Abstract
Stellate pore ornamentation is an unusual feature of angiosperm pollen, so far it is known in only ten genera of Amaranthaceae. The pollen grains of these plants have apertures with large hook‐shaped ektexinous bodies that are stellately arranged. Previous studies interpreted this character complex as a synapomorphy in consideration of its strong specialization. By reconstructing the evolution of stellate pore ornamentation based on phylogenetic trees of Amaranthaceae obtained by parsimony, likelihood, and Bayesian analysis of chloroplast trnK/matK DNA sequences, clear evidence is provided for several independent origins and reversals to less specialized aperture types. In addition to the gomphrenoid genus Pseudoplantago, stellate pore ornamentation evolved several times among achyranthoid genera, which have an African distribution. The most derived apertures, with 5 – 6 large protruding hooks, appear independently in Centemopsis on the one hand, and in Psilotrichum sericeum on the other. In an effort to break down the complex character syndrome of stellate pore ornamentation, we delimited a set of six pollen morphological characters that could be independently traced on the phylogeny. It appears that stellate pore ornamentation was independently derived from apertures with equally spread ektexinous bodies that became hook‐shaped, reduced in number, and symmetrically arranged. Likewise the symmetrically arranged, rectangular ektexinous bodies in Marcelliopsis represent an independent specialization. In view of this pattern of morphological changes, functional significance in the context of specialized insect pollination syndromes and positive selection for stellate pore ornamentation is hypothesized. Stellate pore ornamentation provides an example of a specialized pollen character complex with adaptive significance, and underlines the need for a dense taxon sampling for analyses of character evolution.
The Amaranthaceae‐Chenopodiaceae alliance is well‐established as monophyletic within the angiosperm order Caryophyllales (Rodman Citation1994, Downie et al. Citation1997, Cuénoud et al. Citation2002). It is closely related to Achatocarpaceae and Caryophyllaceae within the Caryophyllales I‐clade (as defined in Hilu et al. Citation2003), including Asteropeiaceae, Simmondsiaceae, Rhabdodendraceae, and the Centrospermae as conventionally delimited (Cronquist & Thorne Citation1994). Detailed molecular analyses of the Amaranthaceae‐Chenopodiaceae‐alliance based on chloroplast rbcL (Kadereit et al. Citation2003), ndhF sequences (Pratt Citation2003) and matK/ trnK (Müller & Borsch Citation2005) show the Amaranthaceae sensu stricto (Schinz Citation1934) as monophyletic but reveal several major lineages of Chenopodiaceae. Exact relationships of these lineages as yet are not fully understood. Therefore, we prefer to use the name Amaranthaceae in the sense of Schinz (Citation1934). This is contrary to the circumscription of the Angiosperm Phylogeny Group (APG2 Citation2003) which includes Chenopodiaceae within the Amaranthaceae s.l.. Amaranthaceae s.s. comprises 77 genera with a total of approximately 840 species (Müller & Borsch Citation2005) and are particularly diverse in the tropics in contrast to the largely temperate Chenopodiaceae.
Phylogenetic analyses within Amaranthaceae revealed a basal grade of Bosea (Macaronesian islands, Cyprus, Himalaya), followed by Charpentiera (endemic to Hawaii and the Australian Ridge) with high support (Kadereit et al. Citation2003, Müller & Borsch Citation2005). While the tribe Celosieae was found to be monophyletic, and appeared as sister to Chamissoa and Amaranthus most other tribes and subtribes (Townsend Citation1993) were revealed to be para‐ or polyphyletic. Molecular data in particular, showed strong support for the polyphyly of Aervinae, which is the most speciose and arguably most heterogeneous subtribe in Townsend's classification. Furthermore, Aervinae includes most of the taxa exhibiting stellate pore ornamentation (cf. ). An achyranthoid, and an aervoid clade composed of former members of Aervinae, were newly resolved in the study of Müller & Borsch (Citation2005) composed of taxa formerly included in Aervinae. However, several “stellate genera” were not included in that study. The genus Pseudoplantago, also with stellate pore ornamentation, is nested within a monophyletic subfamily Gomphrenoideae (Kadereit et al. Citation2003, Müller & Borsch Citation2005). Pseudoplantago shares some morphological features with Amaranthoideae and others with Gomphrenoideae and, therefore, it was suggested that it was in a position that linked both subfamilies, for example, Townsend (Citation1993). The fact that Pseudoplantago is obviously not closely related to other Aervinae suggested independent evolution of stellate pore ornamentation in this genus (Müller & Borsch Citation2005). However, phylogenetic relationships for the majority of genera with stellate pore ornamentation remain unknown.
Pollen of Amaranthaceae is spheroidal ( –); only a few exceptions to this rule occur, for example, the 4‐porate tetrahedral or cuboidal grains of Pseudoplantago. With the exception of this genus and the similarly 4‐porate pollen of Nothosaerva, pollen in Amaranthaceae is pantoporate with pore number ranging from 6 (e.g. Psilotrichum boivinianum) to>250 (e.g. Froelichia floridana) (Borsch Citation1998). Pollen in most genera is tectate, although semitectate pollen is known for some genera with metareticulate pollen (Borsch & Barthlott Citation1998). Pollen with a microspinose, punctuate tectum is the rule, and rarely, the punctae are annulate (e.g. in Mechowia: ) (SP/PT‐Tectum, Nowicke Citation1994). The variation in size, shape, and number of, the ektexinous bodies covering the pores provide most of the morphological variation observed in the pores of Amaranthaceae pollen ( –).
Figure 1. Pollen grains (general view; aperture detail below) of selected Amaranthaceae, arrangement reflecting relationships in : Psilotrichum ferrugineum, Allmaniopsis, and basal achyranthoids. A, B. Psilotrichum ferrugineum; C, D. Allmaniopsis fruticulosa; E, F. Psilotrichum africanum; G, H. Arthraerua leubnitziae; I, J. Calicorema capitata; K, L. Pupalia lappacea. Scale bar – 5 μm (A, C, E, G, I, K); 2 μm (B, D, F, H, J, L).
