Abstract
Pollen grains of the seed plant genera Ephedra L. and Welwitschia Hook. f. (Gnetales) are of similar size, shape, and have a polyplicate exine with alternating thicker and thinner regions. Ephedra pollen is considered inaperturate and the exine is shed during germination, leaving the male gametophyte naked. The shed exine curls up and forms a characteristic structure with transverse striations. Such upcurled exines have been found in situ in Early Cretaceous seeds with affinities to Ephedra. The purpose of this study was to document the germination of Welwitschia pollen and investigate whether they also discard their exine during this process.
The pollen grains of Welwitschia are monoaperturate with a distinct, distal sulcus. During germination, the sulcus splits open and the gametophyte expands to a spherical form that extends out of the exine. The pollen tube starts to grow one or two hours later and as in Ephedra, it is displaced towards one side. The exine is not shed but remains as a “cap” that partly covers the male gametophyte. Thus, in this respect the germination process is distinctly different from that in Ephedra and this study demonstrates that discharging the exine during pollen germination is unique to Ephedra, among the polyplicate pollen producing genera in the Gnetales.
The Gnetales are a small group of seed plants encompassing about 65–75 species in three morphologically and ecologically distinct genera, Ephedra L., Gnetum L. and Welwitschia Hook. f. The group has long been considered a remnant of “former greatness” (Arber & Parkin Citation1908), based on the pronounced differences between the genera. This is supported by a fossil record from the Early Cretaceous and onwards. Early Cretaceous fossils include abundant occurrences of dispersed ephedroid pollen (Crane & Lidgard Citation1989, Crane Citation1996), as well as a growing number of Early Cretaceous megafossils related to Ephedra (Martill et al. Citation1993, Wu Citation1999, Guo & Wu Citation2000, Rydin et al. Citation2004, Yang et al. Citation2005) and to Welwitschia (Crane & Upchurch Citation1987, Rydin et al. Citation2003).
Ephedra is the sister group of the Gnetum‐Welwitschia clade (). It comprises 35–45 species of woody scrubs with reduced leaves, inhabiting arid regions of Eurasia and the Americas (Kubitzki Citation1998). Gnetum is pantropical and comprises 30 species, most of them climbers with vegetative features that are strikingly angiospermous in gross morphology (Kubitzki Citation1998). Welwitschia includes a single species, W. mirabilis Hook. f. endemic to the Namibian desert in South West Africa. It is morphologically unique in that the apical meristem forms only three pairs of buds before being inactivated. These produce one pair of cotyledons, one pair of foliage leaves and one pair of scale‐like leaves (Martens Citation1971). The shoot apex looses its meristematic activity and degrades soon after. New leaves are not produced, even though the largest specimens in Namibia are thought to be two thousand years old (Kubitzki Citation1998). Reproductive shoot systems are formed from the scale body, near the base of the leaves.
Fig. 1. Phylogeny of the Gnetales. Relationships among seed plants have not yet been unambiguously resolved and phylogenetic relationships as well as interpretations of character evolution are uncertain. The clade Conifer II comprises Araucariaceae, Podocarpaceae, Sciadopitycaceae, Taxaceae, Cephalotaxaceae, Cupressaceae and Taxodiaceae.
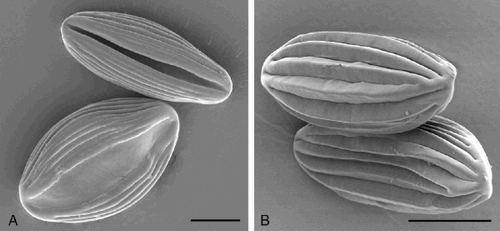
Pollen of Gnetum is small, inaperturate and spherical with a thin, spiny exine (Yao et al. Citation2004), while pollen of Ephedra and Welwitschia are larger and ellipsoid, monosulcate in Welwitschia (), inaperturate in Ephedra (), and characterised by a polyplicate exine of alternating thicker and thinner regions (Steeves & Barghoorn Citation1959, Osborn Citation2000). The morphology and number of ridges varies between species but have also been shown to vary within species, and even within a single microsporangium (El‐Ghazaly & Rowley Citation1997, Ickert‐Bond et al. Citation2003). In Ephedra the pollen exine is shed during germination leaving the male gametophyte naked and the shed exines curl up in a characteristic way (El‐Ghazaly et al. Citation1998).
