Abstract
Triapertury is rare in monocotyledons. The well-defined, regularly spaced, circular porate apertures that occur in Arecaceae: Areca klingkangensis from Borneo, and species of the West African genus Sclerosperma, appear to be unique in monocotyledons. There is evidence to suggest that tripory in Arecaceae has been derived from trichotomosulcy, although in Areca equatorial zonosulcy may have an important role. The apical triporate, and zonosulcate pollen of Areca are described, as well as examples of mono- and trichotomosulcate pollen within the genus. The sub-apical distal triporate pollen of Sclerosperma gilletii and S. mannii are described. Notably, in Sclerosperma pollen, aperture position at post-meiotic tetrad stage follows the rare ‘Garside's rule’ (four groups of three apertures), previously only demonstrated for Proteaceae and Olacaceae. Possible reasons for the occurrence of these rare triporate pollen phenomena in palms are considered. The bearing this may have on the transition from the distal polar position of the single sulcus, to the radial symmetry of the triaperturate condition in many dicotyledons is discussed in comparison with other examples of triapertury in monocotyledons.
Pollen data for Areca Sect. Microareca, acetolysed pollen.
Erdtman's (Citation1969) shape classes adapted to express shape of equatorial outline in monosulcate pollen (Equatorial long axis (EL) divided by equatorial short axis (el)).
Pollen data for Sclerosperma, acetolysed pollen.
Species included, or affiliated to Sect. Microareca (incl. synonomy).
Some monocotyledons with triaperturate pollen.
Triapertury is the most common aperture configuration to occur in the pollen of dicotyledons, while in monocotyledons it is rare. Furthermore, an equivalent of the composite colporate aperture, that is so frequent in dicotyledonous triaperturate pollen, is unknown among monocotyledonous pollen aperture configurations. In monocotyledons triaperturate pollen are recorded in about 20 genera, twelve families, and six orders: Alismatales, Asparagales, Dioscoreales, Liliales, Commelinales and Arecales (Harley: submit.).
The first published description of triporate pollen in Arecaceae is that by Erdtman & Singh (Citation1957) concerning pollen of Sclerosperma, while the original description of triporate pollen in Areca klingkangensis J. Dransf. appears to be that of Ferguson & Harley (Citation1993). Areca and Sclerosperma are both included in the Arecoideae, the largest of the six subfamilies of the Arecaceae. Species belonging to Areca are mostly small to moderate undergrowth palms of tropical rainforest. Only a few tolerate open conditions, for example A. catechu Linn. Many have very precise habitat requirements, and narrow ecological limits (Uhl & Dransfield Citation1987). The species are distributed from India and south China through Malaysia to New Guinea and the Solomon Islands. Sclerosperma is a West African genus; its species grow best in low, wet, swampy areas, although they are also found on higher ground growing in frequently waterlogged sandy loam. The two genera are not closely related, and the morphology of the triporate pollen from each of the two taxa has notable differences.
Contrary to the situation in most other monocotyledons, in the pollen of Areca klingkangensis and of Sclerosperma spp. the small circular pores are well-defined, equally spaced, and constant in number. In all, there are approximately 60 species of Areca; among the 35 species examined so far, triporate pollen grains occur only in A. klingkangensis. It is remarkable that in these two isolated examples of triapertury in the pollen of the Arecaceae that the triporate condition within each genus appears to follow different modes of development.
The morphology of the triporate pollen of Sclerosperma, and of Areca klingkangensis is described. In addition, the zonosulcate pollen from a number of other species are described: A. abdulrahmanii J. Dransf., A. andersonii J. Dransf. and A. chaiana J. Dransf., as well as the trichotomosulcate or monosulcate pollen of Areca minuta Scheff. Areca klingkangensis, A. minuta, and all species of Areca with zonosulcate pollen are included in Sect. Microareca (Dransfield Citation1984), a section restricted to Borneo, with the exception of A. ridleyana, which only occurs in Peninsular Malaysia. Therefore, for comparison, we have included pollen data for all other species in the section (). All of these have the more typical monosulcate, tectate or semi-tectate pollen associated with the majority of Areca species.
Differences between the morphology of triaperturate pollen in other monocotyledonous families, and of Areca klingkangensis, and Sclerosperma spp. are discussed. The apparent variation in development, leading to two types of tripory in palm pollen, is considered in relation to the widespread and conservative retention of distal monosulcate pollen not only within the Arecaceae, but also in the monocotyledons generally. The evidence is discussed, both from an evolutionary and from an ontogenetic point of view.
Taxonomy and systematics
Areca L. and Sclerosperma G. Mann & H. Wendl. are included in subfamily Arecoideae, the largest of the six subfamilies recognised by Uhl & Dransfield (Citation1987). Areca is one of eight genera belonging to subtribe Arecinae, whereas Sclerosperma is the only genus in subtribe Sclerospermatinae (Dransfield & Beentje Citation1995). Opinion varies regarding the number of species recognised in Sclerosperma. Three are suggested by Uhl & Dransfield (Citation1987), while Tuley (Citation1995) comments that although Sclerosperma is a particularly well-defined genus there is no satisfactory treatment at specific level.
Sectional classification of Areca
There is no recent revision of Areca. The latest sectional revision of Areca (Furtado Citation1933) recognises two subgenera, five sections and 43 species. Furtado (Citation1933) based his subgenera largely on the arrangement of the flower and flower groups on the inflorescence axes (rachillae), while details of stamen number, number of orders of branching in the inflorescence, and the distribution of pistillate flowers within the rachillae were used for separating sections. It seems likely that some of the divisions that Furtado (Citation1933) recognised are artificial. A modern revision is required to test the delimitation not only of species, but also of infrageneric groupings. Since Furtado's (Citation1933) account the genus Pichisermollia, with two morphologically very dissimilar species, has been included in Areca (Dransfield Citation1984). Several new species have also been described giving a total of about 60 species. Bornean species were revised and enumerated by Dransfield (Citation1984). Several undescribed taxa have been collected during the last decade, especially in Borneo where the genus is particularly diverse and displays considerable local endemism. Furtado's (Citation1933) suprageneric classification is summarised in the Appendix.