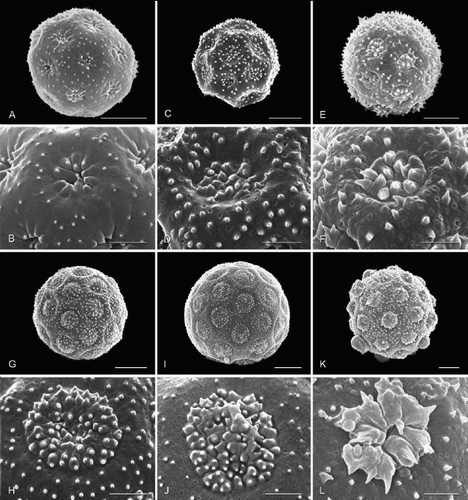
Figure 2. Pollen grains (total view; aperture detail below) of selected Amaranthaceae, arrangement reflecting relationships in : achyranthoids (continued). A, B. Psilotrichum sericeum; C, D. Marcelliopsis splendens; E, F. Kyphocarpa trichinoides; G, H. Sericocoma avolans; I, J. Sericocoma heterochiton; K, L. Sericorema sericea. Scale bar – 5 μm (A, C, E, G, I, K); 2 μm (B, D, F, H, J, L).
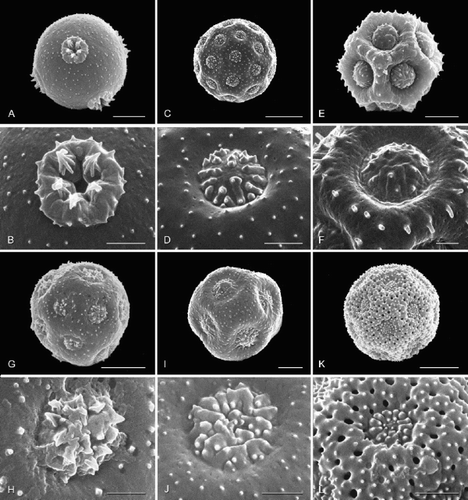
Figure 3. Pollen grains (general view; aperture detail below) of selected Amaranthaceae, arrangement reflecting relationships in : achyranthoids (continued). A, B. Calicorema squarrosa; C, D. Mechowia grandiflora; E, F. Centemopsis micrantha; G, H. Centemopsis trinervis; I Psilotrichum sericeum, grain from same anther as in A, B. Scale bar – 5 μm (A, C, E, G); 2 μm (B, D, F, H, I).
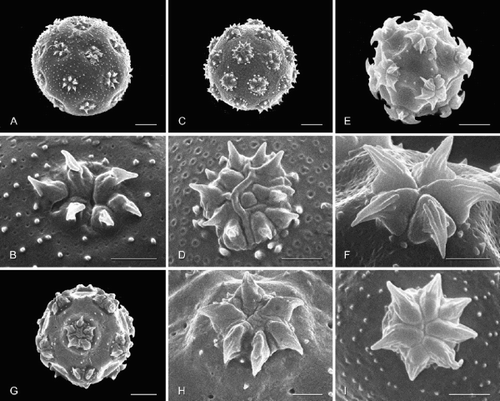
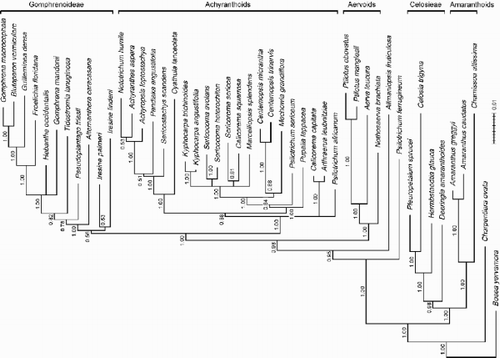
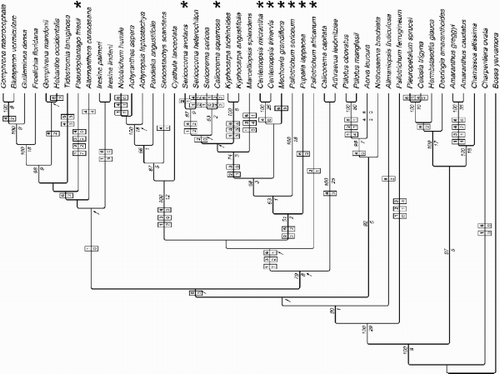
Figure 4. 50%‐majority‐rule‐consensus tree of the Bayesian analysis with posterior probabilities above branches. Mean branch lengths of the trees sampled from the posterior distribution are shown; the scale bar represents 0.01 substitutions per site. The maximum likelihood tree is identical in topology (with polytomies resulting from collapsing very short branches). Clade names follow Müller & Borsch (Citation2005).
Together with Polygonaceae (Nowicke Citation1994), Amaranthaceae exhibits the highest pollen morphological diversity in the Caryophyllales. Therefore, pollen has been viewed as a valuable source of information for taxonomic work in pre‐molecular treatments of the family (Livingstone et al. Citation1973, Townsend Citation1978, Citation1979, Citation1993, Leach et al. Citation1993, Borsch Citation1995, Borsch & Pedersen Citation1997). Based on a survey of pollen morphology in Amaranthaceae (Borsch Citation1998) it has been suggested that, compared to other morphological characters used in Amaranthaceae taxonomy, pollen characters may be less prone to homoplasy (Borsch Citation1998). With the application of molecular phylogenetics to research on Amaranthaceae evolution (Kadereit et al. Citation2003, Müller & Borsch Citation2005), this conjecture has in general been validated. An example is metareticulate pollen with deeply vaulted mesoporia (Borsch & Barthlott Citation1998), which is a synapomorphy for a newly defined core gomphrenoid clade (Kadereit et al. Citation2003, Müller & Borsch Citation2005). However, for other pollen characters, such as aperture number and grain diameter, initial studies (Müller & Borsch Citation2005) have indicated rather homoplastic behaviour. This is also true of the aperture type for which the term “stellate pore ornamentation” was coined (Livingstone et al. Citation1973).
