Abstract
Anther development, microsporogenesis, and microgametogenesis were studied using both light and TEM microscopy in the six accessible subdioecious/cryptically dioecious species of Consolea (Cactaceae). Anther wall development, microsporogenesis, and microgametogenesis are uniform in staminate flowers of all six species, and are typical for Cactaceae. Breakdown of microsporogenesis in male‐sterile anthers occurs early, at the onset of meiosis, and results in anthers bearing no pollen grains. The abortive process follows a common pattern in all investigated species. The tapetum is the first layer to deviate from normal male‐fertile anther development. Tapetal cells in male‐sterile anthers elongate at an early stage and have abundant rER with atypical configurations. Ultimately, the tapetum becomes hypertrophied and non‐functional. Male‐sterility in pistillate flowers appears to be directly related to these anomalies. In addition, other anther layers and tissues are affected, and normal patterns of programmed cell death (PCD) are disrupted. The relationship between these patterns and the pattern of PCD in normal male‐fertile anthers is discussed. We hypothesize a single origin for the cryptically dioecious/subdioecious breeding system of Consolea based on the uniformity of the anther's abortive processes in pistillate flowers.
Dioecious angiosperms are characterized by pistillate (male‐sterile) and staminate (female‐sterile) flowers produced on separate plants. In dioecious species where both staminate and pistillate flowers start as perfect, unisexuality is accomplished by either abolition or developmental inhibition of the alternative sexual organ (Wu & Cheung, Citation2000). Abolition delays and arrests the development of the androecium or gynoecium during early stages (e.g. Melandrium album Mil. Garcke; Farbos et al. Citation1997). Ultimately, flowers will lack one of those structures (e.g. Pistacia vera L. staminate flower; Hormaza & Polito, Citation1996). On the other hand, developmental inhibition would leave behind a more or less atrophied organ such as shriveled anthers with no pollen grains (Chaudhury et al. Citation1994; Strittmatter et al. Citation2002), anthers with non‐functional pollen grains (Anderson & Symon, Citation1989; Caporali et al. Citation2003), or indehiscent anthers (Arabidopsis msH mutant; Dawson et al. Citation1992). According to Wu and Cheung Citation(2000), programmed cell death (PCD) may be involved in either or both pathways. This kind of PCD, termed developmental PCD (Kuriyama & Fukuda, Citation2002), implies a normal developmental activation of a genome‐encoded pathway that will lead to the death of a cell or group of cells where activated (Danon et al. Citation2000).
Male sterility, the process by which male gametophytic function is prevented, can be classified based on its inheritance pattern as genic, cytoplasmic or gene‐cytoplasmic male sterility (Kaul, Citation1988). Genic male sterility (GMS) is chromosomically inherited and follows Mendelian inheritance patterns (Kaul, Citation1988). It is mostly recessive, and it has been suggested that it occurs spontaneously (Gottschalk & Kaul, Citation1974). Cytoplasmic male sterility (CMS) is non‐Mendelian and shows a cytoplasmic, organellar, inheritance pattern. CMS consists of normal (N) and sterile (S) cytoplasm types, and is not very common (Kaul, Citation1988). Genic‐cytoplasmic male sterility (GCMS), as it name implies, has both nuclear and cytoplasmic genes involved; it has N and S cytoplasm types as well as fertility restorer genes that are different than the GMS genes (Kaul, Citation1988). The proposed mechanism of action of GCMS involves nuclear produced peptides that suppress the action of a male sterility causing mitochondrial polypeptide (Schnable & Wise, Citation1998; Wu & Cheung, Citation2000). Most of the best‐studied cases of so‐called CMS are actually GCMS, since the corresponding genic restorer genes have been found. Maize, with various male sterile cytoplasms (T, S, and C types) and a range of nuclear restorers, is one of the most well studied systems (Colhoun & Steer, Citation1981; Kaul, Citation1988).