Fig. 2. Polyplicate pollen grains of the Gnetales (SEM): (A) Welwitschia mirabilis Hook. f. Pollen grains of Welwitschia are monoaperturate with a distinct sulcus; (B) Ephedra altissima Desf. Ephedra grains are inaperturate. Scale bar – 15 μm.
The phenomenon of exine shedding has further been reported for many families in the Conifer II clade: Cephalotaxaceae, Cupressaceae, Sciadopitycaceae, Taxaceae and Taxodiaceae (Wodehouse Citation1935, Southworth Citation1988, Tomlinson Citation1994), and also for Larix and Pseudotsuga of the Pinaceae (Wodehouse Citation1935, Villar et al. Citation1984, Takaso & Owens Citation1994, Slobodnik & Guttenberger Citation2003). However, the conifer grains are not polyplicate and the discarded exines do not curl up to the characteristic striated form of shed Ephedra exines.
During a recent study of coalified seeds from the Early Cretaceous of Buarcos, Portugal (Rydin et al. Citation2004) and Drewry's Bluff, North America (Rydin, Pedersen, Crane, & Friis: in preparation), polyplicate pollen very similar to that of Ephedra was found inside the micropyles of seeds. Some of the pollen grains were split open and curled in a way that matches the shed exines of germinated pollen in extant Ephedra.
Megafossils and dispersed pollen grains showing some features of Welwitschia have previously been found in Drewry's Bluff (Crane & Upchurch Citation1987). Since Welwitschia and Ephedra have similar polyplicate pollen and are both known from the Early Cretaceous, we intended to investigate whether shed polyplicate exines are diagnostic for Ephedra or a shared feature for Ephedra and Welwitschia and perhaps plesiomorphic for the Gnetales. We have therefore carried out experiments in order to document the germination process in Welwitschia.
MATERIAL AND METHODS
The investigations were conducted on freshly harvested anthers from Welwitschia mirabilis Hook. f., cultivated in the Botanical Garden in Berlin‐Dahlem, (voucher: (B) 193‐02‐74‐80). Anthers with pollen were treated with the basal medium described by Brewbaker & Kwack (Citation1963). Two medium temperatures were tested. The medium in one Petri dish was kept at room temperature (18–20°C). The second Petri dish was kept at a temperature of 30–35°C.
The pollen germination and pollen tube growth were examined and documented with light microscope (LM) and scanning electron microscope (SEM).
For SEM studies, germinated pollen was dehydrated in ethanol series (30%, 50%, 70%, 99.5%), and stabilised for 2×3 minutes in Hexamethyldisilazane (HDMS). The grains were mounted on cleaned aluminium stubs, coated with gold in a sputter coater and examined with a Hitachi 4300 scanning electron microscope at 2 kV.
RESULTS
The germination process was notably faster at a temperature of 30–35°C than at room temperature. At the higher temperature pollen germination started after one to three hours and pollen tubes had developed after five hours. In room temperature the grains only started germinating after 16–24 hours.
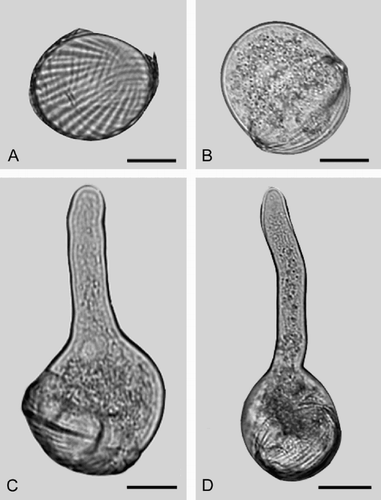
The germination initiated by a split of the sulcus () followed by the gametophyte swelling to a sphere, extending out of the exine (). The exine remains as a “cap” on the gametophyte and persists as the pollen tube continues to grow (). The pollen tube is displaced towards one of the polar ends.