MATERIAL AND METHODS
Pollen for this study was taken from the collections in the Palm Herbarium of the Royal Botanic Gardens, Kew (K), with the exception of one specimen of Areca minuta, which is from the Forest Research Institute of Malaysia at Kuala Lumpur (FRIM). Pollen slide preparations have been incorporated into the Pollen Microscope Slide Collection of the Palynology Unit of the Royal Botanic Gardens, Kew. Standard acetolysis procedures (Erdtman Citation1960) were used for light microscopy (LM), with timing at 100°C restricted to 2 minutes. Preparation methods have been described in more detail elsewhere (Citation CitationHarley Citation1990, 1996, 1999). After acetolysis the pollen samples were mounted in glycerol jelly and examined using a Nikon Optiphot compound microscope. Measurements and other details were recorded using a 100× oil immersion objective, and a crossed micrometer eyepiece graticule. The shape of the equatorial outline of monosulcate pollen is expressed (: column 9) using a modification of the Erdtman (Citation1969) shape class system (): the average of the equatorial long axis (EL) was divided by the average of the equatorial short axis (el). In some taxa the pollen in the slide preparations are always in equatorial view (e.g. ) and, since it was only possible to record the long axis dimension, outline shape could not be accurately assessed. Fresh pollen material for this study was unobtainable, therefore, although not ideal for scanning electron microscopy (SEM) and transmission electron microscopy (TEM), we had to use unacetolysed herbarium material of Areca klingkangensis (Dransfield et al., JD6103, and George, S. 42083), A. abdulrahmanii (Jermy, 13702), A. chaiana (Mohtar et al., S.49276), A. minuta (Dransfield, 5339) and Sclerosperma mannii (Gillett, 279 and Tuley, s.n.). For SEM pollen were examined using an Hitachi S2400 SEM. For TEM, pollen were embedded in London Resin Company, LR White low viscosity resin, and thin-sectioned using a Reichert Ultracut. The sections were then stained in an LKB ultrostainer using standard settings, and subsequently examined in a Hitachi T20 TEM. Thin sections of unacetolysed pollen from all four collections of Sclerosperma examined, show a high percentage of grains that lack cytoplasm (). Therefore, “Alexander's stain” (Alexander Citation1969) was used to check potential viability of unacetolysed pollen from the species of Areca with triporate or zonosulcate pollen. The mono- and trichotomosulcate pollen of Areca minuta were also stained to check for potential viability.
10–19. Sclerosperma spp. 10–12. Close ups to show range of exine types (SEM): (10) S. mannii Gillett, 279, coarsely perforate; (11) S. mannii Tuley s.n., reticulate; (12) S. gilletii Profizi, 84102, rugulate. 13–15. S. mannii Gillett, 279, (LM): (13) pollen in polar view, in mid focus; (14) ibid. in high focus; (15) semi-thin section of unacetolysed pollen grains, stained with toluene blue; note high proportion of “empty” pollen exines. (16) S. mannii Gillett, 279, close up of pore, unacetolysed pollen grain, with operculum in place (SEM). (17) S. mannii Gillett, 279, polar views of two acetolysed pollen grains (SEM): distal face (left), proximal face (right). (18) S. mannii Hall & Enti, GC36150: ultrathin TEM section to show pore with operculum in situ (arrow). (19) S. mannii Gillett, 279, ultrathin TEM section of unacetolysed pollen; note poor preservation of cellular material. LM Figs.: ×150 (in 15); ×1000 (in 13, 14). In SEM & TEM Figs. scale bars: 2.5 μm (in 10–12, 16 & 18); 10 μm (in 17, 19).
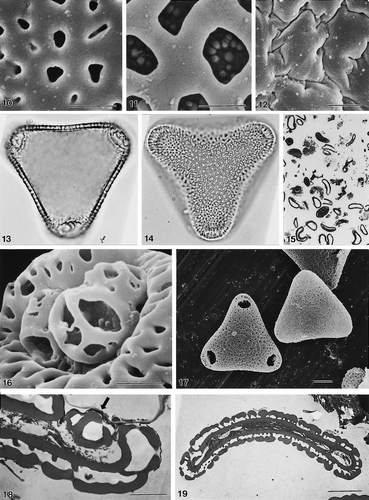
28–37. Other zonosulcate Areca pollen. 28, 29. A. chaiana Mohtar et al., S.49276 (SEM): (28) group of pollen grains at low magnification to show consistency of zonosulcate condition; (29) pollen grain in presumed equatorial view. 30. A. abdulrahmanii Jermy, 13702, pollen grain in presumed equatorial view (SEM). 31. A. chaiana Chai, S.33986, ultrathin section of acetolysed pollen grain (TEM). 32, 33. A. chaiana Mohtar et al., S.49276 (TEM): (32) ultrathin section through zonosulcus, presumed equatorial plane of unacetolysed grain; note thick and channelled intine I, and narrow, homogeneous intine II; (33) ultrathin section, presumed polar plane. (34) A. chaiana Chai, S.33986, pollen grain in high focus (LM). (35) A. andersonii Anderson, S.31937, pollen grain in high-mid focus (LM). (36, 37) A. abdulrahmanii Jermy, 13702; unacetolysed pollen grain (LM): (36) presumed equatorial view, mid-low focus; (37) presumed polar view, mid-low focus. LM Figs.: ×1000 (in 34–37). In SEM/TEM Figs. scale bars: 10 μm (in 29–33); 25 μm (in 28).
Terminology
Pollen terminology follows Punt et al. (Citation1994). Where the word “pollen” is used it is considered to be a plural noun, implying “pollen grains”.
RESULTS
Within Areca triporate pollen or zonosulcate pollen have only been recorded in Bornean species of Sect. Microareca, although within Sect. Microareca, as in other sections of Areca, the majority of species are monosulcate. It is probable that a number of species in Areca have the potential to produce both mono- and trichotomosulcate pollen, although in Sect. Microareca we have such data for only one collection of Areca minuta (Dransfield, JD5339). We have confined our pollen data to the species of Areca in Sect. Microareca (). A full systematic pollen treatment of Areca will be published at a later date. Pollen data for Sclerosperma are taken from two species: S. mannii and S. gilletii. The pollen data for all species examined are summarised in (Areca) & (Sclerosperma). There is no evidence of an acetolysis-resistant endexine in the pollen of any of the taxa examined. Whether the darker staining layer adjacent to the foot layer ( ) is a non-acetolysis resistant endexine or ‘intine 1’ is a matter for debate.
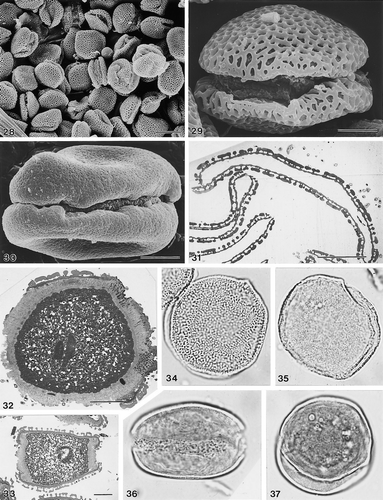
1–9. Areca klingkangensis Dransfield, JD6103, triporate pollen: (1) grain in presumed polar view (SEM); (2) aberrant pollen grain showing 5 of the 6 aperture sites, note larger size of grain, cf. 7–8 (LM); (3) group of grains at low magnification to show consistency of triporate condition (SEM); (4) ultrathin section of pollen grain, polar plane (TEM); (5) ultrathin section through pore, polar plane, note thickened and channelled intine in aperture region and narrow, non-acetolysis resistant, dark-staining layer underlying foot layer (TEM); (6) close up of unacetolysed grain showing aperture and intine “plug” in situ; (7, 8) LM of the whole grain: (7) high focus, (8) low focus; (9) ultrathin section of acetolysed pollen, polar plane; note very reduced ectexinous membrane over aperture, and absence of non ectexinous material in non apertural region (TEM). LM Figs. ×1000 (in 2, 7, 8). In SEM & TEM Figs. scale bars: 2.5 μm (in 5, 6, 9); 10 μm (in 1, 4); 25 μm (in 3).