In pollen with stellate pore ornamentation, the pore membrane bears wedge‐shaped segments in a radial arrangement – ‘flecks of ektexine’ (Livingstone et al. Citation1973, Skvarla & Nowicke Citation1976). In the following account these are termed ‘ektexinous bodies' (Borsch Citation1998). Each of these ektexinous bodies covers a more or less equal area of the pore and is distally elongated into an outwardly projecting hook (e.g., ). Among all angiosperm species examined to date, stellate pore ornamentation has been found to occur only in Amaranthaceae. Until now the function and adaptive value of these exceptional pollen structures remain unclear. Livingstone et al. (Citation1973) discussed the possibility of a functional role in pollination, arguing that the hooks might cling to insect pollinators or to the stigmatic surface. They also thought that a thickened structure over each pore might be advantageous to taxa inhabiting xeric environments. Rapid pore dilatation was observed when pollen with stellate pore structure was immersed in a glycerol/ethanol solution (Livingstone et al. Citation1973).
Initially stellate pore structures were only known from the genera Centemopsis, Psilotrichum, and Pupalia, and, in view of the complexity of the pattern observed, it was considered to be a synapomorphy for these genera (Livingstone et al. Citation1973). All three genera were included in tribe Aervinae (Schinz Citation1934, Townsend, Citation1993). This reasoning led Livingstone et al. (Citation1973) to refute an alternative classification system of Amaranthaceae by Cavaco (Cavaco Citation1962), in which taxa with stellate pore ornamentation are distributed between two tribes (Achyrantheae, Digereae). In a more recent survey of pollen morphology in Amaranthaceae, stellate pore ornamentation was shown to exist in ten genera (Borsch Citation1998), some of which did not appear to be closely related based on a number of other morphological characters ().
Table I. Genera of Amaranthaceae with stellate pore ornamentation (aperture type IV of Borsch Citation 1998 : 131).
In this study our aim was to investigate the evolution of stellate aperture structures, as one of the most curious pollen aperture features known in angiosperms. By doing so, two hypotheses proposed by Livingstone (Citation1973), one of the function and the other on correlation with habitats with stellate pore ornamentation will be discussed in relation to the first hypothesis on phylogenetic relationships of the respective taxa. To achieve this dense sampling was needed, and this also had to include putative relatives from tribe Aervinae that do not have stellate pore ornamentation. Therefore, we extended the existing molecular and pollen morphological datasets, as well as examining further species from genera where stellate pore ornamentation has been reported but where there is no molecular evidence of monophyly.
Material and methods
Sampling design and material
In this study, we use chloroplast trnK intron sequences including the gene matK. An initial 91‐taxa analysis comprised all Amaranthaceae and Chenopodiaceae used in Müller & Borsch (Citation2005) plus a further 15 Amaranthaceae from tribe Aervinae, as well as two more species from Chenopodiaceae, for a better representation of tribe Betoideae. Achatocarpaceae (Phaulothamnus and Achatocarpus) were used as the outgroup. Unlike Müller & Borsch (Citation2005) no further families from Caryophyllales were included, as the sistergroup relationship of Achatocarpaceae to the Amaranthaceae‐Chenopodiaceae clade appeared to be clear. The purpose of analyzing the 91‐taxon dataset was to test if the newly added genera fall within the Amaranthaceae clade and if the hypothesis of Bosea as first branch can be verified (trees not shown here). Since this was the case, we excluded Chenopodiaceae, and used Bosea as the outgroup for the more detailed analyses which aimed to resolve relationships of amaranthaceous taxa with stellate pore ornamentation. This way computational time for the likelihood based analyses could be kept at a minimum. We further reduced the sampling by avoiding multiple representations of genera (e.g. Alternanthera) for which monophyly had already been suggested by previous studies, with the exception of taxa with long branches, for example, Ptilotus. Altogether 47 taxa were retained in the analyses of stellate pore ornamentation.
Material used in the 47‐taxon dataset is listed in . The two additional Chenopodiaceae included in the 91‐taxa‐dataset of matK/trnK are Oreobliton thesioides Dur. & Moq. (Tunisia; P. Stipacek & C. Scheuer s.n. (M); AY875638) and Patellifolia procumbens (C. Sm.) Scott, Ford‐Lloyd & Williams (Tenerife, Canary Islands; K. Müller 752 (BONN); AY875637).
Table II. Taxa for which trnK was sequenced and/or pollen was studied.
Where possible, identical plants were used both for DNA‐extraction and pollen analysis. Material was collected in the field (leaf tissue was dried in silica gel and pollen was preserved in ‘Copenhagen mixture’ (Bridson & Forman Citation1998) or from plants cultivated in the Bonn Botanical Gardens. In some cases, recently collected herbarium specimens were also used for DNA isolation. Field work in South Africa provided material for a number of the relevant taxa.
Electron microscopy of pollen grains
Pollen morphology was studied for all taxa shown in . In some cases, the information was taken from Borsch (Citation1998) or from Eliasson (Citation1988; see ). While for the 19 taxa (see ), new pollen preparations were examined with scanning electron microscopy (SEM).
Air‐dried pollen taken directly from herbarium specimens was used for SEM. Pollen grains were mounted on aluminium stubs previously covered with a Carbon Leit‐Tab (Plano GmbH Marburg) and coated with gold for 1 min 30 s at 20 mA (ca. 25 nm) using a sputter coater (Balzers Union SCD 040, Balzers GmbH, Wiesbaden). Samples were analysed in a Cambridge S 200 SEM equipped with a LaB6 ‐cathode for high resolution.
