Abstract
The extant Antarctic dinoflagellate genus Polarella and the southern European Early Jurassic dinoflagellate cyst Umbriadinium are extremely similar in morphology, particularly in their size, ornamentation and tabulation. Polarella is therefore placed in the subfamily Umbriadinioideae on this morphological evidence. The two genera, however, are maintained as separate entities for several reasons including minor differences in tabulation. This means that the stratigraphical distribution of the subfamily Umbriadinioideae is extended from the Early Jurassic (late Pliensbachian - early Toarcian) to Recent. The two species (Polarella glacialis and Umbriadinium mediterraneense) are separated by around 187 Ma. This large stratigraphical gap is an example of the selectivity of the dinoflagellate fossil record, produced by the loss of the capacity of Polarella/Umbriadinium to produce fossilisable cysts during the early Toarcian. The widely differing records of these genera attests to their longevity and wide geographical and ecological ranges.
Dinoflagellates are a monophyletic group of generally unicellular protists that normally possess a special type of nucleus (a dinokaryon) and a pair of characteristic flagella during at least one stage in their life cycle (Fensome et al. Citation1993). They exhibit an extensive fossil record largely comprising resistant, organic-walled (dinosporin) cysts. All fossils unequivocally recognisable as dinoflagellates are Mesozoic and Cenozoic. However, biochemical and cytological evidence strongly indicates a Proterozoic origin for this important phytoplanktonic group (Citation CitationEvitt Citation1985, Moldowan & Talyzina 1998, Fensome et al. 1999), which had a major radiation during the early Mesozoic (CitationFensome et al. Citation1996, Moldowan et al. 1996). Preservable cysts are produced by c. 13–16% of all living dinoflagellate species (Head Citation1996); however, these data refer to the entire dinoflagellate spectrum. Most fossil dinoflagellates belong to the Subclass Peridiniphycidae (Fensome et al. Citation1993), hence the completeness of the peridiniphycidean fossil record is significantly higher than c. 13–16%. The relationships between the relative proportions of cysts/cells and the number of cyst-producing species varied temporally and geographically (Evitt Citation1985). At the familial and generic levels, cyst production may be restricted to a specific time interval. For example, the extant genus Ceratium does not form preservable resting cysts. However, the fossil record from the latest Jurassic to the latest Cretaceous (c. 146–65 Ma) includes numerous ceratioid cysts (CitationWall & Evitt Citation1975, Bint 1986) and more than 100 species have been described (Williams et al. Citation1998). Therefore, there is a c. 65 Ma gap between the youngest fossil ceratioid cysts and the thecate, nonfossilisable species of modern Ceratium.
The systematic classification of dinoflagellates is phylogenetic and based dominantly on the configuration of plates (tabulation; Fensome et al. Citation1993). Tabulation patterns are of primary importance in dinoflagellate classification because they represent sophisticated morphologies which are extremely unlikely to be duplicated in separate lineages (Fensome et al. Citation1999). Numerous studies demonstrate that tabulation patterns are generally highly conservative. The most conservative regions of the cell are the cingulum (equator) and sulcus (mid ventral region), and the hyposome (posterior hemisphere) is normally more conservative than the episome (anterior hemisphere) (Taylor Citation1987, Citation1999). Dinoflagellate groups that are clearly defined on tabulation tend to be coherent within molecular phylogenetic trees (Fensome et al. Citation1999). Six principal tabulation types define most higher taxa; these are dinophysioid, gonyaulacoid-peridinioid, gymnodinioid, nannoceratopsioid, prorocentroid and suessioid (Fensome et al. Citation1993).