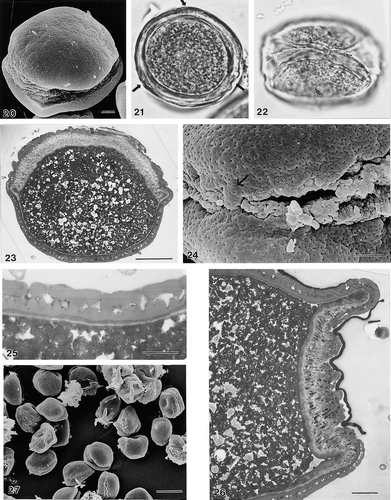
20–27. Areca klingkangensis George, S.42803, zonosulcate pollen: (20) unacetolysed pollen grain tilted, showing presumed equatorially oriented aperture, and one polar face (SEM); (21) unacetolysed pollen grain, presumed polar view, note interruptions (arrows) to sporoderm (LM); (22) unacetolysed pollen grain, presumed equatorial view (LM); (23) very oblique ultrathin section (TEM) through part of zonosulcus (top), and non-apertural exine (bottom); (24) close up to show small ektexinous “hinge” (arrow), linking the two halves of the pollen grain (SEM); (25) ultrathin section through non-apertural wall, note narrow non-acetolysis resistant dark-staining layer, underlying foot layer cf. close similarity of ectexine stratification with (TEM); (26) ultrathin section through zonosulcus, presumed equatorial plane, note thick and channelled intine I, and narrow, homogeneous intine II, unacetolysed (TEM); (27) group of pollen grains at low magnification to show consistency of zonosulcate condition (SEM). LM Figs.: ×1000 (in 21, 22). In SEM/TEM Figs. scale bars: 2.5 μm (in 24–26); 10 μm (in 20, 23); 25 μm (in 27).
Triporate pollen
Areca Linn. ()
Pollen grains oblate ellipsoid in presumed equatorial view; presumed equatorial outline symmetric and slightly rounded triangular. Polarity not established but presumed isopolar. Pores equally spaced and positioned at apices of grain, circular and well-defined, without an operculum. Pore membrane scabrate (), destroyed by acetolysis. Grains in slide preparations always orientated in polar view. Average diameter: (37–) 41.5 (–45) μm. Acetolysed pollen wall thickness: c. 1.75–2.25 μm, tectum and foot layer similar in thickness, infratectum slightly narrower (), columellate. In unacetolysed pollen the intine is very thin and bi-layered under the exine, but greatly thickened and channelled in the pore region (). Exine tectate perforate (). Within the sample a few (3–4) larger pollen grains, with 5–6 irregularly spaced apertures, were recorded (). Grains stain positive for cytoplasm.
Taxon and collection examined. – Areca klingkangensis, Dransfield, JD6103, Malaysia: Sarawak.
Sclerosperma G. Mann & H. Wendl. ()
Pollen grains oblate ellipsoid in equatorial view, heteropolar. Equatorial outline triangular. The polarity of the pollen grains was established in Thanikaimoni (Citation1970), where a post-meiotic tetrahedral tetrad of Sclerosperma mannii is illustrated. Pores positioned sub-apically, on the distal polar face of the grains, circular and well-defined, with an operculum (). Average diameter: (37–) 45.7 (–59) μm. Acetolysed pollen wall: c. 1.75–3.0 μm. Exine perforate, perforate-rugulate, rugulate or reticulate (). In unacetolysed pollen there is a very high percentage of grains with incomplete, or without, cell contents ().
Taxa and collections examined. – S. mannii, Gillett, 279, Zaïre. Hall & Eriti, GC36150, Ghana; Tuley, s.n., Nigeria. S. gilletii, Profizi, 10482, Congo.
Incomplete zonosulcate pollen
Areca ( )
Pollen grains oblate ellipsoid in presumed equatorial view. Polarity not established but presumed isopolar. Equatorial outline circular. Zonosulcus tends to be incomplete with small “hinge” of tectum (), and a rugulate aperture membrane () that is destroyed by acetolysis. Average diameter: (36–) 42.7 (–50) μm. Acetolysed pollen wall thickness: c. 1.0–2.25 μm, tectum and foot layer similar in thickness, infratectum slightly narrower in A. klingkangensis (George, S.42803; ), and notably similar () to A. klingkangensis (Dransfield, JD6103). In A. chaiana (Chai, 33986) the foot layer may be narrower than the tectum () or more or less the same thickness as in Mohtar et al. S.49276 (). In unacetolysed pollen the intine is very thin and bi-layered under the exine, but greatly thickened and channelled in the zonosulcus region ( ). Exine tectate perforate ( ) or finely reticulate (). With the exception of one of the two collections of Areca chaiana (Chai, S.33986) grains of all other collections stain positive for cytoplasm.
Taxa and collections examined. – Areca abdulrahmanii, Jermy, 13702, Malaysia: Sarawak. A. andersonii, Anderson, 31937, Brunei. A. chaiana, Chai, S.33986, Borneo; Mohtar et al., S.49276, Borneo. A. klingkangensis, George, S.42803; Malaysia.
Mono- and trichotomosulcate pollen
Areca ()
Pollen grains oblate, ellipsoid in polar view, mono- or trichotomosulcate and heteropolar. Equatorial outline more or less asymmetric-elliptic (E/e 1.45/1.54), or more or less asymmetric-rounded triangular (E/e 1.07). (NB. Grains with an elliptic sulcus have an elliptic polar outline, while trichotomosulcate grains have a rounded triangular polar outline).
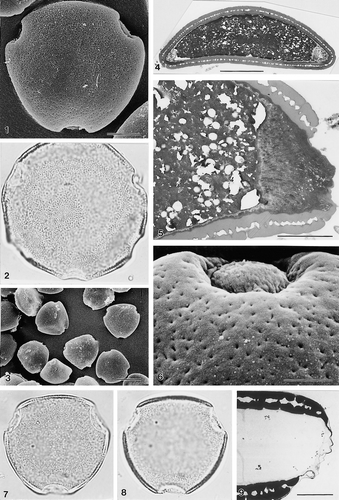
38–43. Areca minuta Dransfield, JD5339: (38) unacetolysed monosulcate pollen grain, oblique polar view, distal face (SEM); (39) tri- and monosulcate pollen grains, polar views, distal faces (LM); (40) group of pollen grains at low magnification showing proportionate representation of mono- versus trichotomosulcate grains within the sample (LM); (41) unacetolysed trichotomosulcate pollen grain, distal face (SEM); (42) ultrathin section of unacetolysed pollen grain (TEM); (43) ultrathin section through sulcus of unacetolysed whole pollen grain, note thick and channelled intine I, and narrow, homogeneous intine II; cf. close similarity of ectexine stratification with (TEM). LM Figs.: ×150 (in 40); ×1000 (in 39). In SEM/TEM Figs. scale bars: 2.5 μm (in 43); 10 μm (in 38, 41, 42).