DNA sequencing, alignment, and Indel Coding
DNA isolation, amplification, and sequencing followed the protocols described in Müller & Borsch (Citation2005). Most of the primers used are those given in Müller & Borsch (Citation2005). lists additional primers that were newly generated for the present study using SeqState (Müller Citation2005), because the newly added species accumulated substitutions at internal binding sites. Alignment of the sequences was performed with help of the alignment editor software QuickAlign (Müller & Müller Citation2003) following rules outlined in Löhne & Borsch (Citation2005). The following regions of ambiguous alignment (mutational hotspots) were excluded from the phylogenetic analysis: positions 413‐427, 602‐637, 690‐793, 1082‐1112, 2903‐2930, and 3073‐3111. The 47‐taxa dataset comprised 2951 characters, out of which 559 were parsimony informative. To incorporate information from length‐mutational events in trnK, we used “simple indel coding” (Simmons & Ochoterena Citation2000). The binary matrix of 132 indel characters encoded according to this coding scheme was generated with SeqState (Müller 2005). Alignment and indel matrix are available upon request.
Table III. Internal sequencing primers not already published in Müller and Borsch ( Citation 2005 ) for the trnK intron in Amaranthaceae.
Phylogenetic analyses
Maximum likelihood and parsimony analyses were conducted using PAUP version 4.0 (Swofford Citation1998). Parsimony ratchet searches (Nixon Citation1999) were performed with the help of PRAP (Müller Citation2004), using ten random addition cycles of 200 ratchet iterations each, with the weight of 25% perturbed characters set to 2. Bootstrapping was used to estimate statistic support for individual nodes found under parsimony, using 10 000 replicates with one simple addition search each. Bremer support (Bremer Citation1988) was also calculated with the help of PRAP, using the reverse constraint method (Bremer Citation1994) and the parsimony ratchet algorithm (Nixon Citation1999).
For Bayesian inference of phylogeny and maximum likelihood analyses, the DNA substitution model of best fit was determined using the Akaike information criterion (Akaike Citation1974) calculated with Modeltest (Posada & Crandall Citation1998). The optimal model was GTR+Γ+I. Bayesian inference of phylogeny was performed with MrBayes 3 (Ronquist & Huelsenbeck Citation2003). Priors were set as follows: all topologies equally probable a priori; branch lengths unconstrained; gamma shape α: uniform (0.05, 50.00); invariable sites: uniform (0.00, 1.00); rate matrix: flat dirichlet; state frequencies: flat dirichlet. Sampling trees from the posterior probability distribution was done using four parallel Markov chains of 1 million generations each, with the temperature of the heated chain set to 0.2. This was repeated three times, each time starting from random trees. After confirming congruence of all three runs, clade posterior probabilities were determined by computing a majority rule consensus tree based on the trees sampled after the burn ins of the individual runs.
Delimitation of and state assignment for pollen characters
Matching DNA‐ and morphological matrices were obtained through the exemplar approach, representing taxa by single species, as opposed to the compartmentalization approach (Mishler Citation1994). For a discussion on advantages and disadvantages of both methods see Doyle et al. (Citation2000). For both pollen and nucleotide data, the states present in these exact individuals retained in the matrix were scored (but see for where specimens differed).
Character complexes (i.e. pollen and aperture types; see Borsch Citation1998) were split up until an independent variation of the individual characters could no longer be hypothesized. We are aware, however, that the independence of characters, to a large extent, can be only safely addressed a posteriori, based on precise knowledge of phylogenetic relationships and tests such as the Concentrated Changes Test (Maddison Citation1990) or a pairwise comparison approach (Maddison Citation2000). Aggravating with respect to the problem of character delimitation is that currently construction principles and ontogenetic pathways in pollen are not yet sufficiently understood, in particular with respect to the ektexine that might also be considered as consisting of a single element of construction.
We delimited six characters (). The characters were unordered to avoid the subjectivity associated with an assignment of differential transformation costs (however, the assumption of equal costs is also ad hoc). For aperture diameter and for number of ektexinous bodies, the distribution of absolute values was first examined and then character states were delimited based on discontinuities apparent in their distribution (Excel files available upon request). The average from several apertures was used to assign states and to get absolute numbers (characters 4 and 6). The pollen character matrix analysed is provided in .
Table IV. Pollen morphological characters and character states related to aperture ornamentation in Amaranthaceae .
Table V. Morphological character matrix for the taxa and pollen characters investigated in this study.
Ancestral state reconstruction of pollen characters
Reconstruction of ancestral character state changes was obtained with the aid of WinClada (Nixon Citation1996). Both the “fast” and “slow” optimisation schemes in the program were examined (assuming accelerated or delayed transitions, respectively). Character state changes obtained under the fast optimisation scheme were plotted on a tree with help of TreeGraph (Müller & Müller Citation2004). The Bayesian tree was chosen for character optimization because it was better resolved than, but congruent with, parsimony and likelihood trees, only differing in places that remained unresolved (collapsed short branches [likelihood], multiple equally short trees [parsimony]) or unsupported (Bootstrap<50). A fully dichotomous topology for character mapping was obtained by showing groups with less than 50% support that are compatible with the groups already included in the 50%‐majority‐rule‐consensus tree. In addition, character histories were traced and branches shaded with MacClade (Maddison & Maddison Citation1992).
Results
Pollen morphology of taxa with stellate pore ornamentation and their close relatives
The pollen grains and apertures of taxa with stellate pore ornamentation and their close relatives were documented with SEM ( –). The arrangement in these figures follows the phylogenetic relationships inferred in this study (, ). In addition, a second grain of Psilotrichum sericeum (from the same anther) is shown in (cf. ) to illustrate pore dilatation caused by harmomegathy.
Figure 5. Consensus tree of the Bayesian analysis, showing groups with less than 50% support that are compatible with the groups already included in the 50%‐majority‐ruleconsensus tree. Character state transformations of pollen morphological characters are indicated in boxes (above branch: character number; below branch: character state that is changed to). Numbers in italics above branches are bootstrap percentages; those below branches are Bremer support values from the parsimony analysis. Clades not resolved in the strict consensus tree or 50%‐majority‐ruleconsensus tree of the parsimony bootstrap analysis are marked with an arrow. Terminals that exhibit the complete syndrome of stellate pore ornamentation are marked with an asterisk (i.e., character 1 state 7, character 2 state 2, character 3 state 3).
