi:10.1002/prep.19970220109.
- Chin, A., D. S. Ellison, S. K. Poehlein, and M. K. Ahn. 2007. Investigation of the decomposition mechanism and thermal stability of nitrocellulose/nitroglycerine based propellants by electron spin resonance. Propellants Explos. Pyrotech 32 (2):117. doi:10.1002/prep.200700013.
- Courty, L., P. Gillard, J. Ehrhardt, and B. Baschung. 2021. Experimental determination of ignition and combustion characteristics of insensitive gun propellants based on RDX and nitrocellulose. Combust. Flame 229:229. doi:10.1016/j.combustflame.2021.111402.
- Ehrhardt, J. 2020. Allumage laser de poudres propulsives à vulnérabilité réduite : influence du taux de nitrocellulose sur les conditions de pyrolyse et d’inflammation, PhD Thesis, Université d’Orléans.
- Ehrhardt, J., L. Courty, P. Gillard, and B. Baschung. 2020. Experimental study of pyrolysis and laser ignition of low-vulnerability propellants based on RDX. Molecules, MDPI 25 (10):2276. doi:10.3390/molecules25102276.
- Fang, X., M. Stone, and C. Stennett. 2020. Pulsed laser irradiation of a nanoparticles sensitised RDX crystal. Combust. Flame 214:387. doi:10.1016/j.combustflame.2020.01.009.
- Gillard, P., L. Courty, S. De Persis, J. F. Lagrange, C. Boulnois, and I. Gökalp. 2018. Combustion properties of a low-vulnerability propellant: An experimental and theoretical study using laser ignition. J. Energetic Mater 36 (3):362. doi:10.1080/07370652.2018.1439126.
- Gillard, P., and F. Opdebeck. 2007. Laser diode ignition of the B/KNO3 pyrotechnic mixture: An experimental study. Combust. Sci. & Tech 179 (8):1667. doi:10.1080/00102200701259833.
- Hiyoshi, R. I., and T. B. Brill. 2002. Thermal decomposition of energetic materials 83. comparison of the pyrolysis of energetic materials in air versus argon. Propellants Explos. Pyrotech 27 (1):23. doi:10.1002/1521-4087(200203)27:1<23:AID-PREP23>3.0.CO;2-B.
- Katoh, K., L. Le, M. Kumasaki, Y. Wada, M. Arai, and M. Tamura. 2005. Study on the spontaneous ignition mechanism of nitric esters (I). Thermochim Acta 431 (1–2):161. doi:10.1016/j.tca.2005.01.067.
- Khichar, M. 2021. Thermal decomposition and combustion modeling of RDX monopropellant and RDX-TAGzT pseudo-propellant. The Pennsylvania State University. PhD dissertation.
- Kuo, K. K. 2005. Principles of combustion, 623–692. Hoboken: John Wiley & Sons. Appendix A.
- Kuo, K. K., J. U. Kim, B. L. Fetherolf, and T. Torikai. 1993. Preignition dynamics of RDX-based energetic materials under CO2 laser heating. Combust. Flame 95 (4):351. doi:10.1016/0010-2180(93)90003-L.
- Liau, Y. C., E. S. Kim, and V. Yang. 2001. A comprehensive analysis of laser-induced ignition of RDX monopropellant. Combust. Flame 126 (3):1680. doi:10.1016/S0010-2180(01)00281-4.
- Melius, C. F. 1990. Thermochemical modeling, II: Application to ignition and combustion of energetic materials. In S. N. Bulusu Chemistry and physics of energetic materials. Chemistry and physics of energetic materials, Vol. 309, 51–78. Springer: Dordrecht. doi:10.1007/978-94-009-2035-4_4.
- Miller, M. S., and W. R. Anderson. 2004. Burning-rate predictor for multi-ingredient propellants: Nitrate-ester propellants. J. Propuls. Power 20 (3):440–54. doi:10.2514/1.10386.
- Patidar, L., and S. T. Thynell. 2017. Quantum mechanics investigation of initial reaction pathways and early ring-opening reactions in thermal decomposition of liquid-phase RDX. Combust. Flame 178:7. doi:10.1016/j.combustflame.2016.12.024.
- Prasad, K., R. A. Yetter, and M. D. Smooke. 1997. An eigenvalue method for computing the burning rates of RDX propellants. Combust. Sci. Technol. 124 (1–6):35. doi:10.1080/00102209708935640.
- Shi, X., Y. Jia, L. Chen, L. Tian, J. Shen, and C. Pei. 2020. Tuning the laser ignition properties of nitrocellulose-nitroglycerine-hexogen propellants via incorporation of carbon nanotubes. Cent. Eur. J. Energ. Mater 18 (3):385. doi:10.22211/cejem/142604.
- Song, Q., W. Cao, X. Wei, J. Liu, J. Yuan, X. Li, X. Guo, and D. Gao. 2021. Laser ignition and combustion of micro- and nano-sized boron under different atmospheres and pressures. Combust. Flame 230:111420. doi:10.1016/j.combustflame.2021.111420.
- Trache, D., and A. F. Tarchoun. 2018. Stabilizers for nitrate ester-based energetic materials and their mechanism of action: A state-of-the-art review. J. Mater. Sci 53 (1):100. doi:10.1007/s10853-017-1474-y.
- Uhlenhake, K. E., D. Collard, M. Gomez, M. Ornek, and S. F. Son 2022. Laser ignition of solid propellants using energetic nAl-PVDF optical sensitizers. AIAA 2022-1744 AIAA SCITECH 2022 Forum.
- Yetter, R. A., F. L. Dryer, M. T. Allen, and J. L. Gatto. 1995. Development of gas-phase reaction mechanism for nitramine combustion. J. Propuls. Power 11 (4):683. doi:10.2514/3.23894.