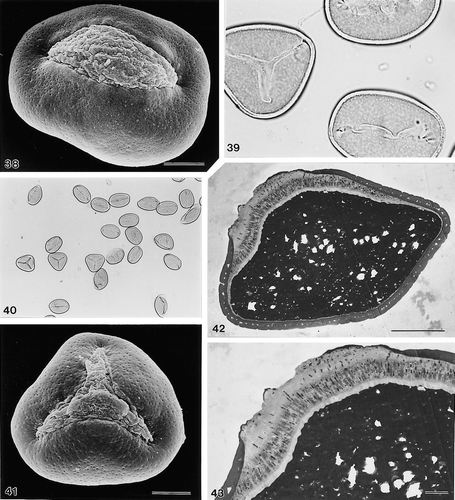
Both the elliptic and the tri-armed sulci have a scabrate to rugulate aperture membrane (), which is destroyed by acetolysis. The size range for the widest diameter of the trichotomosulcate grains is equivalent to that for the long axis of the more frequent monosulcate grains. Average length (long axis) of monosulcate grains: (35–) 39.9 (–58) μm. Acetolysed pollen wall: c. 1.25–2.0 μm. In unacetolysed pollen the intine is very thin and bi-layered under the exine, but greatly thickened and channelled in the sulcus region (). Exine tectate, perforate. Grains stain positive for cytoplasm.
Taxon and collections examined. – Areca minuta Dransfield, JD5339, Borneo; Mohtar et al., S.49323, Borneo; Dewol Sundaling, SAN92378, Malaysia: Sabah; Meijer, SAN141383, Malaysia: Sabah (FRIM).
Pollen morphology of other species in Sect. Microareca ( )
Pollen grains monosulcate, oblate, ellipsoid in equatorial view, long axis, and heteropolar. Equatorial outline more or less asymmetric-elliptic (E/e 1.36 –1.72) or sub-elliptic (E/e 1.18), sulcus elliptic. Average length (long axis): (33–) 41.1 (–49) μm. Acetolysed pollen wall: c. 1.5–2.5 μm. Unacetolysed pollen not examined. Exine tectate perforate or finely reticulate.
Taxa and collections examined. – Areca arundinacea, Dransfield, JD6120, Malaysia: Sarawak. A. brachypoda, Dransfield, JD6011, Malaysia: Sarawak.
A. dayung, Dransfield JD5950, Malaysia: Sarawak. A. furcata, Dransfield, JD5959, Malaysia: Sarawak; Dransfield, JD781, Malaysia: Sarawak. A. ridleyana, Dransfield, JD5136, Peninsular Malaysia.
Pollen morphology of species affiliated to Sect. Microareca ( )
Areca subacaulis
Pollen grains monosulcate, oblate, ellipsoid in equatorial view, long axis, and heteropolar. Equatorial outline more or less asymmetric-elliptic (E/e 1.33) or sub-elliptic (E/e 1.19), sulcus elliptic. Average length (long axis): (35–) 41.1 (–45) μm. Acetolysed pollen wall: c. 1.5–2.0 μm. Unacetolysed pollen not examined. Exine finely reticulate.
Collections examined. – Dransfield, JD4915, Malaysia: Sarawak; Beccari, 3647, Malaysia: Sarawak.
Areca insignis
Pollen grains monosulcate, oblate ellipsoid in equatorial view, long axis, and heteropolar. Equatorial outline symmetric-elliptic (E/e1.60) or sub-elliptic (E/e1.18), sulcus elliptic. Average length (long axis): (26–) 33.5 (–42) μm. Acetolysed pollen wall: c. 1.0–1.25 μm. Unacetolysed pollen not examined. Exine finely sinuous-reticulate.
Collections examined. – A. insignis var. insignis, Ashton, 18383, Malaysia: Sarawak. A. insignis var. moorei, Dransfield, JD738, Malaysia: Sarawak.
DISCUSSION
The transition from distal polar to equatorially positioned apertures
Equatorially triaperturate pollen are rare in the monocotyledons, but widespread in the dicotyledons, whereas the typical distal sulcus in the pollen of monocotyledons is restricted to the ‘primitive’ or ‘basal’ dicotyledons. This fundamental difference between monocot and eudicot pollen has been central to evolutionary studies of flowering plants for many years, for example: CitationKuprianova (Citation1954, 1969), Citation Citation Citation CitationDoyle (Citation1969), Muller (1970), Walker & Doyle (1975), Zavada (1983), Doyle & Hotton (1991).
For a monocotyledonous family Arecaceae have a remarkable range of unusual pollen aperture types. Nevertheless, monosulcate pollen predominates in the family. The variation and configuration of aperture types within the palms (Harley & Baker Citation2001) suggests considerable suppressed developmental potential – why have, at least some of, these aperture types not become more widely established? – equatorial disulcy in the rattan palms being the highly successful exception.
One of the rarest aperture configurations, not only in palms, but for monocotyledons generally, is symmetric triporate. Aperture orientation in the pollen of Areca klingkangensis is presumed equatorial, and in the pollen of Sclerosperma spp. it is confirmed subequatorial. In evolutionary terms, orientation, number, and symmetry are aperture characters of particular interest in the shift from a distal to an equatorial position in monocotyledonous pollen. The ‘move’ from a single distal, to three radially symmetrical equatorial apertures remains “one of the burning questions” (Meeuse Citation1965). It has stimulated a number of hypotheses, for example: Citation Citation CitationWodehouse (Citation1936), Wilson (1964), Meeuse (1965), Chaloner (1970), and Kuprianova (Citation1979). Most authors agree that triporate pollen is a highly evolved condition in angiosperms, for example, Doyle (Citation1969), and Muller (Citation1970). The most frequently discussed possibility for the development of triporate pollen from monosulcate includes a trichotomosulcate intermediary. The transition from distal to equatorial being effected by the arms of the trichotomosulcus extending down to the equator, and a gradual modification of the apices of the arms, into pores. The main ‘body’ of the trichotomosulcus is subsequently ‘lost’. The problem with this hypothesis is that no convincing intermediates have been found in living plants. There is, however, evidence from a range of extinct porotrichotomosulcate fossil grains recovered from the Upper Cretaceous of Gabon (CitationBelsky & Boltenhagen Citation1963, Belsky et al. 1965) that such morphology might have occurred in the past.
The common occurrence of co-existing distal trichotomosulcate and monosulcate pollen in the anthers of palm species is also recorded in other families of monocotyledons (e.g., CitationErdtman Citation1944, Rudall et al. 1997), as well as some primitive dicotyledons. This co-existence is always the result of simultaneous microsporogenesis, as is purely distal trichotomosulcate pollen (Citation CitationRudall et al. Citation1997, Harley 1999, Baker & Harley 2001). Apart from Areca, there are no examples of aperture types, other than monosulcy, associated with trichotomosulcy. Distal monosulcate pollen can result from either successive or simultaneous microsporogenesis. Examples of the co-occurrence of pollen with differing aperture configurations representing both polar and equatorial orientation are unknown. The influence of microsporogenesis type on tetrad configuration and aperture type and position is well recognised (Blackmore & Crane Citation1998), and has been documented for palms (CitationHarley Citation1999, Harley & Baker 2001). According to Blackmore & Crane (Citation1998) spindle orientation of the meiotic cytoskeleton, the relative timing of cytokinesis, and the mode of cytoplasmic partitioning, exert a decisive influence on tetrad configuration and ultimately the outcome of aperture position in relation to the poles.
