Abstract
The role of air pollution has been suspected to be an important factor in the recent increase in the incidence of respiratory diseases. The ambient air, which is the carrier of pollen grains, also incorporates pollutants from the atmosphere, leading to alteration of the pollen proteins. Several studies have reported higher risk of development of pollinosis in the areas of dense vehicular traffic as compared to the less polluted areas. The objective of the present investigation was to study the effect of chemical air pollutants (SO2 and NO {\rm _{2}} ) on the soluble total protein content of the pollen of Ricinus communis taken as a model system. Pollen grains of R. communis were collected and exposed to different (100, 200, 300, 400 and 500 μg/m {\rm ^{3}} ) concentrations of SO {\rm _{2}} and NO {\rm _{2}} individually, as well as together, for 8, 16, 32 and 48 hours. Proteins were extracted from exposed pollen and also unexposed pollen, which acted as control. The variation found in the protein content was analysed statistically. In the exposed pollen, total protein content showed a significant decrease (p<0.05) in the extracts from the unexposed pollen to SO {\rm _{2}} and NO {\rm _{2}} . It varied from 1.84 to 11.01 mg/ml for the extracts from pollen exposed to SO {\rm _{2}} only, whereas NO {\rm _{2}} exposure recorded values for total protein from 0.82 to 3.44 mg/ml only. There was a gradual decrease in the soluble protein content with increase in the concentration and duration of exposure in the pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} together (p<0.05).
Mean extractable soluble protein content (mg/ml of 1:20 w/v) of the Ricinus communis pollen exposed to SO {\rm _{2}} for different concentrations and durations.
Analysis of variance (nested design) for total soluble protein content of (mg/ml, 1:20 w/v) Ricinus communis pollen exposed to SO {\rm _{2.}}
Mean soluble protein content of (mg/ml 1:20 w/v) Ricinus communis pollen exposed to NO {\rm _{2}} for different concentrations and durations.
Analysis of variance (nested design) for total soluble protein content (mg/ml of 1:20) of Ricinus communis pollen exposed to NO {\rm _{2}} .
Variation in the mean soluble protein content (mg/ml of 1:20 w/v) of the Ricinus communis pollen exposed to SO {\rm _{2}} +NO {\rm _{2}} for different concentrations and durations.
Analysis of variance (nested design) for total soluble protein content (mg/ml of 1:20) of Ricinus communis pollen exposed to SO {\rm _{2}} +NO {\rm _{2}} .
While travelling in ambient air, pollen is exposed to a variety of atmospheric substances including air pollutants, which could lead to alteration of the pollen surface and their biochemical properties. This could possibly lead to aggravation of allergic symptoms in susceptible individuals or development of symptoms in non-symptomatic. Various epidemiological studies around the globe have correlated air pollution and increased incidence of pollinosis (Citation Citation CitationIshizaki et al. Citation1987, Nicolai 1997, Schafer & Ring 1997, Wyler et al. 2000). Several researchers have also observed retarded plant growth and low pollen production or production of reduced, non-viable and wrinkled pollen, when exposed to pollution (Citation CitationO'Conner et al. Citation1987, Omura et al. 1989, Renzoni et al. 1990).
Extracts of pollen are complex mixtures of many substances such as proteins, glycoproteins, and fatty acids. Well-characterized pollen antigens responsible for immediate hypersensitivity reactions are mostly proteins (Citation CitationRichman & Gissel Citation1988, Park et al. 1999, Rawat et al. 2000). Studies by Ruffin et al. (Citation1986) have shown that after fumigation of pollen with NO {\rm _{2}} , SO {\rm _{2}} and CO, there is an increase in the free amino acids in the pollen extracted. Pollen morphology is distorted and allergenicity enhanced in animal models due to effect of pollutants on pollen. However, another study from Germany suggested no effect of pollution on the allergenicity of the pollen grains (CitationMajd & Ghanati Citation1995, Helander et al. 1997). Thus the effect of pollution on pollen grains is not yet clearly understood.
Delhi is one of the most polluted cities in India, and concentration of atmospheric pollutants often exceed acceptable limits (80 μg/m {\rm ^{3}} for SO {\rm _{2}} and 120 μg/m {\rm ^{3}} for NO {\rm _{2}} ) due to heavy vehicular traffic and burning of coal in power stations. This paper examines protein variability in the pollen before and after exposure to environmental pollutants.