Fig. 3. LM of germinating pollen grains of Welwitschia mirabilis Hook. f.: (A) Germination starts with a split in the sulcus area. (B) The gametophyte expands out of the exine, to a spherical form. (C–D) The pollen tube is initiated towards one of the polar ends. The exine remains as a “cap” on the gametophyte as the pollen tube continues to grow. Scale bars – 20 μm (A–C); 25 μm (D).
DISCUSSION
The similarities in morphology between Ephedra and Welwitschia pollen are pronounced. However, Welwitschia pollen is monoaperturate with a distinct sulcus, and Ephedra grains are inaperturate. Our studies show that pollen germination differs in important aspects between the two polyplicate pollen producing genera of the Gnetales. In Ephedra, the exine is curled up and shed after about three minutes in the basal medium (El‐Ghazaly et al. Citation1998).
Welwitschia pollen, on the other hand, does not shed their exine prior to, or during the germination process. Germination occurs through the finely striated sulcus () and the exine splits up, but is retained as a “cap” on the gametophyte (–). While the male gametophyte of Ephedra is completely exposed when the pollen tube starts to grow (El‐Ghazaly et al. Citation1998), the Welwitschia gametophyte stays partly covered by the exine. The germination experiments show that discarded, upcurled, polyplicate exines do not occur in Welwitschia but are a unique feature for Ephedra. Even though Ephedra grains and Welwitschia grains are similar in size, in the elliptic shape and in the polyplicate exine, the germination process is different and this is also manifested morphologically in the presence of a sulcus in Welwitschia pollen ().
Fig. 4. SEM of germinating pollen grains of Welwitschia mirabilis Hook. f.: A, B. Male gametophyte partly covered by the exine. C, D. The finely striated sulcus area. E. The exine remains on the gametophyte as the pollen tube continues to grow. Scale bars – 15 μm (A, B); 10 μm (C); 2 μm (D); 30 μm (E).

Shedding of the pollen exine prior to germination found in Ephedra (El‐Ghazaly et al. Citation1998) has also been described for many conifers (Wodehouse Citation1935, Villar et al. Citation1984, Southworth Citation1988, Takaso & Owens Citation1994, Tomlinson Citation1994, Slobodnik & Guttenberger Citation2003). Shed exines of conifers are not polyplicate and upcurled and can not be misinterpreted as Ephedra exines, but the character may nevertheless be a synapomorphy for conifers and the Gnetales, modified in several groups, e.g. in Welwitschia and in conifers with saccate pollen. However, the phylogeny of seed plants is intensely debated and not fully understood (Sanderson et al. Citation2000, Magallón & Sanderson Citation2002, Rydin & Källersjö Citation2002, Rydin et al. Citation2002, Burleigh & Mathews Citation2004), which makes interpretations of character evolution uncertain. Shed exines could also have developed separately in several groups, among them the Ephedra lineage ().
The easily recognised shed exines of Ephedra consist of decay‐resistant sporopollenin and we have found them in situ in fossil Ephedra seeds from the Early Cretaceous (early Aptian to early Albian) (Rydin et al. Citation2004, Rydin, Pedersen, Crane & Friis: in preparation). The circular shape of the micropyle tube, the shape of the outer envelope, and the presence of apical papillae lining the inner surface of the outer envelope supported the affinity of these fossils to Ephedra. Polyplicate grains were found in the upper part of the micropylar tube of the fossils and maceration revealed upcurled and shed exines inside the integument on top of the nucellus (Rydin et al. Citation2004). The shed exines found in these Early Cretaceous fossils are more or less identical to those of living Ephedra, but distinct from those of conifers.
The results presented here indicate that shed, upcurled, polyplicate pollen exines constitute a synapomorphy for living and fossil Ephedra. The clay balls of the Drewry's Bluff locality that yielded some of the Ephedra seeds have been dated on palynological evidence to the basal part of Pollen Zone 1 (earliest Aptian) (Brenner Citation1963, Doyle Citation1992). The habit of shedding the pollen exine during germination is thus a character that has been retained within Ephedra at least since the Aptian (c. 120 Myr).
