Abstract
The pollinium morphology of the two members of the Asclepiadoideae, tribe Fockeeae, Fockea Endl. and Cibirhiza Bruyns, has been studied in detail and compared with that of eight genera of Marsdenieae, the tribe in which Fockea and Cibirhiza were previously accommodated and thus their putative closest relatives, as well as nine genera of Asclepiadeae. Both Fockea and Cibirhiza have several morphological characteristics in common, the most important of which is the absence of well-developed caudicula, which distinguishes them from all other genera of Asclepiadoideae known. The pollinium structure of these two genera, however, differs significantly. Whereas the pollinium of Cibirhiza consists of single pollen grains and is covered by a pollinium wall, as is typical for other Asclepiadoideae, the pollinium of Fockea consists of tetrads and is not covered by a pollinium wall, a condition otherwise typical of Secamonoideae. Fockea, however, has only two pollinia per anther, as does Cibirhiza and all other Asclepiadoideae, whereas the Secamonoideae have four pollinia per anther. Sequence data from two intergenic spacers, trnT-L and trnL-F and the trnL intron of cpDNA was analyzed. The ingroup included three species of Fockea and one species of Cibirhiza. The outgroup taxa consisted of three representatives each of Periplocoideae, and Secamonoideae and 24 species of Asclepiadoideae, including representatives of all tribes, of which eight genera belong to Marsdenieae, as outgroups. The results of the DNA analysis provide strong support for Fockeeae as a monophyletic tribe, distinct from Marsdenieae and, to the rest of the Asclepiadoideae. With the exception of pollen data, all morphological and molecular evidence clearly support recognition of the tribe Fockeeae. The occurrence of two such significantly different types of pollinia structure – characters elsewhere in the family used to distinguish subfamilies – within the small tribe Fockeeae was unexpected, and can perhaps best be understood as yet another attestment to the basal position of the Fockeeae in the nascence of the Asclepiadoideae.
Species and voucher specimens from which pollinia were studied and pollinium size.
Vouchers, locality information, and EMBL accession numbers for plant material used in the molecular studies.
The families Asclepiadaceae and Apocynaceae were recently grouped together into one family, the Apocynaceae s.l. (Endress & Bruyns Citation2000) with five subfamilies recognized: Rauvolfioideae Kostel. (rather than Plumerioideae K. Schum.), Apocynoideae Burnett, Periplocoideae R. Br. Ex Endl., Secamonoideae Endl. and Asclepiadoideae R. Br. Ex Burnett.
Previously in the subfamily Asclepiadoideae five tribes were recognized: Fockeeae H. Kunze, Meve & Liede, Marsdenieae Benth., Stapelieae Decne., Gonolobeae Reichb. ex Don and Asclepiadeae (R. Br.) Duby (Liede & Albers Citation1994). Endress & Bruyns (Citation2000) reduced the tribes to three by abandoning Fockeeae and placing it into the tribe Marsdenieae, abandoning Gonolobeae and placing it within tribe Asclepiadeae; further because of the priority rule, the name Ceropegieae Orb. was adopted for the tribe formerly known as Stapelieae. The three tribes in the subfamily Asclepiadoideae, in their treatment are thus: Marsdenieae, Ceropegieae and Asclepiadeae.
The tribe Fockeeae was established by Kunze et al. (Citation1994), and is comprised of only two genera: Fockea Endl., with six recognized species widespread in arid regions of tropical and southern Africa (Victor et al. Citation2000) and Cibirhiza Bruyns, with two disjunct species: one endemic to the Dhofar Province of Oman, the other restricted to Tanzania and Zambia (Kunze et al. Citation1994). The two key characters of the Fockeeae are: (1) the presence of a gynostegial corona fused into an undulate annulus around the base of the gynostegium, in addition to a staminal corona of free segments [C(is)+Cs using the system of Liede & Kunze Citation1993]; (2) the absence of true caudicula in the pollinarium (the two pollinia are sessile on the dorsal side of the corpusculum; Kunze et al. Citation1994). Cibirhiza and Fockea have a similar habit. Both are twining or scrambling perennial herbs (erect in some species of Fockea) with well-developed leaves, and a woody rootstock, which is often greatly enlarged, turnip-shaped, sometimes partly exposed, and functions as a water storage organ. The tuber of both genera is used by the local inhabitants, either as a source of food (Bruyns Citation1988) or for the water stored in it (van Wyk & Gericke Citation2000). The two genera are also very similar in their floral structure. Perhaps the most easily recognizable difference is that in Fockea the apical anther appendages are conspicuously inflated, whereas in Cibirhiza they are flat and membranous. In addition, the lower part of the corolla tube is campanulate in Cibirhiza, but tubular in Fockea. Despite their considerable disjunct distribution, the two species of Cibirhiza are remarkably similar in their habit, overall appearance and floral structure. The only cited difference between the two species is that the staminal corona is simple in C. dhofarensis and tripartite in C. albersiana (Kunze et al. Citation1994).