THE FAMILY SUESSIACEAE OVERVIEW
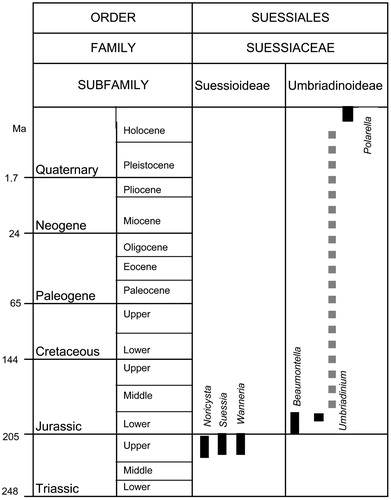
The order Suessiales includes dinoflagellates in which the amphisemal vesicles, usually containing thecal plates, are arranged in seven to ten latitudinal series. This order comprises the families Suessiaceae (fossil dinosporin cysts) and Symbiodiniaceae (extant symbionts; Fensome et al. Citation1993). The family Suessiaceae comprises dinosporin cysts with seven to nine latitudinal paraplate series. This family is known from marine strata of Late Triassic (Carnian-Norian) to Early Jurassic (Pliensbachian-Toarcian) age and includes Beaumontella, Noricysta, Suessia, Umbriadinium and Wanneria. On the basis of the morphologies within the family Suessiaceae, a bipartite subdivision was proposed by Bucefalo Palliani & Riding (Citation2000). The subfamily Suessioideae comprises Late Triassic proximate and proximochorate cyst genera with nontabular or parasutural ornamentation and a combination archaeopyle (Noricysta, Suessia and Wanneria). The subfamily Umbriadinoideae includes Early Jurassic proximochorate and chorate dinoflagellate cysts with intratabular ornamentation and apical or combination archaeopyles (Beaumontella and Umbriadinium). Montresor et al. (Citation1999) described the cells and cysts of Polarella glacialis, a small dinoflagellate from Antarctica using material in culture. Polarella is the only extant species of the family Suessiaceae due to the suessioid tabulation of the vegetative cells, in which the amphiesmal vesicles are arranged into nine latitudinal series.
Polarella and Umbriadinium
Cysts of the extant genus Polarella and the Early Jurassic genus Umbriadinium are similar in morphology. Both are acavate, subspherical to elongate, proximochorate to chorate and relatively small. However, the type species of Umbriadinium, U. mediterraneense Bucefalo Palliani & Riding Citation1997, exhibits a mean diameter of 22 μm, whereas the diameter of Polarella glacialis is c. 10 μm. The autophragm of both cysts is smooth and bears intratabular processes. In Umbriadinium, the intratabular spines indicate the presence of eight or nine latitudinal rows of relatively small, polygonal paraplates. The epicyst of U. mediteraneense comprises four paraplate series; n4, n3, n2 and n1 sensu Below (Citation1987), and four latitudinal series of paraplates have been observed in the hypocyst. The paracingulum is indicated by an extensive equatorial area with rare, randomly inserted spines (Bucefalo Palliani & Riding Citation1997). In Polarella glacialis, there are normally seven series of latitudinal, acicular processes, three on the epicyst and four on the hypocyst, but this configuration is somewhat variable (Montresor et al. Citation1999, p. 189). Processes and other parasutural features are not present in the paracingular area of Polarella. It is not possible to compare the archaeopyle types of these two genera, because the germination of cysts of P. glacialis has not been observed in culture (Montresor et al. Citation1999). The archaeopyle of U. mediterraneense is of combination (disintegration) type, involving the n4, n3 and n2 paraplate series (Bucefalo Palliani & Riding Citation1997).
Montresor et al. (Citation1999) recognised the close similarity of Polarella to members of the family Suessiaceae, but did not recognise the essentially identical morphologies of Polarella and Umbriadinium. However, the provisions of the current International Code of Botanical Nomenclature (ICBN) (Greuter et al. Citation2000), specifically Article 11.7, do not permit the proposal of an emended Umbriadinium is the senior taxonomic synonym of Polarella on the basis that Umbriadinium is the earlier-proposed name. For this action, Umbriadinium would need to be emended to include cysts lacking paracingular ornamentation. Article 11.7 states:
“For purposes of priority, names of fossil taxa (diatoms excepted) compete only with names based on a fossil type representing the same part, life-history stage, or preservational state (see Art. 1.2).”
Thus, names of living plants effectively have priority over names of fossil taxa, irrespective of year of publication. Hence, Polarella cannot legitimately be considered a junior synonym of Umbriadinium. It is better to keep fossil and extant genera apart, thus the separation of Polarella and Umbriadinium is maintained for largely pragmatic and philosophical reasons. Moreover, it is perhaps arguable that the minor tabulation differences (see above) are sufficient to separate these genera. Therefore Polarella and its single species, P. glacialis, are here placed within the subfamily Umbriadinioideae on unequivocal morphological grounds. This subfamily is characterised by acavate, proximochorate to chorate cysts with intratabular ornamentation and a combination or apical archaeopyle (Bucefalo Palliani & Riding Citation2000). The genera constituting this subfamily are Beaumontella, Polarella and Umbriadinium; they are distinguished by their different paracingular morphologies. In Beaumontella, the latitudinal paracingular spines are evenly spaced, whereas in Polarella and Umbriadinium, the paracingulum is indicated by an extensive belt which is devoid of ornamentation or has rare spines respectively. Due to the inclusion of Polarella into the subfamily Umbriadinioideae, the stratigraphical distribution of this subfamily is now Early Jurassic (late Pliensbachian) to Recent ().