Phylogenetic relationships among taxa with stellate pore ornamentation
Results of the likelihood‐based phylogenetic analyses are summarized as a 50%‐majority‐rule‐consensus tree of the Bayesian inference (BI) with posterior probabilities above branches (). Maximum likelihood (ML) analysis yielded one tree with a –ln likelihood of 35654.23953 (parameter estimates: base frequencies A=0.31999, C=0.15167, G=0.17559, T=0.35275; proportion of invariable sites 0.066291; shape parameter α 1.14114; substitution rate matrix AC=1.198351, AG=1.613757, AT=0.258184, CG=0.841617, CT=1.985360). The ML tree was identical in topology to the BI tree (), which also extends to polytomies that result from collapsing very short branches. Parsimony analyses including 132 encoded indels (24 parsimony informative), resulted in 264 shortest trees (CI 0.676, RI 0.690, RC 0.466, HI 0.324). Overall resolution was lower, and clades not resolved in the strict consensus tree, or 50%‐majority‐rule‐consensus tree of the parsimony bootstrap analysis, are marked ().
The core structure of the Amaranthoid‐Celosieae and aervoid clades was found to be similar to Müller & Borsch (Citation2005) (). The extended sampling in this study shows Psilotrichum to be polyphyletic. P. ferrugineum appears as an isolated basal lineage in the tree followed by the monotypic Allmaniopsis, while the two other species of Psilotrichum occupy distant positions in the achyranthoid clade.
Most of the taxa newly sampled for this study fall into the previously recognized achyranthoid clade, therefore, it is considerably extended (). However, resolution is limited among achyranthoids. Centemopsis (stellate pore ornamentation throughout) and Kyphocarpa (no stellate pore ornamentation) appear monophyletic, though not all species were sampled.
Sericocoma is also monophyletic, despite being heterogeneous in pollen morphology (only S. avolans has stellate pore ornamentation; ). It is noted that the pollen shown represents the average appearance and that the hook‐like appearance of ektexinous bodies in S. avolans can be significantly more pronounced than apparent from .
Strong evidence is provided for Calicorema to be polyphyletic. C. capitata (no stellate pore ornamentation; ) is resolved as sister to Arthraerua, while C. squarrosa (stellate pore ornamentation; ) appears in a clade with Sericorema, Sericocoma, Kyphocarpa, and Marcelliopsis, with some evidence for Sericorema being the closest relative.
Evolution of characters connected with aperture ornamentation
Pollen characters were optimised on a consensus tree of the Bayesian analysis, which also included those groups with less than 50% support that were not in conflict with the groups already present in the 50%‐majority‐rule‐consensus tree. This tree () shows character state transformations as boxed numbers.
A character state change towards the conspicuous hooks seen in taxa with stellate pore ornamentation (character 1, state 7; ) cannot be unambiguously optimised. According to the fast scheme, the character state was acquired in the common ancestor of the achyranthoid clade above Calicorema capitata / Arthraerua (). It was later lost in the subclade of the achyranthoids that includes Cyathula. According to the slow scheme, the character state evolved twice in parallel: (i) in Psilotrichum africanum; (ii) in the subclade extending from Pupalia to Sericocoma in . In either optimisation, the character state was subsequently independently reversed four times (), namely in Marcelliopsis (), Kyphocarpa (), Sericorema (), and Sericocoma heterochiton (). The state was independently acquired in Pseudoplantago.
Ektexinous bodies (character 2) exceeding pore radius (state 2) were independently derived twice: once in the branch leading to Pupalia, and once in the branch leading to Pseudoplantago (). Fast and slow optimisations agree in a reversal of state 2 in the ancestor of the clade embracing Marcelliopsis to Sericocoma (), with a later reacquisition in Calicorema.
As to the arrangement of ektexinous bodies on the pore, the reconstructed pattern is similar to that of character 1: Either two parallel events in Psilotrichum africanum, and in the subclade from Pupalia to Sericocoma (), or one state change in the last common ancestor of the achyranthoid clade with the exception of Calicorema capitata and Arthraerua, followed by a loss in the subclade of the achyranthoids that includes Cyathula (). The same stellate arrangement is unequivocally reconstructed to have occurred a second time in Calicorema squarrosa.
Higher numbers of 7 – 16 ektexinous bodies have been reduced to 5 – 6 in most species with stellate pore ornamentation (character 4) near terminal branches of the tree (). Heterogeneity of size in ektexinous bodies (Borsch Citation1998) is a derived character state in Ptilotus (character 5). Pore diameter (character 6) is highly homoplastic and exact mapping of ancestral states greatly depends on the optimisation scheme. Except for Psilotrichum africanum (<3 μm on average, ), all species with stellate pore ornamentation share an aperture diameter greater than 3 but less than 4.2 μm.
Discussion
Towards understanding relationships of Amaranthaceae‐Aervinae
Sequence data of matK/trnK clearly show whether genera of the Aervinae belong to the achyranthoid clade or occupy other positions on the tree. However, sequences of additional variable genomic regions will be required to fully resolve internal relationships of the achyranthoids. With respect to rather high posterior probabilities (), numerous studies have indicated that caution is needed due to a likely overcredibility of Bayesian clade probabilities (e.g. Suzuki et al. Citation2002, Simmons & Miya Citation2004, Pickett & Randle Citation2005). Resolution and support based on parsimony analysis are generally lower. Nevertheless, some lineages can be recognized within achyranthoids based on matK/trnK. One of these comprises Cyathula, Sericostachys, Pandiaka, Achyranthes, Achyropsis, and Nototrichium, which is equally well‐supported in both parsimony‐ and likelihood‐based analyses. Stellate pore ornamentation is absent from this clade.