MATERIAL AND METHODS
We selected Ricinus communis (Castor bean) pollen to study the protein variability, if any, before and after exposure to SO {\rm _{2}} and NO {\rm _{2}} the most common pollutants in Delhi air. Pollen of Ricinus communis is an important allergen and is well characterized in India and elsewhere (Citation CitationSingh et al. Citation1992, 1993, Garcia-Gonzalez et al. 1999). It has been established that R. communis can cause allergic symptoms in 5–30% of the symptomatic population in India (Citation CitationShivpuri et al. Citation1979, Shivpuri 1980, Parui et al. 1999).
Collection and storage of Ricinus communis pollen
To procure pure pollen, undehisced inflorescences were collected from the field in relatively unpolluted areas around Delhi during December to February. The dried inflorescences were crushed gently and the pollen grains released were sieved through 100, 200 and 300 mesh/cm {\rm ^{2}} sieves to obtain >95% pure pollen for experimentation. The pollen grains were stored at 4°C in airtight containers for further experimentations.
Exposure of pollen with pollutants
The pollen grains were fumigated with SO {\rm _{2}} and NO {\rm _{2}} individually and together in concentrations of 100, 200, 300, 400 and 500 μg/m {\rm ^{3}} of 8, 16, 32 and 48 hour durations. Pollen grains were placed on a Petri dish and a fine smear was made to avoid overcrowding of grains. The Petri plate containing pollen was kept inside a glass chamber specially fabricated for the purpose over an elevated platform (). The same glass chamber was used for exposure one after the other sequentially for various durations and concentrations as stated above. For the controlled release of NO {\rm _{2}} and SO {\rm _{2}} in the glass chamber, the following chemical reactions were performed and the amount of chemical reagents required for desired concentrations were calculated stoichiometrically as follows:
Diagrammatic sketch of the Glass Chamber used for the exposure of Ricinus communis pollen with pollutants SO2 and NO2.
For SO {\rm _{2}}
Na {\rm _{2}} SO {\rm _{3}} +2HCl⇒2NaCl+SO {\rm _{2}} +H {\rm _{2}} O
FOR NO2
4ZN+4HNO {\rm _{3}} ⇒NO {\rm _{2}} +NO+4ZN (NO {\rm _{3}} ) {\rm _{2}} +H {\rm _{2}} O
During the exposure, the glass chamber was always kept airtight by applying an adhesive and humidity level in chamber was controlled with CaCl {\rm _{2}} kept in a Petri dish along with the pollen powder and chemical reagents. Unexposed pollen grains of R. communis as obtained from the field were used for the control sample.
Preparation of pollen extracts
Defatting – Pollen grains were defatted to remove the lipids and non-specific irritants, with diethyl ether three to four times the volume of pollen material with fresh lots of ether by repeated changes. The pollen, thus defatted, was dried in vacuum desiccators containing calcium chloride for 24–48 hours. These were then stored in dry airtight container at 4°C till extraction.
Extraction of pollen proteins – Defatted, 1 g dry pollen was suspended in 20 ml (1:20 Weight/ Volume) phosphate buffer saline, and extracted at 4°C by continuous stirring for 20 hours. The suspension was cleared by centrifugation at 10,000 rpm for 30 minutes at 4°C. The supernatant was dialyzed against distilled water in viscin dialysis tubing (with cut off point of 7000 Daltons) for 24 hours with frequent changes of distilled water. The antigenic extracts were dispensed in small aliquots, lyophilized and stored at −70°C for future analysis.
Estimation of protein from pollen extracts
The soluble protein content of all the pollen extracts was estimated by Lowry's method with slight modification (Lowry et al. Citation1951). Proteins were precipitated using phosphotungstic acid (15% phosphotungstic acid in 10% hydrochloric acid) and the precipitate was then dissolved in 2 ml of 2% sodium hydroxide solution. Serial dilutions of bovine serum albumin (BSA) were used as standard to calibrate the optical density values for measuring the amount of protein present in the pollen extracts. To minimize the error in total protein estimation of all the extracts, the experiment was repeated five times. The reproducibility within 15% was accepted.