The stratigraphical ranges (solid black lines) of the genera belonging to the subfamilies Suessioideae and Umbriadinoideae (adapted from Bucefalo Palliani and Riding Citation2000; fig. 2). The stratigraphical gap between the Early Jurassic and Recent occurrences of Umbriadinium and Polarella is indicated by a discontinuous grey line. The geological ages are taken from de Graciansky et al. (Citation1989).
DISCUSSION
Beaumontella and Umbriadinium are separated from Polarella by approximately 187 Ma (). It appears that cysts of Umbriadinium/Polarella type ceased producing fossilisable cysts during the Pliensbachian (Early Jurassic) and this case is an example of the selectivity of the dinoflagellate fossil record. The consequently strongly disjunct differing records of the Umbriadinioideae indicate the longevity and wide geographical and ecological ranges of this subfamily. Umbriadinium originated in the Early Jurassic of the Mediterranean region (Greece, Hungary, Italy and Portugal) around 25° N to 30° N (Bucefalo Palliani & Riding Citation1997, Citation2000). The type species, U. mediterraneense, is confined to the late Pliensbachian to early Toarcian of the Tethyan Realm. During the Jurassic, the climate of southern Europe was generally equable and virtually subtropical (Rees et al. Citation2000). On the basis of its palaeogeographical distribution, U. mediterraneense, was a low latitude, warm water species (Bucefalo Palliani & Riding Citation1997). The early evolution of the family Suessiaceae has been related to the evolution of scleractinian corals; a symbiotic life strategy has been hypothesised for the Late Triassic-Early Jurassic representatives (CitationLoeblich III & Sherley Citation1979, Bucefalo Palliani & Riding 2000). Polarella glacialis, by contrast, is a free-living dinoflagellate which alternates between motile and encysted phases in seasonal, ice-covered, southern polar seas (Stoecker et al. Citation1997, Citation1998). It is a photosynthetic, cryo- and halotolerant species, especially abundant in land-fast ice, where vegetative cells are found at low temperatures (−1°C to −7°C) and salinity from 20 psu to 140 psu (CitationStoecker et al. Citation1998, Montresor et al. 1999).
Therefore, the evolution of these closely related genera during the last 187 Ma produced substantial changes in ecology and geographical distribution. This suggests how relationships between morphology and palaeoecology of dinoflagellate cysts should not be regarded as consistently uniformitarian, especially where the taxa are unusually long-ranging. The analyses of the SSU ribosomal RNA gene of Polarella glacialis suggests that this species, despite a common phylogenetic pathway with Symbiodinium, underwent a significantly older and independent speciation event (Montresor et al. Citation1999). This is consistent with the Early Jurassic inception of Umbriadinium, which may represent a link between the fossil Suessiales (Late Triassic to Early Jurassic), and the extant members of this order.
ACKNOWLEDGEMENTS
The authors are grateful to Dr Roberto Rettori (University of Perugia) for helpful discussions on taxonomy and systematics during the preparation of the initial draft of this article. Dr Robert A. Fensome of the Geological Survey of Canada (Atlantic) kindly pointed out the existence and implications of Article 11.7 of the ICBN and critically reviewed the manuscript. Drs Stewart G. Molyneux and Michael H. Stephenson (British Geological Survey) also reviewed the manuscript. J. B. Riding publishes with the permission of the Executive Director, British Geological Survey (NERC).
REFERENCES
- Below , R. 1987 . Evolution und Systematik von Dinoflagellaten-Zysten aus der Ordnung Peridiniales. I. Allgemeine Grundlagen und Subfamilie Rhaetogonyaulacoidae (Familie Peridiniaceae). . – Palaeontographica Abt. B , 205 : 1 – 178 .
- Bint , A. N. 1986 . Fossil Ceratiaceae: a restudy and new taxa from the mid-Cretaceous of the Western Interior, U.S.A. . – Palynology , 10 : 135 – 180 .
- Bucefalo Palliani , R. and Riding , J. B. 1997 . Umbriadinium mediterraneense gen. et sp. nov. and Valvaeodinium hirsutum sp. nov.: two dinoflagellate cysts from the Lower Jurassic of the Tethyan Realm. . – Palynology , 21 : 197 – 206 .
- Bucefalo Palliani , R. and Riding , J. B. 2000 . Subdivision of the dinoflagellate cyst family Suesiaceae and discussion of its evolution. . – J. Micropalaeont. , 19 : 133 – 137 .