The combined data show that Psilotrichum is undoubtedly polyphyletic, which is not surprising in view of the highly heterogeneous pollen morphology. There are also several non‐pollen characters (such as elongated spikes with densely arranged flowers) that seem to separate at least some Asian species, here represented by P. ferrugineum, from the remainder of the genus. P. ferrugineum has a unique pollen type in Amaranthaceae () but was not included by Borsch (Citation1998). Similarly, Calicorema is polyphyletic. Examination of the pollen of C. capitata in the present study shows that pollen morphology in C. capitata is closest to that of Arthraerua among all specimens examined (). Thus, pollen morphology supports the close affinity (100% support) between C. capitata and Arthraerua indicated by molecular data. Sericocoma is monophyletic, despite its rather heterogeneous pollen morphology (). The divergent pollen morphology within the genus is particularly noteworthy in view of the fact that the two species of Sericocoma are very difficult to distinguish in the field. Commonly used diagnostic features for S. heterochiton in comparison to S. avolans are: slightly smaller petals, somewhat longer and thinner leaves, and more slender inflorescences. The concept of Sericocoma we follow here is that of Cavaco (Citation1962) where he excludes the strongly deviating morphology of S. pungens Fenzl, for which he established the monotypic genus Pseudosericocoma Cavaco. So far we have not succeeded in amplifying trnK, neither have we been able to obtain mature pollen from specimens of Pseudosericocoma.
Multiple origins of stellate pore ornamentation
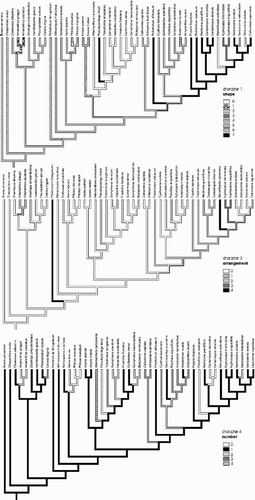
Stellate pore ornamentation provides a complex set of characters (characters no. 1–6). It is clear from the optimisations, and from –, that individual character states may vary to some extent. This is especially true for pore diameter (character 6) and for number of ektexinous bodies (character 4). The size of single ektexinous bodies also varies (character 2, state 2). Most taxa have ektexinous bodies which overlap the perimeter of the pore, but in a few taxa the ektexinous bodies are smaller and do not overlap the perimeter (Psilotrichum africanum, Sericocoma avolans). However, the stellate arrangement (character 3, state 2) and hook‐like shape of ektexinous bodies (character 1, state 7) are constant in all taxa initially identified as possessing stellate pore ornamentation. Exceptionally, in Sericocoma avolans, there is no stellate arrangement and the hooks are less pronounced.
The reconstruction of stellate pore evolution depends to some extent on the optimisation scheme. For the hook‐shaped ektexinous bodies (state 7 of character 1), fast optimisation (plotted in ) shows two independent gains and five independent reversals, while with slow optimisation three independent gains and four reversals are reconstructed. Three independent gains are hypothesized for the particular orientation of ektexinous bodies (character 3, irrespective of optimisation type), and two (four) losses (slow optimisation in braces).
Traced character histories for shape, arrangement, and number of ektexinous bodies are shown in . It appears that stellate pore ornamentation derived independently from apertures with many randomly spread ektexinous bodies of rectangular or roundish shape. These became hook‐shaped, reduced in number, and symmetrically arranged. Similarly the symmetrical arrangement of Marcelliopsis also represents an independent specialization.
Figure 6. Traced histories for characters 1, 3, and 4. 1. Shape of ectexinous bodies: 0 – Rectangular, sinuous or elongate in outline, 1.5 – 4 times as long as broad, with 2 – 3 distinct microspines; 1 – Tri‐ or quadrangular in outline; 2 – Rounded or polyhedral, wider than high, with 1 – 2 distinct microspines; 3 – Plate‐like, with 1 – 2 distinct microspines in centre; 4 – Drop‐shaped, +‐gradually elongated into spine; 5 – Tooth‐ or cone‐shaped, gradually elongated into spine, straight erect, more or less forming an isosceles triangle in lateral view; 6 – Tooth‐ or cone shaped, elongated into spine, more or less curved not an isosceles triangle in lateral view, but tip never forming an outwardly projecting hook; 7 – Distally elongated into an outwardly projecting hook. Arrangement on pore: 0 – Mosaic pattern, closely adjoined, but separated; 1 – Evenly spread, distinctly separated from each other (much space in between); 2 – Radially arranged, closely adjoined, each covering an equal segment of the circular area of the pore; 3 – Margins fused into a slightly raised ridge; 4 – Radially arranged, short sides of rectangles pointing to centre of pore, distinctly separated from each other and not covering an equal segment. Number on each pore: 0 – up to 6; 1 – 7–16; 2 – 17–30; 3 – 31–50; 4 – >50.
Stellate pore ornamentation: occurrence and function?
In view of multiple origins and losses, functional significance and positive selection for stellate pore ornamentation can be hypothesized. A function in pollination seems likely since the hooks may cling to insect pollinators or to the stigmatic surface (Livingstone Citation1972, Livingstone et al. Citation1973, Borsch Citation1998). However, data on pollination biology of Amaranthaceae are scarce and entirely lacking for the species with stellate pore ornamentation. Contrary to the prevailing idea of Amaranthaceae as being wind pollinated (Townsend Citation1993), which may stem from observing widespread genera such as Amaranthus, many genera are in fact frequently visited by insects, for example, in the case of Herbstaedtia, various Lepidoptera (Müller & Thiel, pers. obs.). Vividly coloured tepals in taxa with stellate pore ornamentation also suggest insects as pollen vector (e.g. Mechowia). There are genera, however, with even more conspicuous flowers among close relatives that do not have pollen with stellate pore ornamentation, for example, Marcelliopsis. Stellate pore ornamentation cannot be considered the only means by which insect pollination may be achieved among achyranthoids, but may be the result of further specialization to particular vectors. Further observations in the field are needed to clarify this. Stellate pore ornamentation may also have a function in pollination biology via the pollen‐stigma interaction. The hooks may cling to the stigmatic surface. In preliminary investigations, no general differences in stigma morphology (such as size of papillae) were apparent in taxa with stellate pore ornamentation. We also observed different stages of pore expansion in Psilotrichum species (; Borsch, pers. obs.). These initial observations call for further experiments that may help to elucidate the selection pressures behind the evolution of this unique pore type. Additional insights will also be gained from the TEM studies of the apertures currently underway. An operculum in the sense of one distinctly delimited ektexinous structure covering the apertural endexine (Wodehouse Citation1935, Punt et al. Citation1994) as opposed to isolated ektexinous bodies does not seem to exist in Amaranthaceae.