It has been shown in several independent studies, both morphological and molecular, that Fockea may represent an intermediate stage between Secamonoideae and the rest of the Asclepiadoideae ( Citation CitationKunze Citation1993, 1994, 1996; Citation Citation CitationCiveyrel et al. Citation1998, Civeyrel & Rowe 2001, Fishbein 2001, Potgieter & Albert 2001). In each analysis Fockea appears at the base of the Asclepiadoideae. Relatively little is known about the genus Cibirhiza, however, and it has never been included in any cladistic analysis. In the analysis of Sennblad & Bremer (Citation2000) the plant used as Cibirhiza was actually Fockea multiflora (see details in Material and Methods). Both Cibirhiza and Fockea have never been included in the same cladistic analysis. A logical next step is to do a combined morphological and molecular study including both genera. The study presented here combines a comparative analysis of the pollinia of Fockea and Cibirhiza with an analysis based on the two non-coding intergenic spacers trnT-L and trnL-F and the trnL intron of the two ingroup genera and 30 outgroup taxa. Our main objectives are: (1) to provide additional information on these two genera; (2) to elucidate their position in the Asclepiadoideae; and (3) to determine if the Fockeeae is monophyletic.
MATERIAL AND METHODS
Palynology
Pollinia were obtained from herbarium specimens (). Flowers were rehydrated in 3% phosphate-buffered glutaraldehyde. For scanning electron microscopy (SEM) pollinia were removed from flowers and put into 100% ethanol. The pollinia were air dried and mounted on stubs using double-sided tape, coated with gold and examined with a JEOL Winsem 6400 microscope at 10 kV. Measurements of pollinia were done with the SEM using the measurement facility of the microscope. For transmission electron microscopy (TEM) the rehydrated pollinia were postfixed in 2% osmium tetroxide, stained with 0.5% uranyl acetate, dehydrated in an alcohol series and embedded in Spurr's low-viscosity resin (Spurr Citation1969). Sections were stained with uranyl acetate, followed by lead citrate, and examined with a Philips CM 100 electron microscope at 60 kV.
Terminology
A pollinium is the entire contents of one microsporangium (pollen sac) and forms one pollen unit. A pollinarium is the apparatus for the transport of pollinia and consists of a translator plus the pollinia. Translators are formed out of hardened substances that are secreted in special grooves on the periphery of the uppermost part of the gynoecium in the Apocynaceae. In the Asclepiadoideae the translator consists of a corpusculum and two caudicula. The corpusculum is a hard, usually brown to black body. It is the first part of the translator to be secreted. It is composed of two halves, which are joined at the top to form a hard clamp, into which the leg or mouthpart of the pollinator becomes wedged. The caudicula are secreted later in development and thus retain a softer consistency. They form two slender, flexible arms, which are more or less perpendicular to the corpusculum. One end of a caudiculum is attached to the corpusculum and the other end adheres to the pollinium, after the anther has dehisced. Translators in the Secamonoideae are similar to those in the Asclepiadoideae, except that caudicula are often absent or only poorly developed, so that the pollinia are usually more or less sessile on the corpusculum. In most Secamonoideae the pollinia are more or less sessile on the corpusculum. They are attached to the dorsal side of the corpusculum by secreted substances. These secreted substances may be scarcely more than a layer of adhesive, or they form a short stalk, which has been called a caudiculum. In the genus Secamonopsis, however, well-developed caudicula are present (Citation CitationOmlor Citation1996, Civeyrel & Rowe 2001, Civeyrel et al. 1998). Translators in Periplocoideae, in contrast, consist of a firm but flexible spoon-shaped structure, which can be roughly divided into three morphological parts: a pollen receptacle lined with adhesive on the abaxial side, a stalk, and sticky disc lined with adhesive on the adaxial side, by which the translator becomes attached to the pollinator (Citation Citation CitationDemeter Citation1922, Schill & Jäkel 1978, Schick 1982, Endress 2001).
The terminology used for the walls of the pollinium is as follows: In Fockea the distal walls are the tetrad walls that face towards the outside (periphery) of the pollinium. The proximal walls are the walls that separate tetrads on the inside of the pollinium. The inner walls are the walls that separate individual pollen grains of a tetrad.
Molecular analysis
Taxa. – Two species of Fockea (F. edulis and F. multiflora), and Cibirhiza dhofarensis constitute the ingroup. The plant used for F. multiflora (Specks 248) had been identified erroneously as Cibirhiza albersiana and was included in the analysis of chloroplast rbcL sequences by Sennblad & Bremer (Citation2000) under this name. In addition, the three Fockea collections used by Potgieter & Albert (Citation2001) were included in a second analysis because Potgieter & Albert (Citation2001) had analyzed only the trnL intron and the trnL-F spacer. Unfortunately, two of their three collections are synonymous (F. edulis and F. cylindrica; Court Citation1987), so that only F. sinuata complements the present dataset. As most distant outgroup, three members of Periplocoideae were selected; further outgroups are three representatives of Secamonoideae and 24 members of Asclepiadoideae. Each tribe of Asclepiadoideae and all subtribes of Asclepiadeae (CitationLiede Citation2001, Liede & Täuber 2002) are represented by at least one collection (; refer there for names of authors of species). Eleven representatives of eight genera of Marsdenieae, the tribe to which Fockea and Cibirhiza were previously attributed, have been analyzed.