- de Graciansky P.-C. Hardenbol J. Jacquin T. Vail P. R. (Eds). 1998 Mesozoic and Cenozoic sequence stratigraphy of European basins. – Sp. Publ. No. 60. Soc. Sed. Geol. SEPM, Tulsa OK
- Evitt W. R. 1985 Sporopollenin dinoflagellate cysts: their morphology and interpretation. – AASP, Dallas TX
- Fensome R. A. Taylor F. J. R. Norris G. Sarjeant W. A. S. Wharton D. I. Williams G. L. 1993 A classification of living and fossil dinoflagellates. – Micropaleontology, Sp. Pap. No. 7. Am. Mus. Nat. Hist., New York
- Fensome , R. A. , MacRae , R. A. , Moldowan , J. M. , Taylor , F. J. R. and Williams , G. L. 1996 . The early Mesozoic radiation of dinoflagellates. . – Paleobiology , 22 : 329 – 338 .
- Fensome , R. A. , Saldarriaga , J. F. and Taylor , F. J. R. “Max”. 1999 . Dinoflagellate phylogeny revisited: reconciling morphological and molecular based phylogenies. . – Grana , 38 : 66 – 80 .
- Greuter W. McNeill J. Barrie F. R. Burdet H. M. Demoulin V. Filgueiras T. S. Nicolson D. H. Silva P. C. Skog J. E. Trehane P. Turland N. J. Hawksworth D. L. (Eds). 2000 International Code of Botanical Nomenclature (Saint Louis Code) adopted by the XVI Int. Bot.. Congr., St. Louis 1999. – Regn. Veg. 138. Koeltz Sci. Books, Königstein (G)
- Head M. J. 1996 Chapter 30. Modern dinoflagellate cysts and their biological affinities. – In: Palynology: Principles and applications, Vol. 3 (ed J. Jansonius & D. C. McGregor), pp. 1197–1248. – AASP, Dallas TX
- Loeblich III , A. R. and Sherley , J. L. 1979 . Observations on the theca of the motile phase of free-living and symbiotic isolates of Zooxanthella microadriatica (Freudenthal) comb. nov. . – J. Mar. Biol. Assoc. UK , 59 : 195 – 205 .
- Moldowan , J. M. and Talyzina , N. M. 1998 . Biogeochemical evidence for dinoflagellate ancestors in the Early Cambrian. . – Science , 281 : 1168 – 1170 .
- Moldowan , J. M. , Dahl , J. , Jacobson , S. R. , Huizinga , B. J. , Fago , F. J. , Shetty , R. , Watt , D. S. and Peters , K. E. 1996 . Chemostratigraphic reconstruction of biofacies: molecular evidence linking cyst-forming dinoflagellates with pre-Triassic ancestors. . – Geology , 24 : 158 – 162 .
- Montresor , M. , Procaccini , G. and Stoecker , D. K. 1999 . Polarella glacialis, gen. nov., sp. nov. (Dinophyceae): Suessiaceae are still alive! . – J. Phycol. , 35 : 186 – 197 .
- Rees P. M. Ziegler A. M. Valdes P. J. 2000 Jurassic phytogeography and climates: New data and model comparisons. – In: Warm Climates in Earth History (ed. B. T. Huber, K. G. MacLeod & S. L. Wing), pp. 297–318 – Cambridge Univ. Press, Cambridge UK
- Stoecker , D. K. , Gustafson , D. E. , Merrel , J. R. , Black , M. M. D. and Baier , C. T. 1997 . Excystment and growth of chrysophytes and dinoflagellates at low temperatures and high salinities in Antarctic sea-ice. . – J. Phycol. , 33 : 585 – 595 .
- Stoecker , D. K. , Gustafson , D. E. , Black , M. M. D. and Baier , C. T. 1998 . Population dynamics of microalgae in the upper land-fast sea ice at a snow-free location. . – J. Phycol. , 34 : 60 – 69 .
- Taylor F. J. R. 1987 Dinoflagellate morphology. – In: Biology of dinoflagellates (ed. F. J. R Taylor), pp. 24–91 – Blackwell, Oxford
- Taylor , F. J. R. 1999 . Morphology (tabulation) and molecular evidence for dinoflagellate phylogeny reinforce each another. . – J. Phycol. , 35 : 1 – 3 .
- Wall , D. and Evitt , W. R. 1975 . A comparison of the modern genus Ceratium Schrank, 1793, with certain Cretaceous marine dinoflagellates. . – Micropaleontology , 21 : 14 – 44 .
- Williams G. L. Lentin J. K. Fensome R. A. 1998 The Lentin and Williams index of fossil dinoflagellates 1998 edition. – AASP Contrib. Ser. No. 34 Dallas TX