It is of particular interest to note in Sclerosperma, from the post-meiotic tetrad stage (Thanikaimoni Citation1970), that the pores are arranged according to Garside's rule (Garside Citation1946): apertures form in groups of three at four points in the tetrad. Previously this arrangement has only been demonstrated for the pollen of Proteaceae and Olacaceae (CitationGarside Citation1946, Blackmore & Barnes 1995). The usual arrangement for triaperturate pollen at the tetrad stage conforms to Fischer's rule (Erdtman Citation1952), where the apertures form in pairs at six points in the tetrad. Klaus (Citation1979) described fossil pollen from the Triassic with a distal trichotomosulcate and three trisaccate apertures. He suggested that the aperture system conformed to Garside's rule. Blackmore & Barnes (Citation1995) commented that distal apertures are not strictly comparable with equatorial apertures, even the long-armed trichotomosulcate apertures in some species of Schisandra (Praglowski Citation1976) where the arms can extend, from their polar position down over the equator. Nevertheless, it interesting to note that in Schisandra the additional 3 short apertures are positioned according to Fischer's rule (Huynh Citation1976). Chaloner (Citation1970) examined the trichotomosulcate pollen of the cocoid palm Elaeis guineensis Jacq. and observed of the trichotomosulcus that, “… the three arms along which germination may occur are radial in position – that is, they correspond in position to that of a trilete mark, if such were present (but of course on the opposite face).” He further comments that the radial position is unusual and corresponds to ‘Garside's rule’, whereas, in the majority of angiosperms (that correspond to ‘Fischer's rule’), the three apertures related to tetrad configuration have an inter-radial position. That is to say the apertures lie between the arms of the hypothetical trilete mark.
Triporate Areca pollen
It is tempting to imagine that there must be an intermediate aperture configuration between the zonosulcate and triporate configuration in Areca klingkangensis, but this assumes an evolutionary change over time, rather than a developmental switch that, although unusual, would probably be relatively simple. So far the type of microsporogenesis has not been demonstrated for Areca klingkangensis but, given the common occurrence in the genus of asymmetric mono- and, occasionally, trichotomosulcate pollen, it is likely that microsporogenesis is simultaneous. If the change from one aperture configuration to another in Areca klingkangensis is not complex, then why is it not recorded in any of the other species of Areca that have zonosulcate pollen? The perfect symmetry of the triporate grains encourages speculation that this triporate form has evolved over aeons of time. Counter to this, the occurrence within the same species of a population with zonosulcate pollen suggests that the condition is not stable. Further support for this possible instability is provided by slight evidence, from one or two of the zonosulcate Areca klingkangensis pollen grains, of a partially developed triporate state (). What advantages would triporate apertures give to the pollen grain? One possibility might be an improved breeding opportunity by providing a better distribution of pollen tube outlets, while providing greater protection from dessication of the developing male gametophyte by reducing aperture size. If this were true why, to our knowledge, is this aperture configuration apparently restricted to one population of a single species? The incomplete zonosulcate condition seems to be well-established in a number of species of Areca, but the occurrence of three, presumed equatorially positioned, pores within the genus remains enigmatic. A zonosulcate aperture provides ample opportunity for germination of the developing pollen tube. It also has the possibility that the two halves of the pollen grain have a clam-like action. This would seem to offer a more effective protection from dessication than three non-operculate pores. Curiously, however, although Sclerosperma pollen have opercula we have found a high percentage of sterile pollen in our samples (see next section). Developmental work with zonosulcate pollen of species of Areca is needed to ascertain how the triporate condition might have arisen. It is highly unlikely that the zonosulcate and triporate pollen that occur in Areca klingkangensis will have differing orientations in relation to polarity, although confirmation of this must await results from developmental studies. Zonosulcate and triporate pollen have not been found within the same plant but, given the evidence so far, this is not beyond question. We are not aware of any fossil records of this triporate pollen type.
Triporate Sclerosperma pollen
The uniqueness of tripory in the pollen of Sclerosperma, and the isolated position of the genus within the arecoid palms, does not provide obvious clues regarding the origin of tripory within the genus. Nevertheless, the position of the three pores on the distal face of the pollen grain, combined with simultaneous microsporogenesis, provide strong support to the theory of Erdtman & Singh (Citation1957). This theory proposes that the distal triporate configuration of Sclerosperma pollen apertures is an unusual variant of the more frequent trichotomosulcate condition found in many arecoid palms. Erdtman & Singh (Citation1957) comment that, “…some of the younger grains are ‘crypto-trichotomosulcate’.” We have not observed this condition, and the wall () does not suggest this to be the case in mature pollen grains.
Sclerosperma is in tribe Areceae where species tend to have monosulcate pollen or, if trichotomosulcate pollen occur, it is in association with monosulcate grains. Furthermore, both the mono- and the trichotomosulcate pollen grains often tend to be asymmetric. However, in another arecoid tribe, Cocoeae, Elaeis guineensis (Elaeidinae), and a number of genera in subtribe Bactridinae: (Acrocomia, Bactris, Gastrococos and Astrocaryum) have species with exclusively symmetric trichotomosulcate pollen. It is probable that exclusively symmetric trichotomosulcy is a development from asymmetric monosulcy and, it does not seem unreasonable to speculate that the distal triporate pollen of Sclerosperma represents a rare modification.
In Sclerosperma the triporate condition is apparently stable. There are reliable fossils of Sclerosperma pollen, for example, from Senegal (Medus Citation1975), although many records purporting to be of fossil Sclerosperma pollen are much less convincing (Harley Citation1996, Harley & Srivastava: in prep.) One further, and interesting, point regarding the pollen of the four Sclerosperma collections examined in our study is their apparent sterility. In all collections there is a high percentage, at least 70%, of grains with little or no cellular material (). Whether this is reflected in the reproductive success rate of the genus has not been demonstrated to our knowledge. However, it is noteworthy that these acaulescent palms have a suckering stem habit in all described species (Tuley Citation1995), which suggests that Sclerosperma is not wholly dependant on pollen for reproduction.
Zonosulcate pollen
The occurrence of incompletely or completely zonosulcate pollen in the palms is very interesting. Although not unique to palms – for example, it is quite common in Araceae (Grayum Citation1992) – it is not a common pollen aperture type either in the monocots or in the dicots. There are three other genera of Arecaceae that have species with zonosulcate pollen. Most Salacca species, as many as one third of Korthalsia species (Calamoideae), and the monotypic subfamily Nypoideae (Nypa fruticans Wurmb.). In these three genera the pollen appears to be the result of successive, rather than simultaneous microsporogenesis, although so far demonstrated conclusively only for Nypa fruticans (CitationDavis Citation1966, Harley 1999). Furthermore, the zonosulcus in Nypa pollen has been conclusively demonstrated to be meridionally orientated by observation of the post-meiotic tetrad (Harley Citation1999), whereas in Areca it may well be equatorial, although this remains to be demonstrated unequivocally. It is of interest to note here that there is a striking morphological difference between the exine of the calamoid/nypoid zonosulcates, and the incomplete zonosulcates in Areca. In Areca species the exine is tectate-perforate, or finely reticulate, while in Salacca and Nypa fruticans it is tectate-perforate and spiny or, in Korthalsia, intectate and clavate or spiny clavate (CitationHarley Citation1996, Harley & Baker 2001).