DNA Extraction and PCR. – DNA was isolated from fresh or dried leaf tissue using a modified CTAB protocol (Doyle & Doyle Citation1987). PCR primers and protocol for the plastid trnT-trnL and trnL-trnF spacers (primers “a”, “b” and “e”, “f”, respectively) and the trnL intron (primers “c” and “d”) correspond exactly to Taberlet et al. (Citation1991). Sequences were obtained on an ABI Prism Model 310 Version 3.0 sequencer. Of the 33 accessions, 17 have been sequenced for this study; the remaining 16 had been deposited at EMBL in the course of earlier studies of SL (accession numbers see ). Three accessions (trnL intron and trnL-F spacer only) were included from the study of Potgieter & Albert (Citation2001).
Data Analysis. – Sequences were pre-aligned with Perkin Elmer Sequence Navigator Version 1.0.1 and subsequently adjusted manually. Indels were coded as missing characters throughout; no separate indel coding was performed, because of doubtful homology in several cases. Phylogenetic analysis and tests for clade support were performed using PAUP version 4.0b8 (PPC) (Swofford Citation1998). Phylogenies were generated using Fitch parsimony as implemented in PAUP* employing heuristic searches, with 1,000 replicates, random stepwise addition, MULPARS off, and tree-bisection-reconnection (TBR) branch swapping. The resulting trees were then used as starting trees for a second round of search with MULPARS on. Our search strategy aimed at finding as many different islands of trees as possible. Sets of equally parsimonious trees recovered from each analysis were summarized by strict consensus. Decay indices (CitationBremer Citation1988, Donoghue et al. 1992) and bootstrap values (Felsenstein Citation1985) derived from 1,000 replicates with random addition sequence and 100-addition sequence replicates (saving a maximum of 100 trees per replicate) were calculated as measures of support for individual clades. Decay analyses were performed with AutoDecay 4.0 (Eriksson Citation1998) in combination with the reverse constraint option of PAUP*.
RESULTS
Palynology
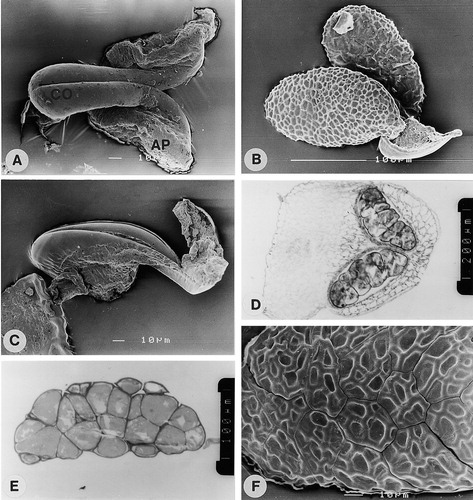
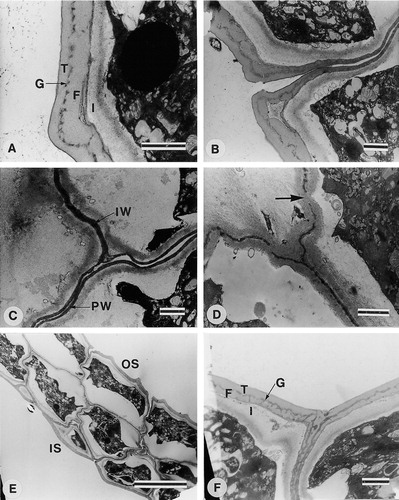
Flowers of Fockea and Cibirhiza have five pollinaria, each of which consists of two pollinia attached to a translator. In Fockea the translator consists of a narrow corpusculum, which is divided by a longitudinal cleft. On the ventral part of the corpusculum adhesive material is present, which spreads out to form two lateral adhesive pads, which anchor the corpusculum to the anthers (). Each of the two pollinia is attached to the upper region of the corpusculum by a short obscure caudiculum (adhesive material; ). The pollinia are almost sessile on the corpusculum. In each anther there are two pollinia (). The pollinia are 164–235 μm long and 106–149 μm in width () and consist of calymmate tetrads, which are decussate or rhomboidal (). The tetrads are coherent, but not fused, and the pollinium is not covered by a pollinium wall (). No pores were observed in any walls of the pollinium. The pollinium wall shows angular depressions on outer surface. The two surfaces of the pollinium are different in that the inner surfaces (the two facing each other) are smoother in appearance than the outer surfaces (). The smoother appearance is the result of a continuous distal wall on the inner surface (). The outer distal surface is discontinuous where tetrads join (). The distal wall structure of the inner () and outer surfaces () are however similar. Civeyrel (Citation1996) describes this for F. capensis as the tectum being continuous on the inner surface, but discontinuous on the outer surface, and this was the first report of a heterogeneous tectum in asclepiads. In the wall structure of the tetrads forming the pollinium there is also a difference between the distal and the proximal walls. The distal wall consists of a tectum (distal exine layer), thin granular layer, foot layer, and intine ( A). The tectum, granular layer and foot layer together are 0.5 to 0.8 μm thick. The proximal walls between tetrads are separated by a space (gap) and consist of only a granular exine layer subtended by an intine ( B, C). At the surface of the pollinium where two tetrads join, the tectum, thin granular layer and foot layer are distinguishable in the proximal wall. However, the tectum becomes progressively thinner towards the inside of pollinium and gradually disappears ( B). On the inside of the pollinium it is not possible to distinguish the thin granular layer and foot layer in proximal walls. The inner walls, which separate individual grains within a tetrad, consist of a granular exine layer subtended by an intine ( C, D). The inner walls are interrupted by intine bridges ( D).