CONCLUSIONS
Our study supports the conclusion that shedding a polyplicate pollen exine prior to germination (El‐Ghazaly et al. Citation1998) is a unique Ephedra character. Though similar to Ephedra pollen in many respects, Welwitschia grains possess an aperture, a sulcus, which splits open and allows the gametophyte to expand. The exine remains as a “cap”, which partly covers the gametophyte during the pollen tube formation. The habit of shedding a polyplicate exine prior to male gametophyte growth and pollen tube formation is not present in Welwitschia and it is evidently a unique feature for fossil and extant Ephedra, established at least by the Aptian. Some conifers also shed the exine during germination but these exines are not polyplicate and can easily be distinguished from those of Ephedra. Upcurled, polyplicate pollen exines can be used as a diagnostic feature for Ephedra, for instance in molecular dating analyses.
Acknowledgements
The authors are grateful to Beat E. Leuenberger at the Botanischer Garten, Berlin‐Dahlem for sending plant material, to Elisabeth Grafström, Swedish Museum of Natural History, for technical assistance and to Kaj Raunsgaard Pedersen, University of Aarhus, for valuable discussion. We also thank S. Blackmore, Royal Botanic Garden at Edinburgh, for useful comments to the work. The study was supported by a grant from the Swedish Research Council to EMF.
References
REFERENCES
- Arber , EAN and Parkin , J . 1908 . Studies on the evolution of angiosperms: the relationship of the angiosperms to the Gnetales. . – Ann. Bot. , 22 : 489 – 515 .
- Brenner , GJ . 1963 . The spores and pollen of the Potomac Group of Maryland. . – Maryland Dept Geol., Mines Water Resourc. Bull. , 27 : 1 – 215 .
- Brewbaker , JL and Kwack , BH . 1963 . The essential role of calcium ion in pollen germination and pollen tube growth. . – Am. J. Bot. , 50 : 859 – 865 .
- Burleigh , JG and Mathews , S . 2004 . Phylogenetic signal in nucleotide data from seed plants: Implications for resolving the seed plant tree of life. . – Am. J. Bot. , 91 : 1599 – 1613 .
- Crane , PR . 1996 . The fossil history of the Gnetales. . – Int. J. Pl. Sci. , 157 (Suppl 6) : S50 – S57 .
- Crane , PR and Lidgard , S . 1989 . Angiosperm diversification and palaeolatitudinal gradients in Cretaceous floristic diversity. . – Science , 246 : 675 – 678 .
- Crane , PR and Upchurch , GR . 1987 . Drewria potomacensis gen. et sp. nov., an Early Cretaceous member of Gnetales from the Potomac Group of Virginia. . – Am. J. Bot. , 74 : 1722 – 1736 .
- Doyle , JA . 1992 . Revised palynological correlations of the lower Potomac Group (USA) and the Cocobeach sequence of Gabon (Barremian‐Aptian). . – Cretaceous Res. , 13 : 337 – 349 .
- El‐Ghazaly , G and Rowley , JR . 1997 . Pollen wall of Ephedra foliata. . – Palynology , 21 : 7 – 18 .
- El‐Ghazaly , G , Rowley , JR and Hesse , H . 1998 . Polarity, aperture condition and germination in pollen grains of Ephedra (Gnetales). . – Pl. Syst. Evol. , 213 : 217 – 231 .
- Guo , S‐X and Wu , X‐W . 2000 . Ephedrites from latest Jurassic Yixian Formation in western Liaoning, Northeast China. . – Acta Palaeontol. Sin. , 39 : 81 – 91 .
- Ickert‐Bond , SM , Skvarla , JJ and Chissoe , WF . 2003 . Pollen dimorphism in Ephedra L. (Ephedraceae). . – Rev. Palaeobot. Palynol. , 124 : 325 – 334 .