Statistical analysis
The amounts of protein present in extracts from exposed and unexposed pollen (expressed as mg/ml in 1:20 w/v concentration of extract) were statistically analysed using Analysis of Variance (ANOVA). Significance of the observed difference in the protein content (μg/ml) of all the extracts including those of the replications for each exposed pollen sample and control were subjected in a nested design (Zar Citation1999). Influence of four parameters on the protein content was studied as (1) Variation among five replicates of all the extracts and; (2) Variation due to different concentrations of exposed pollutants; (3) Variation due to different time duration of exposure; and (4) Variation due to interaction among concentration of pollutants with duration of exposure (interaction effect).
RESULTS
The average protein content in the antigenic extract prepared from the control (unexposed) R. communis pollen was 5.8 mg/ml in 1:20 (w/v) concentration of the pollen extract.
Pollen exposed to SO {\rm _{2}}
– After 8 hour exposure, all treatment levels showed increased extractable protein compared to the control. At later times the amount of extractable protein was reduced to less than the control (, ). Maximum of 11.01 mg/ml soluble protein was estimated from pollen exposed to 300 μg/m {\rm ^{3}} for 8 hours. Further, with increase in the duration of exposure to 16 hours there was a substantial decrease () in the average protein values as compared to the control and at 8 hours exposure. Apparently, further increase in the duration of exposure did not show much variation in protein content (). The minimum amount of protein (1.08 mg/ml) was recorded from the pollen exposed to 100 μg/m {\rm ^{3}} of SO {\rm _{2}} for 48 hours. There was a significant increase (p<0.05) in the average protein content after 8 hours exposures with different concentrations of SO {\rm _{2}} used except 200 μg/m {\rm ^{3}} , which was 6.0 mg/ml ().
Line diagram depicting total soluble protein content (mg/ml) in 1:20 (w/v) extracts of Ricinus communis pollen exposed to: (A) SO2; (B) NO2; (C) SO2+NO2 of different concentrations and durations of exposures.
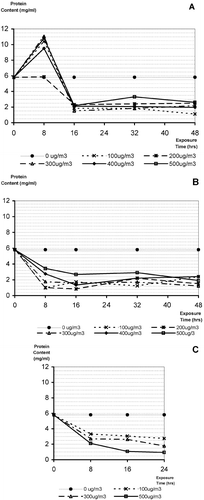
There was a significant (p<0.05) variation amongst five replicates of extracts from pollen exposed to SO {\rm _{2}} () showing experimental conditions varied with time. However, the interaction effect (concentration of SO {\rm _{2}} and duration of exposure taken together as source of variation) was highly significant (p<0.01). This suggests that effect of concentration of SO {\rm _{2}} is not independent of duration of exposure and has cumulative effect on protein content. There was a significant variation (p<0.01) in the values with reduced protein content after an initial increase at 8 hour exposures with SO {\rm _{2}} .
Pollen exposed to NO {\rm _{2}}
– Unlike pollen exposed to SO {\rm _{2}} , there was an immediate significant decline in the total soluble protein content in pollen exposed to NO {\rm _{2}} for 8 hours as compared to unexposed control (). After initial exposure at 100 μg/m {\rm ^{3}} , the value of protein was 0.98 mg/ml after 8 hours, which further decreased to 0.82 mg/ml, the lowest at 200 μg/m {\rm ^{3}} concentrations. The maximum amount of protein (3.44 mg/ml) among exposed pollen was recorded from pollen exposed to 500 μg/m {\rm ^{3}} for 8 hours. The variation in the protein content showed a stabilizing trend with increasing concentration as well as duration of exposure after initial significant decline in protein values at 8 hours of 100 μg/m {\rm ^{3}} of NO {\rm _{2}} ().
As in pollen exposed to SO {\rm _{2}} , a significant decrease in protein content (p<0.01) was also recorded in pollen exposed to NO {\rm _{2}} with increased concentration and duration of exposure together (interaction effect). A significant variation (p<0.01) in protein value was also observed due to increasing concentration of exposure to NO {\rm _{2}} , independently. However, increased duration of exposure alone did not show a significant (p>0.05) variation ().
Pollen exposed to SO {\rm _{2}} +NO {\rm _{2}}
– We studied only three concentrations and durations of exposure of SO {\rm _{2}} and NO {\rm _{2}} together on pollen to observe the effect on protein variability. Average protein content in extract of pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} together showed a substantial decrease as compared to the control (). The estimated values for protein was maximum 3.32 mg/ml in pollen exposed to 100 μg/m {\rm ^{3}} of SO {\rm _{2}} +NO {\rm _{2}} for 8 hours, and minimum 0.93 mg/ml for the pollen exposed to 500 μg/m {\rm ^{3}} for 24 hours. Unlike exposure of pollen with SO {\rm _{2}} and NO {\rm _{2}} independently, a conspicuous decrease in protein content in the pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} together was recorded ().