A–F.: (A) SEM of Fockea edulis (Olivier 3042); Translator showing corpusculum (CO) with adhesive pads (AP); (B) SEM of F. multiflora (Giess & Wiss 3310); Pollinarium with two pollinia showing attachment to upper region of corpusculum and difference in the two surfaces of pollinium; The distal, reticulate wall surface of the lower pollinium and the proximal, uneven wall of the upper pollinium are shown; (C) SEM of F. angustifolia (Vorster & Jackson 2160); Attachment of pollinium to corpusculum by adhesive material; D–E. LM of F. sinuata (Smook 6854): (D) Section of anther showing two pollinia; (E) Section of pollinium showing tetrads. (F) SEM of F. angustifolia (Vorster & Jackson 2160); Part of pollinium showing tetrads and absence of pollinium wall. Scale bars – 10 μm (in A, C & F); 100 μm (in B, E); 200 μm (in D).
A–F. TEM of Fockea multiflora (Giess & Wiss 3310): (A) Distal wall of outer surface showing tectum (T), granular layer (G), foot layer (F) and intine (I); (B) Distal and proximal walls at joining of tetrads; (C) Proximal walls (PW) between tetrads and inner wall (IW) separating individual grains of a tetrad; (D) Inner walls interrupted by wall bridges (arrow); (E) Part of pollinium showing outer (OS) and inner surface (IS); (F) Distal wall of inner surface showing wall structure (F=foot layer, G=granular layer, I=intine, T=tectum) and continuous wall where tetrads join. Scale bars – 1 μm (in A–D & F) and 10 μm (in E).
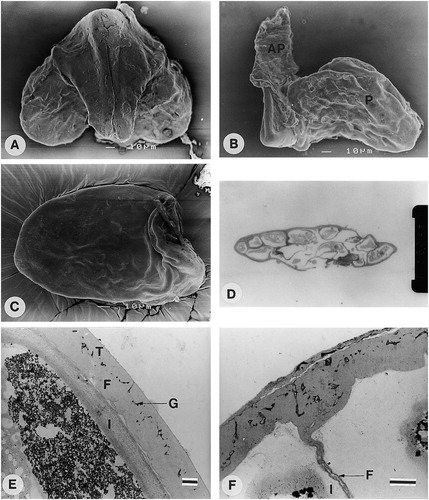
In Cibirhiza the translator consists of a corpusculum, which is broad on the end where the pollinia are attached and narrow at the other end ( A). The other characteristics, namely adhesive pads and attachment of pollinia to corpusculum ( B) are similar to those in Fockea. The pollinia are 114–181 μm long and 73–102 μm in width (). The pollinium consists of single pollen grains, which lack apertures, and the pollinium is covered by a smooth pollinium wall ( C, D). The pollinium wall consists of a tectum (distal exine layer), thin granular layer, foot layer and intine ( E). The tectum, granular layer and foot layer together are 1.7–2.2 μm thick. The inner walls separating the individual pollen grains from each other, consist of a granular layer (electron light) which is flanked, on both sides by an electron dense layer (foot layer) and intine ( F).
A–F.: (A) SEM of Cibirhiza dhofarensis (Miller 7592); Pollinarium showing corpusculum and two pollinia. B–F. C. albersiana (White 6969): (B) SEM. Side view of pollinarium showing corpusculum, pollinium (P) and adhesive pad (AP); (C) SEM. Pollinium with smooth pollinium wall; (D) LM. Section of pollinium showing single pollen grains and pollinium wall; (E) TEM section of pollinium wall showing tectum (T), granular layer (G), foot layer (F) and intine (I); (F) TEM of inner wall separating individual pollen grains showing granular layer (electron light), flanked on both sides by foot layer (F) and intine (I). Scale bars – 10 μm (in A–C); 100 μm (in D); 1 μm (in E, F).