Evidence from Areca suggests that, in palms, possible modifications to the aperture in the transition between monosulcate and zonosulcate, probably includes trichotomosulcate and extended monosulcate. It seems probable that the genes responsible for aperture development require only minor changes to their coding, and perhaps timing, to effect notable morphological differences. It is interesting to note that, systematically, zonosulcy is one of only two unusual aperture types – extended sulcate being the other – that occur in both the calamoid and the arecoid palms. However, observations of extended monosulcate pollen in some species from subfamilies Coryphoideae, Calamoideae and Arecoideae (CitationHarley Citation1999, Harley & Baker 2001) have shown it to result from successive microsporogenesis. The post-meiotic tetrad stage has not been examined for any of the species of Areca. The unusual co-occurrence, within tribe Arecinae, of symmetric monosulcate pollen, and pollen with an extended monosulcus, as well as asymmetric monosulcate pollen and pollen with a trichotomosulcus, suggests that zonosulcy is a labile condition that, like monosulcy, may result from successive or from simultaneous microsporogenesis.
Interesting observations published by Citation CitationPozhidaev (Citation1998, 2000 a, b) report on chance in vivo malformation of apertures in the pollen of a systematically widespread range of over 50 angiosperm families (dicotyledons and monocotyledons). Citation CitationPozhidaev (Citation1998, 2000 a, b) has demonstrated that, where deviant pollen are recorded within different taxa, however widely separated the taxa may be, the variety of deviant pollen aperture forms can be arranged in closely similar series. Monocot examples represent eight families, zonosulcate pollen are represented by a genus with successive microsporogenesis: Rhodospatha (Araceae), as well as one with simultaneous microsporogenesis: Rapatea (Rapateaceae). From his results Pozhidaev (Citation2000 b) hypothesised that the basal aperture configuration, from which all other aperture configurations have derived, is zonosulcate rather than monosulcate. Furthermore, he concludes that all described bi- and radially symmetric aperture configurations result from changes taking place during the developmental process, rather than slow evolutionary adaptation. However, Citation CitationPozhidaev's (Citation1998, 2000 a, b) work is based on mature pollen, and any relationship between malformation and ontogenetic changes requires testing by developmental studies.
Aperture variation within species
Aperture variation within species is not as uncommon as might be supposed. There are many examples, some of which are reviewed by Borsch & Wilde (Citation2000). Nilsson, in Dahlgren & Clifford (Citation1982) observed that, “…among dicotyledonous taxa with pollen aperture configurations bordering on the monocotyledonous pollen type…”, Nymphaeaceae (Nelumbo excluded) (Nymphaeales), may occasionally produce 2-, 3-, or 4-sulculate grains as well as the more usual zonosulcate grains. He observed that: “This may be the case in Euryale, Victoria and even in Nymphaea, although it is not often commented on in the literature (see Erdtman Citation1952)”.
In a study of pollen and anther development in Nelumbo Kreunen & Osborn (Citation1999) observed that aperture formation was first detectable during early free microspore stage, a later stage of development than for the initiation of apertures in many angiosperms. Although most of the pollen grains are triaperturate, a few other aperture conditions were also recorded, of which the most commonly encountered was zonosulcate. At the tetrad stage tetrads were predominantly tetrahedral, although a few tetragonal tetrads were also observed. It would be very interesting to know if there is a correlation between aperture type and tetrad type in Nelumbo but, because aperture development is only detectable at post-tetrad stage, such a correlation would be very difficult to detect. Kreunen & Osborn (Citation1999) conclude that aperture variability in Nelumbo may be correlated with lateness of aperture ontogeny in the genus, although the degree to which aperture variation in the genus can be attributed to phylogeny vs. ontogeny would require additional study.
Pollen morphology and Sect. Microareca
Within Sect. Microareca three Bornean species form a rather distinctive group (Dransfield Citation1984): A. abdulrahmanii, A. andersonii and A. klingkangensis, and this is strongly supported by the incomplete zonosulcate pollen aperture morphology that is found in all three taxa. Stamen number (nine) distinguishes Areca klingkangensis from A. andersonii (six) and A. abdulrahmanii (c. 16). Furthermore, the inflorescences of A. klingkangensis are smaller than in the other two species (Dransfield, Citation1984). The pollen morphologies of A. arundinacea, A. brachypoda, and A. dayung are closely similar (). A. chaiana is regarded as an aberrant member of Areca (Dransfield Citation1984). Its flowers and fruit indicate an affinity with the genus, but it is the only member of the genus with a truly spicate inflorescence. It is particularly interesting to note, therefore, that it also has incomplete zonosulcate pollen, a pollen aperture type not known in Areca, outside section Microareca. Finely reticulate pollen are recorded in a number of species in Areca but not the fine sinuous reticulate exine morphology that appears to be peculiar to A. insignis (A. insignis var. insignis and A. insignis var. moorei).
Pollen characteristics of Bornean species of Areca
All aperture characteristics so far recorded for the genus Areca occur among the Bornean species, with the exception of oblate monoporate pollen, known only in A. caliso, a Philippine endemic in Sect. Axonianthe. However, it is only among the Bornean species in Sect. Microareca that triporate and incomplete zonosulcate pollen are found.
Triaperturate pollen in other monocotyledons
In the monocotyledons triaperturate pollen have been recorded in at least some species of about 20 genera, twelve families, and six orders (; Harley: submit.). Of these, records of triapertury in Dioscorea and Rajanea (Erdtman Citation1952) have not been substantiated by the more recent work of Caddick et al. (Citation1998) who describe pollen of these two genera as “…usually bisulcate……occasionally monosulcate…”. However, there are a number of characteristics that set apart the triporate pollen of Arecaceae from triaperturate (porate or sulcate) pollen of other monocotyledons (Harley: submit.). The most important are the equatorial or subequatorial position, the regular spacing and number (3) of the pores, the uniformity of pore size, a well-defined aperture margo and, with few other exceptions, notably Vanilla (Orchidaceae), simultaneous microsporogenesis (although not confirmed for Areca). All other genera of monocotyledons that may produce pollen with three apertures have successive microsporogenesis.
CONCLUSIONS
Two rare examples of triporate pollen, one in Areca and the other in Sclerosperma have been described for Arecaceae, as well as the co-occurrence of zonosulcy in Areca. So far, within the angiosperms, Areca is the only demonstrable example of a genus where both trichotomosulcy and, presumed, equatorial triapertury occur. The possible orientation of the apertures in relation to the polarity of the pollen grains has been considered, and reasons why tripory should occur within the palms have been discussed, with particular emphasis on trichotomosulcy.