Summarized observation for all the extracts from pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} together are provided in . The variance among the five replicates of protein content of each extract taken together was not significant (p>0.05). When pollen grains were exposed to SO {\rm _{2}} and NO {\rm _{2}} , together, the interaction effect of duration and concentration of pollutants was not found to be significant (p>0.05), but when both these parameters were considered independently, showed a significant decrease (p<0.01) in the soluble protein content in pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} .
DISCUSSION
Epidemiological studies all over the world have reported a significant increase in the incidence of allergic disorders such as bronchial asthma, allergic rhinitis, atopic dermatitis and Urticaria, during the last few decades (Citation CitationVishwanathan Citation1964, Azpiri et al. 1999, Charpin et al. 2000). In India itself the reported cases of respiratory disorders have increased from 10% in 1964 (Vishwanathan Citation1964) to 30% in year 2000 (CitationChhabra et al. Citation1998, Anonymous 2000). However, it is too short a period for any genetic change to occur. Therefore, environmental factors (including air pollution) seem to be likely explanation for the phenomenon, beside genetic predisposition. Correlation between increased morbidity due to respiratory diseases and increased levels of air pollution has also been shown by various workers (Citation Citation CitationIshizaki et al. Citation1987, Nicolai 1997, Wyler et al. 2000, Ostro et al. 2001). Pollution may affect the respiratory system directly, or indirectly by modifying the allergy causing agents like pollen grains and fungal spores (Citation Citation CitationKoenig et al. Citation1985, Molfino et al. 1991, Rusznak et al. 1994, 1996). The effect of air pollution on the respiratory system of the animals and human is very well documented by various researchers (Citation Citation CitationMatsumara Citation1970, Molfino et al. 1991, Knorst et al. 1994, Kitabatake et al. 1995). Some researchers have reported that, SO {\rm _{2}} , O {\rm _{3}} and NO {\rm _{2}} might damage the pollen grain, not only morphologically but also by reducing fertility and production, leading to reduced sized grains (Citation CitationO'Conner et al. Citation1987, Omura et al. 1989, Majd & Ghanati 1997). Cerceau (1986) reported that the amount of certain mineral exine elements in Dactylis glomerata pollen were changed due to exposure to pollutants and these might also affect the allergenic behaviour of the pollen grains (Cerceau Citation1986). Altered allergenicity of the pollen grains is also a consequence of the interaction of pollen with the air pollution (Citation Citation Citation CitationCerceau Citation1986, Majd & Ghanati 1997, Thomas et al. 1997, Parui et al. 1998, Streenberg et al. 1999). Extracts of pollen are complex mixtures of many substances like proteins and glycoproteins, but well characterized pollen extracts responsible for immediate hypersensitivity reaction are mostly proteins (Citation Citation CitationPuttonen & Pilstrom Citation1980, Richman & Gissel 1988, Park et al. 1999, Rawat et al. 2000). Significant decrease (p<0.05) in protein content from pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} , either individually or together, was recorded for different concentrations and durations. Lower duration (8 hours) of exposure to all the concentrations of SO {\rm _{2}} was however an exception.
This unique phenomenon could be due to damage of the pollen wall by SO {\rm _{2}} to the extent that, even the proteins are released and eluted in the extraction fluid. The initial increase in the protein content was also reported by Parui et al. (Citation1998) and Behrendt et al. (Citation1999) with respect to Argemone mexicana and grass pollen (Phleum pratense), respectively.
However, with increase in the duration of exposure, greater damage to the pollen wall might have occurred which facilitated the faster release of the proteins from the damaged wall. It is hypothesized that the loss of proteins from pollen exposed to pollutants in the atmosphere could be deposited on the ultra-fine, (<1μm) respirable dust particles thus rendering them allergenic (CitationSpieksma et al. Citation1990, Schäppi et al. 1996).