The pollinaria of the eight representatives of Marsdenieae (Dischidia R.Br., Gongronema (Endl.) Decne., Gunnessia P.I. Forst., Hoya R. Br., Marsdenia R. Br., Rhyssolobium E. Mey., Stigmatorhynchus Schltr., and Telosma Coville) and nine representatives of Asclepiadeae (Cynanchum L., Glossonema Decne., Matelea Aubl., Microloma R. Br., Oxystelma R. Br., Pentarrhinum E. Mey., Sarcostemma R. Br., Tylophora R. Br. and Xysmalobium R. Br.) were examined with SEM. All were found to have normally developed caudicula and to have the pollinia covered by a pollinium wall.
The major differences between the pollinia of Cibirhiza and Fockea are: pollinium consisting of tetrads and not covered by a pollinium wall in Fockea vs. pollinium consisting of single pollen grains and covered by a pollinium wall in Cibirhiza.
Molecular analysis
The alignment (available from the authors and from TreeBASE (study accession number=S715, matrix accession number=M1140, M1141, Sanderson et al. Citation1994) comprises 36 taxa and 2,079 characters [1,023 sequence characters in the trnT-trnL spacer (primers a–b), 550 in the trnL intron (primers c–d), and 506 in the trnL-trnF spacer (primers e–f)]. The trnT-L spacer sequence of two Fockea edulis accessions and F. sinuata is unknown, and so are 66 positions at the end of the trnL intron, and both ends of the trnL-T spacer for the same taxa. Remarkably, these three accessions differ in lengths of poly-chains from the Fockea sequences of the Bayreuth lab, supporting the reservations of Liede (Citation2001) against coding varying lengths of such chains as indel characters.
In a first analysis, the 33 taxa for which complete sequence data are available were analyzed; in a second analysis, the three accessions of Potgieter and Albert (Citation2001) were added and analysis restricted to the 988 positions for which data of all taxa were available. Parsimony analysis of the 33 taxa for which complete sequence data were available (203 parsimony informative characters) resulted in 21 trees of 576 steps ( A). The consistency index is 0.742 (excluding uninformative characters) and the retention index is 0.869. Parsimony analysis of trnL intron and trnL-F spacer for all 36 taxa (85 parsimony informative characters) resulted in 406 trees, of 232 steps ( B). The consistency index is 0.769 (excluding uninformative characters), and the retention index is 0.889. While homoplasy measures change only slightly, the loss of 118 parsimony informative characters between the first and the second analysis is reflected in less resolution and lower support for most clades. Nevertheless, Fockeeae remain monophyletic with good support, and also maintain their position at the base of Asclepiadoideae. The other asclepiadoid tribes, Marsdenieae, Ceropegieae and Asclepiadeae, are likewise well supported, and fall into two major well-supported clades, one composed of Marsdenieae+Ceropegieae, and the other comprised solely by the Asclepiadeae. Support for Microloma at the base of Asclepiadeae is derived exclusively from the trnT-L spacer, it switches – unsupported – to the base of the Marsdenieae in the second analysis ( B), a very unlikely position considering its pendent pollinia.
A. Strict consensus of 21 most parsimonious trees resulting from cladistic analysis of 33 taxa for which complete sequence data were available [203 parsimony informative characters, l=576 steps, CI=0.742 (excluding uninformative characters), RI=0.869]. B. Strict consensus of 406 trees resulting from parsimony analysis of the trnL intron and trnL-F spacer for 36 taxa [85 parsimony informative characters, 1=232 steps, CI=0.769 (excluding uninformative characters), RI=0.889]. Numbers indicate bootstrap percentages of 1,000 replicates and decay values.