Furthermore, Livingstone et al. (Citation1973) thought that a thickened ornamentation over each pore might be advantageous for taxa inhabiting xeric environments since it could help to prevent water loss. Livingstone (Citation1973) observed rapid pore dilatation when pollen with stellate pore ornamentation was immersed in a glycerol/ethanol solution. A correlation with xeric habitats is not apparent based on field observations and data from the literature, as many species which are adapted to dryness lack stellate pore ornamentation, for example, Arthraerua while other, less xerophilous taxa have stellate pores, for example, Psilotrichum africanum. Both species of Sericocoma dwell in almost identical semi‐desert habitats, but only in S. avolans have the ektexinous bodies on the pore membranes developed hooks. It follows, now that more is known about the distribution of pollen with stellate, hooked ektexinous bodies among the species of Amaranthaceae, that the Livingstone et al. (Citation1973) correlation with xeric habitats is no longer justified. It seems more likely that stellate pore ornamentation has evolved in association with more specialized insect pollination syndromes.
Acknowledgements
We are indebted to G. F. Smith (Pretoria, South Africa) for his support during a collecting trip (KM) in South Africa. M. Jordaan (Pretoria, South Africa) and T. Thiel (Bonn) also provided much help during fieldwork on this trip. Fruitful and inspiring discussions with M. Hesse and M. Weber (Vienna) regarding pollen morphology are appreciated. Thanks are due to G. Kadereit (Mainz), H. Freitag, K. Weising (Kassel), D. B. Pratt (Nacogdoches, Texas) for discussions on phylogenetics of Amaranthaceae‐Chenopodiaceae. M. Harley (Kew), H. Sauquet (Stockholm), and D. Pratt carefully checked the manuscript and made valuable suggestions to improve the text. We would also like to thank E. Fischer (Koblenz) for material of Sericostachys, G. Zizka of the Herbarium Senckenbergianum (FR) for material of Pandiaka, F. Schuhwerk of the Botanische Staatssammlung München (M) for material of Calicorema, and the late T. M. Pedersen (Mburucuya, Argentina) for material of Pseudoplantago. We are indebted to W. Barthlott (Bonn) for his enduring support and discussions on the topic. Financial support by the Deutsche Forschungsgemeinschaft (grant BO 1815/1‐1 and 1‐3 to TB) is gratefully acknowledged.
References
References
- Akaike , H . 1974 . A new look at the statistic model identification. . – IEEE Trans. Autom. Contr. , 19 : 716 – 723 .
- APG . 2003 . An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. . – Bot. J. Linn. Soc. , 141 : 399 – 436 .
- Borsch , T . 1995 . Three new combinations in Pfaffia (Amaranthaceae) from the New World tropics. . – Novon , 5 : 230 – 233 .
- Borsch , T . 1998 . Pollen types in the Amaranthaceae. Morphology and evolutionary significance. . – Grana , 37 : 129 – 142 .
- Borsch , T and Barthlott , W . 1998 . Structure and evolution of metareticulate pollen. . – Grana , 37 : 68 – 78 .
- Borsch , T and Pedersen , TM . 1997 . Restoring the generic rank of Hebanthe Martius (Amaranthaceae). . – Sendtnera (München) , 4 : 13 – 31 .
- Bremer , K . 1988 . The limits of amino acid sequence data in angiosperm phylogenetic reconstructions. . – Evolution , 42 : 795 – 803 .
- Bremer , K . 1994 . Branch support and tree stability. . – Cladistics , 10 : 295 – 304 .
- Bridson D Forman L 1998 The Herbarium Handbook. 3 {\rm ^{rd}} ed. – R. Bot. Gards, Kew
- Cavaco , A . 1962 . Les Amaranthaceae de l'Afrique au sud du tropique du Cancer et de Madagascar. . – Mém. Mus. Natl Hist. Nat. B Sér. , 13 : 1 – 251 .
- Cronquist A Thorne RF 1994 Nomenclatural and taxonomic history. – In: Caryophyllales. Evolution and systematics (ed. H. D. Behnke & T. J. Mabry), pp. 5–25. – Springer, Berlin
- Cuénoud , P , Savolainen , V , Chatrou , L , Powell , M , Grayer , RJ and Chase , M . 2002 . Mol. phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. . – Am. J. Bot. , 89 : 132 – 144 .
- Downie , SR , Katz‐Downie , DS and Cho , KY . 1997 . Relationships in the Caryophyllales as suggested by phylogenetic analyses of partial chloroplast DNA ORF2280 homolog sequences. . – Am. J. Bot. , 84 : 253 – 273 .
- Doyle JA Bygrave P Le Thomas A 2000 Implications of molecular data for pollen evolution in Annonaceae. In: Pollen and spores: Morphology and biology (ed. M. M. Harley, C. M. Morton, & S. Blackmore), pp. 259–284. – R. Bot. Gards, Kew
- Eliasson , UH . 1988 . Floral morphology and taxonomic relations among the genera of Amaranthaceae in the New World and the Hawaiian Islands. . – Bot. J. Linn. Soc. , 96 : 235 – 283 .
- Hilu , KW , Borsch , T , Müller , K , Soltis , DE , Soltis , PS , Savolainen , V , Chase , M , Powell , M , Alice , LA , Evans , R , Sauquet , H , Neinhuis , C , Slotta , TA , Rohwer , JG , Campbell , CS and Chatrou , L . 2003 . Angiosperm phylogeny based on matK sequence information. . – Am. J. Bot. , 90 : 1758 – 1776 .