Significant decrease in the protein concentrations in pollen exposed to NO {\rm _{2}} individually, and those exposed to SO {\rm _{2}} +NO {\rm _{2}} together was observed. Exposure to pollution may lead to biochemical changes in the form of breakage of peptide bonds in the proteins and an increase in free amino acids as observed by Ruffin et al. (Citation1986) in red oak pollen. They also observed the lowering of proteins in exposed pollen extracts as compared to the unexposed ones.
The protein content in pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} individually showed that variation is neither dose nor time dependent. Undefined fluctuations in the protein content could possibly be due to change in moisture conditions inside the chamber that was not completely controlled.
Extracts of pollen exposed to SO {\rm _{2}} and NO {\rm _{2}} , independently, showed significant (p<0.05) variation in protein, indicating that the experimental conditions varied with time. Therefore, it is definite that effect of concentration of SO {\rm _{2}} is not independent of duration of exposure and has a cumulative effect on the protein content. However, extracts from pollen exposed to both the pollutants together, variance was not significant (p>0.05), indicating that the conditions of all the five experiments for all extracts were homogenous and standardized.
Although, it is almost impossible to create a laboratory model that will simulate the real environmental situation, only one or two factors can be studied at a time. Not only does the atmosphere contain a number of different particulate and gaseous pollutants, but also their concentration varies from time-to-time and from one location to another. Therefore, the interaction of pollen – pollution is not only controversial but also complicated. However, our results have shown clearly that pollutants change the protein composition of pollen grains.
ACKNOWLEDGEMENTS
Authors are extremely thankful to Dr. C. K. Gupta for statistical analysis of the data and Mr. Pawan Kumar for technical help. One of the authors (A. Bist) acknowledges the receipt of Research Fellowship from UGC.
REFERENCES
- Anonymous 2000 All India coordinated project on aeroallergens and human health. – Rep. Min. Environ. For. (India), New Delhi
- Azpiri , A. , Gamboa , P. M. , Fernandez , E. , Fernandez de Corres , L. , Alonso , E. , Escobar , A. , Jauregui , I. , Audicana , M. I. , Munoz , D. and Antepara , I. 1999 . Prevalence of pollinosis in Basque country. . – Allergy , 54 : 1100 – 1104 .
- Behrendt , H. , Tomczok , J. , Sliwa-Tomczok , W. , Kasche , A. E. , Eschenbach , C. E. von , Becker , W. M. and Ring , J. 1999 . Timothy grass (Phleum pratense L.) pollen as allergen carriers and initiators of an allergic response. . – Int. Arch Allergy Immunol. , 118 : 414 – 418 .
- Cerceau-Larrival M. T. 1986 Recherches biopalynologiques sur Dactylis glomerata L. – In: Pollen of cockfoot (Dactylis glomerata L.) and their environment, Stockholm 1986. Fr.-Sw. Symp. Rep. (ed. Org.Comm.), pp. 13–18 & pl. II, III. – Rep. 51. AFSR. Stockholm
- Charpin , D. , Raherison , D. , Dutau , H. and Taytard , A. 2000 . Epidemiology of respiratory allergies: current data. . – Rev. Mal. Respir. , 17 : 139 – 158 .
- Chhabra , S. K. , Gupta , C. K. , Rajpal , S. and Chhabra , P. 1998 . Prevalence of asthma in schoolchildren in Delhi. . – J. Asthma , 3 : 291 – 296 .
- Garcia-Gonzalez , J. J. , Bartolomé-Zavala , B. , Trigo , P. M.d.M. , Barcelo-Munoz , J. M. , Fernandez-Melendez , S. , Negro-Carrasco , M. A. , Carmona-Bueno , M. J. , Vega-Chicote , J. M. , Munoz , R. C. , Palacios-Pelaez , R. , Cabezudo-Artero , B. and Martinez-Quesada , J. 1999 . Pollinosis to Ricinus communis (Castor bean): an aerobiological, clinical and immunochemical study. . – Clin. Exp. Allergy , 29 : 1265 – 1275 .
- Ghanati F. Majd A. 1997 Ultrastructural variations in certain pollen grains exposed to polluted air. – In: Aerobiology. 5 {\rm ^{th}} Int. Aerobiol. Conf., Bangalore 1994. Proc. Vol. (ed. S.N. Agashe et Coll.), pp. 427–437. – Sci. Publ., Enfield NH/New Delhi
- Helander , M. L. , Savolainen , J. and Ahlholm , J. 1997 . Effects of air pollution and other environmental factors on birch pollen allergens. . – Allergy , 52 : 1207 – 1214 .