For the present, inaccessibility of young stage inflorescences precludes ontogenetic pollen studies of either Areca klingkangensis, or Sclerosperma spp. Developmental work would permit the observation of the developing microspores, and possible differences in the timing and mechanism of cytokinesis, and of aperture initiation. Microsporogenesis type for Areca klingkangensis could also be ascertained. There is a high probability that both the zonosulcate and the triporate pollen of Areca klingkangensis will prove to be equatorially orientated, although this too awaits results from developmental studies. If these data can be provided they may help to clarify the basis for the apparent differences between the two types of triporate pollen that occur in palms. In the wider context of angiosperm pollen evolution, they may also throw light on the transition of apertures from a distal to an equatorial position.
APPENDIX. SECTIONAL CLASSIFICATION OF ARECA (FURTADO Citation1933)
Subgenus Blumeoareca Furt. (correctly Subgenus Areca)
1. Sect. Arecella Wendl. et Drude
[1. A. borneensis Becc., 2. A. latiloba Ridley, 3. A. laxa Buch.-Hamilt., 4. A. montana Ridl., 5. A. alicae F. Muell., 6. A. triandra Roxb., 7. A. hutchinsoniana Becc., 8. A. vidaliana Becc., 9. A. laosensis Becc.]
2. Sect. Oeotheanthe Scheff. (correctly Sect. Areca)
[10. A. catechu L., 11. A. celebica Burr., 12. A. concinna Thw., 13. A. costulata Becc., 14. A. kinabaluensis Furtado, 15. A. macrocarpa Becc., 16. A. oxycarpa Miq., 17. A. parens Becc., 18. A. whitfordii Becc.]
Subgenus Beccarioareca Furtado
3. Sect. Axonianthe Scheff.
[19. A. caliso Becc., 20. A. camariensis Becc., 21. A. congesta Becc., 22. A. glandiformis Lam, 23. A. ipot Becc., 24. A. jobiensis Becc., 25. A. ledermaniana Becc., 26. A. macrocalyx Burr., 27. A. nannospadix Burr., 28. A. niga-solu Becc., 29. A. rechingeriana Becc., 30. A. torulo Becc., 31. A. warburgiana Becc.]
4. Sect. Microareca Furtado
[32. A. arundinacea Becc., 33. A. bongayensis Becc., 34. A. hewittii Furtado, 35. A. amdjahi Furtado, 36. A. hullettii Furtado, 37. A. minuta Scheff., 38. A. furcata Becc., 39. A. ridleyana Becc.]
5. Sect. Mischophloeus (Scheff.) Becc.
[40. A. guppyana Becc., 41. A. henrici Furtado, 42. A. novo-hibernica Becc., 43. A. paniculata (Miq.) Scheff.]
Acknowledgements
We would like to thank Carol Furness (RBG, Kew) for her time in demonstrating the LR White resin embedding protocols to MH, and Bill Chaloner for stimulating comments on the pollen apertures of Elaeis guineensis. Also, we greatly appreciated the helpful and constructive comments from our three anonymous reviewers.
REFERENCES
- Alexander , M.P. 1969 . Differential staining of aborted and non-aborted pollen. . – Stain Technol. , 44 : 117 – 122 .
- Angiosperm Phylogeny Group. 1998 . An ordinal classification for the families of flowering plants. . – Ann. Mo. Bot. Gard. , 85 : 531 – 553 .
- Belsky , C.Y. and Boltenhagen , E. 1963 . Sporomorphes de position taxonomique incertaine du Cretace superieur du Gabon. . – Grana Palynol. , 4 : 262 – 270 .
- Belsky , C.Y. , Boltenhagen , E. and Potonié , R. 1965 . Sporae dispersae der Oberen Kreide von Gabun, Äquatoriales Afrika. . – Paläontol. Z. , 39 : 72 – 83 .
- Blackmore , S. and Barnes , S. 1995 . Garside's rule and the microspore tetrads of Grevillea rosmarinifolia A. Cunningham and Dryandra polycephala Bentham (Proteaceae). . – Rev. Palaeobot. Palynol. , 85 : 111 – 121 .
- Blackmore S. Crane P.R. 1998 The evolution of apertures in the spores and pollen grains of embryophytes. – In: Reproductive Biology (ed. S.J. Owens & P.J. Rudall), pp. 159–182. – R. Bot. Gard., Kew
- Borsch T. Wilde V. 2000 Pollen variability within species, populations, and individuals, with particular reference to Nelumbo. – In: Pollen and spores: Morphology and biology (ed. M.M. Harley, C. Morton & S. Blackmore), pp. 285–299. – R. Bot. Gard., Kew
- Caddick , L.R. , Furness , C.A. , Stobart , K.L. and Rudall , P.J. 1998 . Microsporogenesis and pollen morphology in Dioscoreales and allied taxa. . – Grana , 37 : 321 – 336 .
- Chaloner , W.G. 1970 . The evolution of miospore polarity. . – Geoscience & Man , 1 : 47 – 56 .
- Cranwell , L.M. 1953 . New Zealand Pollen Studies. The monocotyledons. . – Bull. Auckland Inst. Mus. , 3 : 1 – 91 .
- Dahlgren R.M.T. Clifford H.T. 1982 The monocotyledons: A comparative study. – Acad. Press, London
- Davis G.L. 1966 Systematic embryology of the angiosperms. – Wiley, New York
- Doyle , J.A. 1969 . Cretaceous angiosperm pollen of the Atlantic Coastal Plain and its evolutionary significance. . – J. Arnold Arbor. , 50 : 1 – 35 .
- Doyle J.A. Hotton C.L. 1991 Chapter 9. Diversification of early angiosperm pollen in a cladistic context. – In: Pollen and spores: Patterns of diversification (ed. S. Blackmore & S. Barnes), pp. 169–195. – Clarendon Press, Oxford
- Dransfield , J. 1984 . The genus Areca (Palmae: Arecoideae) in Borneo. . – Kew Bull. , 39 : 1 – 22 .
- Dransfield J. Beentje H.J. 1995 The Palms of Madagascar. – R. Bot.Gard., Kew & The International Palm Society
- Erdtman , G. 1944 . Pollen morphology and plant taxonomy II. Notes on some monocotyledonous pollen types. . – Sv. Bot. Tidskr. , 38 : 163 – 168 .
- Erdtman G. 1952 Pollen Morphology and Plant Taxonomy: Angiosperms. – Almqvist & Wiksell, Stockholm
- Erdtman , G. 1960 . The acetolysis method. A revised description. . – Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Erdtman G. 1969 Handbook of Palynology. An introduction to the study of pollen grains and spores. – Munksgaard, Copenhagen
- Erdtman , G. and Singh , G. 1957 . On the pollen morphology in Sclerosperma mannii. . – Bull. Jard. Bot. Etat. (Bruxelles) , 27 : 217 – 220 .
- Ferguson , I.K. and Harley , M.M. 1993 . The significance of new and recent work on pollen morphology of the Palmae. . – Kew Bull. , 48 : 205 – 243 .
- Furtado , C.X. 1933 . The limits of Areca and its sections. . – Repert. Sp. Nov. Regni Veg. , 33 : 217 – 239 .
- Garside , S. 1946 . The developmental morphology of the pollen of Proteaceae. . – J. S. Afr. Bot. , 12 : 27 – 34 .