- Kadereit , G , Borsch , T , Weising , K and Freitag , H . 2003 . Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C {\rm _{4}} photosynthesis. . – Int. J. Plant Sci. , 164 : 959 – 986 .
- Leach , GJ , Townsend , CC and Harley , MM . 1993 . Omegandra, a new genus of Amaranthaceae from Australia. . – Kew Bull. , 48 : 787 – 793 .
- Livingstone DA 1972 The pollen morphology of African Amaranthaceae. – In: 3rd Ann. Meet. AASP (American Association of Stratigraphic Palynologists), Toronto 1970. Proc. & Abstr. (ed. B. F. Perkins), p. 132. – Geosci. Man 4. USL, Baton Rouge LA
- Livingstone , DA , Tomlinson , M , Friedman , G and Broome , R . 1973 . Stellate pore ornamentation in pollen grains of the Amaranthaceae. . – Pollen Spores , 15 : 345 – 351 .
- Löhne , C and Borsch , T . 2005 . Phylogenetic utility and molecular evolution of the petD group II intron in basal angiosperms. . – Mol. Biol. Evol. , 22 : 317 – 332 .
- Maddison , WP . 1990 . A method for testing the correlated evolution of two binary characters: are gains and losses concentrated on certain branches of a phylogenetic tree? . – Evolution , 44 : 539 – 557 .
- Maddison , WP . 2000 . Testing character correlation using pairwise comparisons on a phylogeny. . – J. Theor. Biol. , 202 : 195 – 204 .
- Maddison WP Maddison DR 1992 MacClade. – Sinauer Associates, Sunderland
- Mishler , BD . 1994 . The cladistic analysis of Mol. and morphological data. . – Am. J. Phys. Anthropol. , 94 : 143 – 156 .
- Müller , J and Müller , K . 2003 . QuickAlign: A new alignment editor. . – Pl. Mol. Biol. Rep. , 21 : 5
- Müller , J and Müller , K . 2004 . TreeGraph: automated drawing of complex tree figures using an extensible tree description format. . – Mol. Ecol. Notes , 4 : 786 – 788 .
- Müller , K . 2004 . PRAP – computation of Bremer support for large data sets. . – Mol. Phylogenet. Evol. , 31 : 780 – 782 .
- Müller , K . 2005 . SeqState ‐primer design and sequence statistics for phylogenetic DNA data sets. . – Appl. Bioinform. , 4 : 65 – 69 .
- Müller , K and Borsch , T . 2005 . Phylogenetics of Amaranthaceae based on matK/trnK sequence data – evidence from parsimony, likelihood, and Bayesian analyses. . – Ann. Mo. Bot. Gard. , 92 : 96 – 102 .
- Nixon KC 1996 WINCLADA. – Software Auth. distrib.: www.cladistics.com
- Nixon , KC . 1999 . The Parsimony Ratchet, a new method for rapid parsimony analysis. . – Cladistics , 15 : 407 – 414 .
- Nowicke JW 1994 Pollen morphology and exine ultrastructure. – In: Caryophyllales. Evolution and systematics (ed. H. D. Behnke & T. J. Mabry), pp. 168–221. – Springer, Berlin
- Pickett , KM and Randle , CP . 2005 . Strange bayes indeed: uniform topological priors imply non‐uniform clade priors. . – Mol. Phylogenet. Evol. , 34 : 203 – 211 .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution. . – Bioinformatics , 14 : 817 – 818 .
- Pratt DB 2003 Phylogeny and morphological evolution of the Chenopodiaceae‐Amaranthaceae alliance. – Ph.D. Thes., Iowa St. Univ., Ames IA
- Punt W Blackmore S Nilsson S Le Thomas A 1994 Glossary of pollen and spores terminology – LPPF Ser. 1.LPP Found., Utrecht
- Rodman JE 1994 Cladistic and phenetic studies. – In: Caryophyllales. Evolution and systematics (ed. H. D. Behnke & T. J. Mabry), pp. 279–301. – Springer, Berlin
- Ronquist , F and Huelsenbeck , JP . 2003 . Mr Bayes 3: Bayesian phylogenetic inference under mixed models. . – Bioinformatics , 19 : 1572 – 1574 .
- Schinz H 1934 Amaranthaceae. – In: Die natürlichen Pflanzenfamilien. 2nd amend. ed. Bd. 16c (ed. A. Engler & K. Prantl contd. H. Harms), pp. 7–85, 46 figs & 585–586. – W. Engelmann, Leipzig
- Simmons , MP and Miya , M . 2004 . How meaningful are Bayesian support values? . – Mol. Biol. Evol. , 21 : 188 – 199 .
- Simmons , MP and Ochoterena , H . 2000 . Gaps as characters in sequence‐based phylogenetic analyses. . – Syst. Biol. , 49 : 369 – 381 .
- Skvarla , JJ and Nowicke , JW . 1976 . Ultrastructure of pollen exine in centrospermous families. . – Pl. Syst. Evol. , 126 : 55 – 78 .
- Suzuki , Y , Glazko , GV and Nei , M . 2002 . Overcredibility of molecular phylogenetics obtained by Bayesian phylogenetics. . – Proc. Natl. Acad Sci. USA , 99 : 16138 – 16143 .
- Swofford DL 1998 PAUP*. Phylogenetic analysis using parsimony (*and other Methods). – Sinauer Assoc., Sunderland
- Townsend , CC . 1978 . Notes on Amaranthaceae. 4. The genera Dasysphaera and Volkensinia. . – Kew Bull. , 33 : 417 – 419 .
- Townsend , CC . 1979 . Notes on Amaranthaceae. 8. The generic position of Centema stefanii Chiov. . – Kew Bull. , 34 : 237 – 238 .
- Townsend CC 1993 Amaranthaceae. – In: Families and genera of vascular plants Vol. 2 (ed. K. Kubitzki, J. G. Rohwer & V. Bittrich), pp. 70–91. – Springer, Berlin/Heidelberg
- Wodehouse R 1935 Pollen grains, their structure, identification and significance in science and medicine. – McGraw‐Hill., New York