- Ishizaki , T. , Koizumi , K. , Ikemori , R. , Ishiyama , Y. and Kushibiki , E. 1987 . Studies of prevalence of Japanese cedar pollinosis among the residents in a densely cultivated area. . – Ann. Allergy , 58 : 265 – 270 .
- Kitabatake , M. , Yamamoto , H. , Yuan , P. F. , Manjural , H. , Maurase , S. and Yamauchi , T. 1995 . Effect of exposure of NO {\rm _{2}} and SO {\rm _{2}} on bronchopulmonary reaction induced by Candida albicans in Guinea pigs. . – J. Toxicol. Environ. Health , 45 : 75 – 82 .
- Knorst , M. M. , Kienast , K. , Riechelmann , H. , Muller-Quernheim , J. and Ferlinz , R. 1994 . Effect of sulfur dioxide on mucocilliary activity and cilliary beat frequency in guinea pig trachea Int. . – Arch. Occup. Environ. Health , 65 : 325 – 328 .
- Koenig , J. Q. , Morgan , M. S. , Horike , M. and Pierson , W. E. 1985 . The effect of sulfur dioxide on nasal and lung function in adolescents with extrinsic asthma. . – J. Allergy Clin. Immunol. , 76 : 813 – 818 .
- Lowry , O. , Rosenborough , N. , Farr , A. and Randall , R. 1951 . Protein measurement with Folin's phenol reagent. . – J. Biol. Chem. , 193 : 263 – 275 .
- Majd , A. and Ghanati , F. 1995 . The effect of air pollution on the allergenicity of Pinus elderica (Pinaceae) pollen. . – Grana , 34 : 208 – 211 .
- Matsumura , Y. 1970 . Effects of Ozone, Nitrogen dioxide and sulphur dioxide on the experimentally induced allergic respiratory disorder in Guinea pigs. I. The effect on sensitization with albumin through the airway. . – Am. Rev. Respire. Dis. , 102 : 430 – 437 .
- Molino , N. A. , Wright , S. C. , Katz , I. , Taro , S. , Silverman , F. , Mclean , P. A. , Salami , J. P. , Arizona , M. , Slushy , A. S. and Camel , N. 1991 . Effect of low concentrations of ozone on inhaled allergen responses in asthmatic subjects. . – Lancet , 338 : 199 – 203 .
- Nicolai , T. 1997 . Epidemiology of pollution induced airway disease: urban / rural differences in East and West Germany. . – Allergy , 52 (Suppl. 38) : 26 – 29 .
- O'Conner , C. J. , Ashford , K. P. and Speeding , D. J. 1987 . Germination and metabolism of Pinus radiata pollen in the presence of sulfur dioxide. . – J. Plant Physiol. , 126 : 373 – 378 .
- Omura , M. , Matsuta , N. , Mariguchi , T. , Kazaki , I. and Akihama , T. 1989 . Variation in physiological and genetic characteristics and pollen grain number in Japanese pear depending on the growing conditions. . – Bull. Fruit Tree Res. , 16 : 11 – 24 .
- Ostro , B. , Lipsett , M. , Mann , J. , Braxton-Owens , H. and White , M. 2001 . Air pollution and exacerbation of asthma in African-American children in Los Angeles. . – Epidemiology , 12 : 200 – 208 .
- Park , J. W. , Ko , S. H. , Kim , C. W. , Jeoung , B. J. and Hong , C. S. 1999 . Identification and characterization of the major allergen of the Humulus japonicus pollen. . – Clin. Exp. Allergy , 29 : 1080 – 1086 .
- Parui , S. , Mondal , A. K. and Mandal , S. 1998 . Protein content and patient skin test sensitivity of the pollen of Argemone mexicana on exposure to SO {\rm _{2}} . . – Grana , 37 : 121 – 124 .
- Parui , A. , Mondal , A. K. and Mandal , S. 1999 . Identification and partial characterization of the allergenic properties of Ricinus communis L. pollen- a new approach. . – Grana , 38 : 311 – 315 .
- Puttonen , E. and Pilstrom , L. 1980 . Purification of birch pollen extract by gel filtration. Chemical and immunological characterization of the fractions. . – Int. Arch. Allergy Appl. Immunol. , 61 : 299 – 307 .