![A. Strict consensus of 21 most parsimonious trees resulting from cladistic analysis of 33 taxa for which complete sequence data were available [203 parsimony informative characters, l=576 steps, CI=0.742 (excluding uninformative characters), RI=0.869]. B. Strict consensus of 406 trees resulting from parsimony analysis of the trnL intron and trnL-F spacer for 36 taxa [85 parsimony informative characters, 1=232 steps, CI=0.769 (excluding uninformative characters), RI=0.889]. Numbers indicate bootstrap percentages of 1,000 replicates and decay values.](/cms/asset/eaad4771-73ca-443a-92e8-5aa86c979600/sgra_a_9613920_o_-4.jpg)
DISCUSSION
The subfamilies Periplocoideae, Secamonoideae and Asclepiadoideae have long been distinguished by androecial-based characters. In the Periplocoideae tetrads are formed in most of the genera. However, in seven genera, pollinia (four per anther) are present, which consist of tetrads and are not covered by a pollinium wall (Verhoeven & Venter Citation2001). The tetrads and pollinia are presented on a spoon-shaped translator, which is attached to the pollinator by means of an adhesive disk (Schick Citation1982, Citation CitationVerhoeven & Venter Citation1994, 1997, 1998). The Secamonoideae are characterized by pollinia (four per anther), which consist of tetrads and are not covered by a pollinium wall. The pollinia are, however, attached to a corpusculum, which attaches to the pollinator via a clamping mechanism. In Secamone R. Br. the translator was traditionally thought to have no caudicula; the four pollinia were considered to be attached directly onto the back of the corpusculum. Citation CitationCiveyrel (Citation1994, 1995, 1996), however, demonstrated that in some species the secreted substances form a short projection between the pollinia and corpusculum, which she called a “caudicle” (see also Verhoeven & Venter Citation2001, A–B). And in Secamonopsis the presence of well-developed caudicula was demonstrated by both Civeyrel (Citation1996) and Omlor (Citation1996). The Asclepiadoideae are characterized by two pollinia per anther. The pollinia consist of single pollen grains and are covered by a pollinium wall. The pollinia are attached to a corpusculum by a caudiculum (Citation Citation CitationEl-Gazzar & Hamza Citation1973, El-Gazzar et al. 1974, Schill & Jäkel 1978, Dannenbaum & Schill 1991). In the Asclepiadoideae the translator is attached to the pollinator by means of a clasping mechanism as it is in the Secamonoideae.
The general pollinium structure of the genus Fockea differs from that found in other Asclepiadoideae in that the pollinium consists of tetrads, and further, that it is not covered by a pollinium wall; in addition the attachment of pollinium to corpusculum is not by well developed caudicula (Verhoeven & Venter Citation2001). The pollinia are almost sessile on the upper region of the corpusculum, being attached only by a thin layer of adhesive. The pollinia have been described as being attached to the corpusculum via a ‘dorsal process' (Kunze Citation1993) or unshaped adhesive secretion (Kunze et al. Citation1994). Although C shows some kind of caudiculum (very similar to the structure shown in Kunze Citation1993, ), it is not a well differentiated structure as is found in other Asclepiadoideae. The pollinium consisting of tetrads and not being covered by a pollinium wall, and the attachment of pollinium to corpusculum by a poorly differentiated caudiculum are all characteristics reminiscent of those found in the Secamonoideae. Fockea, however, has only two pollinia per anther, as is typical of the Asclepiadoideae, whereas the Secamonoideae have four pollinia per anther. The structure of the distal pollinium wall in Fockea also differs from that found in the Secamonoideae in that it lacks a well-developed granular layer. Rather, it exhibits the typical pollinium wall structure found in the Asclepiadoideae, which consists of a tectum, thin granular layer, foot layer and intine. Thus, based on anther morphology (only two pollen sacs) Fockea is clearly a member of the Asclepiadoideae, whereas the detailed pollinium morphology suggests a position closer to Secamonoideae. The above mentioned characters support an intermediate position for Fockea between the Secamonoideae and the more advanced Asclepiadoideae as was suggested by Citation CitationKunze (Citation1993, 1994, 1996). Furthermore, the pollinia consisting of tetrads and pollinium not covered by a pollinium wall, which characterize Fockea, support the recognition of the tribe Fockeeae, and does not conflict with its placement as the basalmost tribe of the Asclepiadoideae, as has been shown in previous molecular analyses (Citation Citation CitationCiveyrel et al. Citation1998, Civeyrel & Rowe 2001, Fishbein 2001, Potgieter & Albert 2001). In Cibirhiza the attachment of the pollinium to the corpusculum by a scarcely developed caudiculum is otherwise known only in Fockea, and nowhere else in the Asclepiadoideae. The pollinium of Cibirhiza, consisting of single pollen grains and being covered by a pollinium wall, however, is in agreement with the pollinium of all Asclepiadoideae, except Fockea (CitationSchill & Jäkel Citation1978, Dannenbaum & Schill 1991, Verhoeven: unpubl. results). Cibirhiza and Fockea resemble each other in the presence of adhesive pads and absence of a well developed caudiculum. According to Kunze et al. (Citation1994) the structure of the translators presents the most convincing link between the two genera: the absence of true caudicula, attachment of the pollinia to the dorsal side of the corpusculum and the basal adhesive pads affixing the corpusculum to the anther wings, are common to both genera. The pollinium structure, however, is very different in the two genera. Based on detailed pollinium structure (pollinium composed of single grains and covered by a pollinium wall), Cibirhiza occupies a more advanced position than does Fockea, and based on this palynological character alone, would appear to be better placed within the tribe Marsdenieae. According to Bruyns (Citation1988) Cibirhiza is unlike any other genus in the Marsdenieae and either occupies an isolated position within the tribe, or fits into none of the tribes comfortably.