- Kubitzki K 1998 The families and genera of vascular plants. – In: Pteridophytes and Gymnosperms. (ed. K. U. Kramer & P. S. Green) – Springer Berlin/Heidelberg
- Magallón , S and Sanderson , MJ . 2002 . Relationships among seed plants inferred from highly conserved genes: sorting conflicting phylogenetic signals among ancient lineages. . – Am. J. Bot. , 89 : 1991 – 2006 .
- Martens P 1971 Les gnétophytes. 2nd ed. by W. Zimmermann, S. Carlquist, P. Ozenda & H. D. Wulff. – Gebr. Borntraeger Berlin/Stuttgart
- Martill DM Brito PM Wenz S Wilby PR 1993 Fossils of the Santana and Crato Formations, Brazil. – In: Field guides to fossils series. 5. (ed. E. A. Jarzembowski) – Palaeontol. Assoc. pp. 1–159 London
- Osborn JM 2000 Pollen morphology and ultrastructure of gymnospermous anthophytes. – In: Pollen and spores: Morphology and biology. (ed. M. M. Harley, C. M. Morton & S. Blackmore) pp. 163–185 – R. Bot. Gards Kew
- Rydin , C and Källersjö , M . 2002 . Taxon sampling and seed plant phylogeny. . – Cladistics , 18 : 485 – 513 .
- Rydin , C , Källersjö , M and Friis , EM . 2002 . Seed plant relationships and the systematic position of Gnetales based on nuclear and chloroplast DNA: conflicting data, rooting problems and the monophyly of conifers. . – Int. J. Pl. Sci. , 163 : 197 – 214 .
- Rydin , C , Mohr , B and Friis , EM . 2003 . Cratonia cotyledon gen. et sp. nov.: a unique Cretaceous seedling related to Welwitschia. . – Biol. Lett. R. Soc. London , 270 : 29 – 32 .
- Rydin , C , Pedersen , KR and Friis , EM . 2004 . On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. . – Proc. Natl Acad. Sci. USA , 101 : 16571 – 16576 .
- Sanderson , MJ , Wojciechowski , MF , Hu , JM , Sher Khan , T and Brady , SG . 2000 . Error, bias, and Long‐branch attraction in data for two chloroplast photosystem genes in seed plants. . – Molec. Biol. Evol. , 17 : 782 – 797 .
- Slobodnik , B and Guttenberger , H . 2003 . Pollination mechanism of European larch (Larix decidua). . – Biologia , 58 : 95 – 102 .
- Southworth , D . 1988 . Isolation of exines from gymnosperms. . – Am. J. Bot. , 75 : 15 – 21 .
- Steeves , MW and Barghoorn , ES . 1959 . The pollen of Ephedra. . – J. Arnold Arbor. , 40 : 221 – 255 .
- Takaso , T and Owens , JN . 1994 . Effects of ovular secretions on pollen in Pseudotsuga menziesii (Pinaceae). . – Am. J. Bot. , 81 : 504 – 513 .
- Tomlinson , PB . 1994 . Functional morphology of saccate pollen in conifers with special reference to Podocarpaceae. . – Int. J. Pl. Sci. , 155 : 699 – 715 .
- Villar , M , Knox , RB and Dumas , C . 1984 . Effective pollination period and nature of pollen‐collecting apparatus in the gymnosperm Larix leptolepis. . – Ann. Bot. , 53 : 279 – 284 .
- Wodehouse RP 1935 Pollen grains; their structure, identification and significance in science and medicine. – McGraw‐Hill New York
- Wu S‐Q 1999 A preliminary study of the Jehol flora from western Liaoning. – Palaeoworld 11: 7–37 (in Chinese with English summary)
- Yang , Y , Geng , B‐Y , Dilcher , DL , Chen , Z‐D and Lott , TA . 2005 . Morphology and affinities of an Early Cretaceous Ephedra (Ephedraceae) from China. . – Am. J. Bot. , 92 : 231 – 241 .
- Yao , Y‐F , Xi , Y‐Z , Geng , B‐Y and Li , C‐S . 2004 . The exine ultrastructure of pollen grains in Gnetum (Gnetaceae) from China and it's bearing on the relationship with the ANITA Group. . – Bot. J. Linn. Soc. , 146 : 415 – 425 .