- Rawat , A. , Singh , A. , Gaur , S. N. , Kumar , L. , Roy , I. , Ravindran , P. and Singh , A. B. 2000 . Clinical and immunologic evaluation of Cedrus deodara pollen: a new allergen from India. . – Allergy , 55 : 620 – 626 .
- Renzoni , G. C. , Viegi , L. , Stefani , A. and Onnis , A. 1990 . Difference in vitro germination responses in Pinus pinea pollen from two localities with different levels of pollution. . – Ann. Bot. Fenn. , 27 : 87 – 90 .
- Richman , P. G. and Gissel , D. S. 1988 . A procedure for total protein determination with special application to allergenic extract standardization. . – J. Biol. Stand. , 16 : 225 – 238 .
- Ruffin , J. , Liu , M. Y. G. , Sessoms , R. , Banerjee , S. and Banerjee , U. C. 1986 . Effects of certain atmospheric pollutants (SO {\rm _{2}} , NO {\rm _{2}} and CO) on the soluble amino acids, molecular weight and antigenicity of some airborne pollen grains. . – Cytobios , 46 : 119 – 129 .
- Rusznak , C. , Devalia , J. L. and Davies , R. J. 1994 . The impact of pollution in allergic disease. . – Allergy , 49 : 21 – 27 .
- Rusznak , C. , Devalia , J. L. and Davies , R. J. 1996 . The airway response to asthmatics to inhaled allergen after exposure to pollutants. . – Thorax , 51 : 1105 – 1108 .
- Schafer , T. and Ring , J. 1997 . Epidemiology of allergic disease. . – Allergy , 52 : 14 – 22 .
- Schäpii , G. F. , Monn , C. , Wuthrich , B. and Wanner , H. U. 1996 . Direct determination of allergens in ambient aerosols. Methodological aspects. . – Int. Arch. Allergy Immunol. , 110 : 364 – 370 .
- Shivpuri , D. N. 1980 . Pollen, fungal and insect allergens for nasobronchial allergy patients. . – Asp. Allergy Appl. Immunol. , 13 : 19–23.4
- Shivpuri , D. N. , Singh , A. B. and Babu , C. R. 1979 . New allergenic pollens of Delhi state, India and their clinical significance. . – Ann. Allergy , 42 : 49 – 52 .
- Singh , A. B. , Malik , P. , Gangal , S. V. and Babu , C. R. 1992 . Intraspecific variations in pollen extracts of Ricinus communis prepared from different source materials. . – Grana , 31 : 229 – 235 .
- Singh , A. B. , Malik , P. , Parkash , D. and Gangal , S. V. 1993 . Identification of specific IgE binding proteins in Castor Bean (Ricinus communis) pollen obtained from different source materials. . – Grana , 3 : 376 – 380 .
- Spieksma , F. Th. M. , Kramps , J. A. , Van der Linden , A. C. , Nikkels , B. H. , Plomp , A. , Koerten , H. K. and Dijkman , J. H. 1990 . Evidence of grass-pollen allergenic activity in the smaller micronic atmospheric aerosol fraction. . – Clin. Exp. Allergy , 20 : 273 – 280 .
- Streenberg , P. A. , Dormans , J. A. , van Doorn , C. C. , Middendorp , S. , Vos , J. G. and van Loveren , H. 1999 . A pollen model in the rat for testing adjuvant activity of air pollution components. . – Inhal. Toxicol. , 11 : 1109 – 1122 .
- Thomas , P. , Strube , D. and Przybilla , B. 1997 . Altered skin prick test reactivity and histamine release with extracts from pollen exposed to pollutants. . – Int. Arch. Allergy Immunol. , 113 : 264 – 265 .
- Vishwanathan , R. 1964 . Definition, incidence, etiology and natural history of asthma. . – Ind. J. Chest Dis. , 6 : 108 – 124 .
- Wyler , C. , Fahrlander , B. , Kunzil , N. , Schindler , C. , Liebrich , U. , Perruchoud , A. P. , Leuenberger , P. and Wuthrich , B. 2000 . Exposure to motor vehicle traffic and allergic sensitization. The Swiss study on air pollution and lung diseases in adults (SAPALDIA) team. . – Epidemiology , 11 : 450 – 456 .
- Zar H. J. 1999 Biostatistical analysis. 4 {\rm ^{th}} ed. (pp. 303–311). – Prentice Hall, New Jersey