The results of the molecular analysis presented here, however, are much more clear-cut. They show that: (1) despite their very different pollinium structure, Cibirhiza and Fockea (and thus the tribe Fockeeae) form a strongly supported monophyletic group; (2) that the Fockeeae is at the base of, and sister to, all other Asclepiadoideae; and (3) that all other Asclepiadoideae fall into two major clades: one comprised of the Marsdenieae+Ceropegieae, the other of solely the Asclepiadeae. These results support the pattern found previously in other large cladistic analyses, in which, however, the Fockeeae were represented only by the genus Fockea (Citation Citation CitationSennblad & Bremer Citation2000, Civeyrel & Rowe 2001, Fishbein 2001, Potgieter & Albert 2001).
Morphologically Cibirhiza and Fockea are very similar in their overall habit, floral structure (including the type of corona), and, most importantly, they share a type of translator structure found nowhere else in the Asclepiadoideae. This makes it all the more surprising that Cibirhiza has a very different pollinium type than does Fockea. The molecular findings clearly support the close relationship of these two genera as predicted by the majority of the morphological data. Thus, the significant differences in pollinium structure in these two genera, seem best interpreted as a taxonomic red herring.
ACKNOWLEDGEMENTS
The financial support from the National Research Foundation and the University of the Free State is gratefully acknowledged. All the herbaria mentioned are thanked for the kind loan of their specimens. Dr. D. Goyder and the Royal Botanic Gardens, Kew, are thanked for providing material of Cibirhiza.
REFERENCES
- Bremer , K. 1988 . The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. . – Evolution , 42 : 795 – 803 .
- Bruyns , P. V. 1988 . Studies in the flora of Arabia XXI: Cibirhiza, a new genus of Asclepiadaceae from Oman. . – Notes R. Bot. Gard. Edinburgh , 45 : 51 – 54 .
- Civeyrel , L. 1994 . Variation et évolution des types polliniques du genre Secamone (Asclepiadaceae, Secamonoideae). . – C. R. Acad. Sci. (Paris) , 317 : 1159 – 1165 .
- Civeyrel L. 1995 Pollen morphology and ultrastructure of the genus Secamone in Africa. – In: 2 Symp. African Palynol., Tervuren, Belgium (ed. A. Le Thomas & E. Roche), pp. 207–215. – CIFEG, Orléans
- Civeyrel L. 1996 Phylogenie des Asclepiadaceae: Approche palynologique et moleculaire. – Ph.D. Thes., Montpellier Univ., Montpellier
- Civeyrel , L. and Rowe , N. 2001 . Phylogenetic relationships of Secamonoideae based on the plastid gene matK, morphology, and biomechanics. . – Ann. Mo. Bot. Gard. , 88 : 583 – 602 .
- Civeyrel , L. , Le Thomas , A. , Ferguson , K. and Chase , M. 1998 . Critical re-examination of palynological characters used to delimit Asclepiadaceae in comparison to the molecular phylogeny obtained from plastid matK sequences. . – Mol. Phylogen. Evol. , 9 : 517 – 527 .
- Court , D. 1987 . Fockea Endl. – an African genus. . – Asklepios , 40 : 69 – 74 .
- Dannenbaum , C. and Schill , R. 1991 . Die Entwicklung der Pollentetraden und Pollinien bei den Asclepiadaceae. . – Bibl. Bot. , 141 : 1 – 138 .
- Demeter , K. 1922 . Vergleichende Asclepiadeenstudien. . – Flora , 115 : 130 – 176 .
- Donoghue , M. J. , Olmstead , R. G. , Smith , J. F. and Palmer , J. D. 1992 . Phylogenetic relationships of Dipsacales based on rbcL sequences. . – Ann. Mo. Bot. Gard. , 79 : 333 – 345 .
- Doyle , J. J. and Doyle , J. L. 1987 . A rapid DNA isolation procedure for small quantities of fresh leaf tissue. . – Phytochem. Bull. , 19 : 11 – 15 .
- El-Gazzar , A. and Hamza , M. K. 1973 . Morphology of the twin pollinia of Asclepiadaceae. . – Pollen Spores , 15 : 459 – 470 .
- El-Gazzar , A. , Hamza , M. K. and Badawi , A. A. 1974 . Pollen morphology and taxonomy of Asclepiadaceae. . – Pollen Spores , 16 : 227 – 238 .
- Endress , M. E. 2001 . Apocynaceae and Asclepiadaceae: united they stand. . – Haseltonia , 8
- Endress , M. E. and Bruyns , P. V. 2000 . A revised classification of the Apocynaceae s.l. . – Bot. Rev. (Lancaster) , 66 : 1 – 56 .
- Eriksson T. 1998 AutoDecay 4.0. – Stockholm University, Stockholm
- Felsenstein , J. 1985 . Confidence limits on phylogenies: An approach using the bootstrap. . – Evolution , 39 : 783 – 791 .
- Fishbein , M. 2001 . Evolutionary innovation and diversification in the flowers of Asclepiadaceae. . – Ann. Mo. Bot. Gard. , 88 : 603 – 623 .