- Grayum M.H. 1992 Comparative external pollen ultrastructure of the Araceae and putatively related taxa. – Monogr. Syst. Bot. 43. Mo. Bot. Gard., St. Louis MO
- Halbritter , H.-M. 1992 . Morphologie und systematische Bedeutung des Pollens der Bromeliaceae. . – Grana , 31 : 197 – 212 .
- Harley , M.M. 1990 . Occurrence of simple, tectate, monosulcate or trichotomosulcate pollen grains within the Palmae. . – Rev. Palaeobot. Palynol. , 64 : 137 – 147 .
- Harley M.M. 1996 Palm Pollen and the fossil record. – Ph.D. Thes. E. London Univ./R. Bot. Gard., Kew
- Harley M.M. 1999 Tetrad variation: its influence on pollen form and systematics in the Palmae. – In: The evolution of plant architecture (ed. M.H. Kurmann & A. Hemsley), pp. 289–304.– R. Bot. Gard., Kew
- Harley , M.M. and Baker , W.J. 2001 . Pollen aperture morphology in Arecaceae: application within phylogenetic analyses, and a summary of the fossil record of palm-like pollen. . – Grana , 40 : 45 – 77 .
- Huynh , K.-L. 1976 . L'arrangement du pollen du genre Schisandra (Schisandraceae) et sa signification phylogénique chez les angiospermes. . – Beitr. Biol. Pflanzen , 52 : 227 – 253 .
- Klaus , W. 1979 . Zur entwicklungsgeschichtlichen Bedeutung triadischer, angiospermider Pollenapertur- und Struckturanlagen. . – Beitr. Paläont. Österr. , 6 : 135 – 177 .
- Kosenko V.N. 1990 Possible trends of evolution of pollen grain wall with special reference to the genus Tulipa (Liliaceae). – Bot. Zh. (Moscow & Leningrad) 75: 929–942+2pls. (In Russian)
- Kreunen , S.S. and Osborn , J.M. 1999 . Pollen and anther development in Nelumbo (Nelumbonaceae). . – Am. J. Bot. , 86 : 1662 – 1676 .
- Kuprianova L.A. 1954 Sur la phylogénie des monocotylédones (d'après les données palynologiques). – Ess. Bot. I. Ed. l'Academie des Sciences de L'URSS, pp. 119–127, Leningrad
- Kuprianova , L.A. 1967 . Apertures of pollen grains and their evolution in angiosperms. . – Rev. Palaeobot. Palynol. , 3 : 73 – 80 .
- Kuprianova , L.A. 1969 . On the evolutionary levels in the morphology of pollen grains and spores. . – Pollen Spores , 2 : 333 – 351 .
- Kuprianova , L.A. 1979 . On the possibility of the development of tricolpate pollen from monosulcate. . – Grana , 18 : 1 – 4 .
- Martin , J. , Pedrola , J. and Caujapé . 1993 . Pollen morphology and biometry in Androcymbium (Colchicaceae). . – Can. J. Bot. , 71 : 1369 – 1374 .
- Medus , J. 1975 . Palynologie de sédiments Tertiaires du Sénégal méridional. . – Pollen Spores , 17 : 545 – 601 .
- Meeuse , A.D.J. 1965 . The message of the pollen grains. . – Adv. Fronts Plant Sci. , 11 : 112 – 133 .
- Muller , J. 1970 . Palynological evidence of early differentiation of angiosperms. . – Biol. Rev. , 45 : 417 – 450 .
- Poole , M.M. and Hunt , D.R. 1980 . Pollen morphology and the taxonomy of the Commelinaceae: an exploratory survey. . – Kew Bull. , 33 : 639 – 660 .
- Pozhidaev , A. 1998 . Hypothetical way of pollen aperture patterning 1. Formation of 3-colpate patterns and endoaperture geometry. . – Rev. Palaeobot. Palynol. , 104 : 67 – 83 .
- Pozhidaev , A. 2000 a . Hypothetical way of pollen aperture patterning 2. Formation of polycolpate patterns and pseudoaperture geometry. . – Rev. Palaeobot. Palynol. , 109 : 235 – 254 .
- Pozhidaev A. 2000 b Pollen variety and aperture patterning. – In: Pollen and spores: Morphology and biology (ed. M.M. Harley, C. Morton & S. Blackmore), pp. 205–225. – R. Bot. Gard., Kew
- Praglowski J. 1976 Schisandraceae Bl. – World Pollen and Spore Flora 5. Almqvist & Wiksell, Stockholm
- Punt W. Blackmore S. Nilsson S. Le Thomas A. 1994 Glossary of pollen and spore terminology. – LPP Contrib. Ser.1. LPP Foundation, Utrecht
- Rowley , J.R. and Dahl , A.O. 1962 . The aperture of the pollen grain in Commelinantia. . – Pollen Spores , 4 : 221 – 232 .
- Rudall , P.J. , Furness , C.A. , Chase , M.W. and Fay , M.F. 1997 . Microsporogenesis and pollen sulcus type in Asparagales. . – Can. J. Bot. , 75 : 408 – 430 .
- Schulze , W. 1975 . Beiträge zur Taxonomie der Liliifloren. II. Colchicaceae. – Wiss. Z. Friedrich Schiller-Univ. . (Jena) Math.-Nat. R. , 24 : 417 – 428 .
- Schwanitz , G. 1967 . Untersuchungen zur postmeiotischen mikrosporogenese des Ruppia pollen. . – Pollen Spores , 9 : 9 – 48 .
- Simpson , M.G. 1983 . Pollen ultrastructure of the Haemodoraceae and its taxonomic significance. . – Grana , 22 : 79 – 103 .
- Sousa , G.M. , Wanderley , M.G.L. and Barros , M.A.V. 1997 . Morfologia polínica de espécies de Aechmea Ruiz & Pav. (Bromeliaceae) de Pernambuco, Brasil. . – Bol. Bot. Univ. S. Paulo. , 16 : 21 – 30 .
- Thanikaimoni G. 1970 Les Palmiers: Palynologie et Systematique. – Trav. Sect. Sci. Techn. 11. Inst. Franc., Pondichèry
- Tuley P. 1995 The palms of Africa. – Trendrine Press, Zennor, St Ives, Cornwall
- Uhl N.W. Dransfield J. 1987 Genera Palmarum. A classification of the palms based on the work of H.E. Moore Jr. The L.H. Bailey Hortorium and International.Palm Society. – Allen Press, Lawrence KS
- Walker , J.W. and Doyle , J. 1975 . The bases of angiosperm phylogeny: Palynology. . – Ann. Mo. Bot. Gard. , 62 : 664 – 723 .
- Wilson , T.K. 1964 . Comparative morphology of the Canellaceae. III. Pollen. . – Bot. Gaz. (Chicago) , 125 : 192 – 197 .
- Wodehouse . 1936 . Evolution of pollen grains. . – Bot. Rev. (Lancaster) , 2 : 67 – 84 .
- Zavada , M. 1983 . Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structures. . – Bot. Rev. (Lancaster) , 49 : 331 – 379 .