- Kunze , H. 1993 . Evolution of the translator in Periplocaceae and Asclepiadaceae. . – Plant Syst. Evol. , 185 : 99 – 122 .
- Kunze , H. 1994 . Ontogeny of the translator in Asclepiadaceae s.str. . – Plant Syst. Evol. , 193 : 223 – 242 .
- Kunze , H. 1996 . Morphology of the stamen in the Asclepiadaceae and its systematic relevance. . – Bot. Jahrb. Syst. , 118 : 547 – 579 .
- Kunze , H. , Meve , U. and Liede , S. 1994 . Cibirhiza albersiana, a new species of Asclepiadaceae, and establishment of the tribe Fockeeae. . – Taxon , 43 : 367 – 376 .
- Liede , S. 1997 . American Cynanchum (Asclepiadaceae) – a preliminary infrageneric classification. . – Novon , 7 : 172 – 181 .
- Liede , S. 2001 . Subtribe Astephaninae (Apocynaceae – Asclepiadoideae) reconsidered: New evidence based on cpDNA spacers. . – Ann. Mo. Bot. Gard. , 88 : 657 – 668 .
- Liede , S. and Albers , F. 1994 . Tribal disposition of Asclepiadaceae genera. . – Taxon , 43 : 201 – 231 .
- Liede , S. and Kunze , H. 1993 . A descriptive system for corona analysis in Asclepiadaceae and Periplocaceae. . – Plant Syst. Evol. , 185 : 275 – 284 .
- Liede , S. and Täuber , A. 2002 . Circumscription of the genus Cynanchum (Apocynaceae – Asclepiadoideae). . – Syst. Bot. , 27 : 789 – 800 .
- Omlor , R. 1996 . Do Menabea venenata and Secamonopsis madagascariensis represent missing links between Periplocaceae, Secamonoideae and Marsdenieae (Asclepiadaceae)? . – Kew Bull. , 51 : 695 – 715 .
- Potgieter , K. and Albert , V. A. 2001 . Phylogenetic relationships within Apocynaceae s.l. based on trnL intron and trnL-F spacer sequences and propagule characters. . – Ann. Mo. Bot. Gard. , 88 : 523 – 549 .
- Sanderson , M. J. , Donoghue , M. J. , Piel , W. and Eriksson , T. 1994 . TreeBASE: a prototype database of phylogenetic analyses and an interactive tool for browsing the phylogeny of life. . – Am. J. Bot. , 81 : 183
- Schick , B. 1982 . Zur Morphologie, Entwicklung, Feinstruktur und Funktion des Translators von Periploca L. (Asclepiadaceae). . – Trop. Subtrop. Pflanzenwelt , 40 : 1 – 45 .
- Schill , R. and Jäkel , U. 1978 . Beitrag zur Kenntnis der Asclepiadaceen-Pollinarien. . – Trop. Subtrop. Pflanzenwelt , 22 : 1 – 122 .
- Sennblad , B. and Bremer , B. 2000 . Is there a justification for differential a priori weighting in coding sequences? A case study from rbcL and Apocynaceae s.l. . – Syst. Biol. , 49 : 101 – 113 .
- Spurr , A. A. 1969 . A low-viscosity epoxy resin embedding medium for electron microscopy. . – J. Ultrastr. Res. , 26 : 31 – 43 .
- Swofford D. L. 1998 PAUP*. Phylogenetic analysis using parsimony (*and other methods). Vers. 4. – Sinauer Assoc. Publ., Sunderland MA
- Taberlet , P. , Gielly , L. , Pautou , G. and Bouvet , J. 1991 . Universal primers for amplification of three non-coding regions of chloroplast DNA. . – Plant Mol. Biol. , 17 : 1105 – 1109 .
- Van Wyk B. E. Gericke N. 2000 People's plants. A Guide to useful plants of Southern Africa. – Briza Publ., Pretoria
- Verhoeven , R. L. and Venter , H. J. T. 1994 . Pollen morphology of the Periplocaceae from Madagascar. . – Grana , 33 : 295 – 308 .
- Verhoeven , R. L. and Venter , H. J. T. 1997 . The translator of Raphionacme (Periplocaceae). . – S. Afr. J. Bot. , 63 : 46 – 54 .
- Verhoeven , R. L. and Venter , H. J. T. 1998 . Pollinium structure in Periplocoideae (Apocynaceae). . – Grana , 37 : 1 – 14 .
- Verhoeven , R. L. and Venter , H. J. T. 2001 . Pollen morphology of the Periplocoideae, Secamonoideae and Asclepiadoideae (Apocynaceae). . – Ann. Mo. Bot. Gard. , 88 : 569 – 582 .
- Victor J. E. Bredenkamp C. L. Venter H. J. T. Bruyns P. V. Nicholas A. 2000 Apocynaceae. – In: Seed plants of southern Africa: families and genera (ed. O. A. Leistner). – Strelitzia (Natl. Bot. Inst., Pretoria)10: 71–98.