Male sterility (GMS, CMS and GCMS) has been extensively studied in monoecious and dioecious crops, in ornamental plants, and in naturally occurring and induced mutants of hermaphroditic crops and its cultivars (Sawhney & Shukla, Citation1994). Recently, emphasis has shifted towards the understanding of stamen and male gametophyte development, and the genes involved in those processes in Arabidopsis thaliana (Chaudhury et al. Citation1994; Sanders et al. Citation1999; Zhang et al. Citation2002). In order to determine the timing and place of the male sterility process, anatomical studies were first performed at the light microscope level (Singh & Rhodes, Citation1961; Dubey & Singh, Citation1965; Brooks et al. Citation1966; Joppa et al. Citation1966; Kaul & Singh, Citation1966; Chauhan & Singh, Citation1966; Pritchard & Hutton, Citation1972; Graybosch et al. Citation1984; Yonggen & Rutger, Citation1984; Sawhney & Bhadula, Citation1988; Kini et al. Citation1994) and later at the ultrastructural level (Overman & Warmke, Citation1972; Warmke & Lee, Citation1977; Horner, Citation1977; Lee et al. Citation1979, 1980; Pollak, Citation1992; Loukides et al. Citation1995). Recent studies combined inheritance, molecular, and ultrastructure techniques for a more comprehensive analysis of male sterility (Dawson et al. Citation1992; Chaudhury et al. Citation1994; Jin et al. Citation1997; Sanders et al. Citation1999). In most of these studies, some abnormality in the tapetum, the innermost layer of the anther wall, has been implicated as a direct or indirect cause of microspore mother cell (mmc), tetrad, microspore, or pollen grain abortion (Laser & Lersten, Citation1972). Since CMS has been linked to defective or aberrant mitochondria, most ultrastructural studies of CMS and GCMS systems concentrate on the tapetal cell cytoplasm and its organelles, specifically the mitochondria (Warmke & Lee, Citation1977; Horner, Citation1977; Lee & Warmke, Citation1979; Hanson & Conde, Citation1985; Pollak, Citation1992).
Male sterility has not been studied in species of no economic importance. In this paper we focus on male sterility and its role in sex determination in a cryptically dioecious/subdioecious breeding system. The Caribbean endemic Consolea (Opuntioideae, Cactaceae) has nine species (Areces‐Mallea, Citation2001), the six accessible species were studied and found to be subdioecious/cryptically dioecious (C. spinosissima – Strittmatter et al. Citation2002; C. corallicola – Negrón‐Ortiz & Strittmatter, Citation2004; all the other species – unpubl. data). In those six species, pistillate flowers possess no pollen bearing anthers and staminate flowers have aborted ovules at anthesis. Developmentally both types initiate as perfect flowers. We investigated anther differentiation and pollen development in anthers of pistillate (male‐sterile/female‐fertile) and staminate (male‐fertile/female‐sterile) flowers of C. millspaughii (Britton) A. Berger, C. moniliformis (L.) A. Berger, C. nashii (Britton) A. Berger, C. rubescens (Salm‐Dyck ex DC.) Lem, C. picardeae (Urb.) Areces, and C. spinosissima (Mill.) Lem using light and transmission electron (TEM) microscopy. Very few studies have investigated anther wall development and microsporogenesis in the Cactaceae (Neumann, Citation1935; Tiagi, Citation1954; Tiagi, Citation1961; Strittmatter et al. Citation2002; Negrón‐Ortiz & Strittmatter, Citation2004) and none have done so from an ultrastructural perspective. Thus this study is, to our knowledge, the first one to describe anther wall development and microsporogenesis in the Cactaceae using transmission electron microscopy (TEM).
The purpose of our study is to describe anther development, microsporogenesis and microgametogenesis in male‐fertile and male‐sterile anthers of Consolea. We focus on the male‐sterility process to determine what is the critical stage at which Consolea anthers became non‐functional from an ultrastructural standpoint, as well as to determine which tissue/s are involved in the process. In addition, we examine whether the pathway to male sterility is similar in all studied species. The results are compared to similar studies in crop plants and Arabidopsis.
Material and methods
Pistillate and staminate flowers at varying developmental stages of C. moniliformis, C. millspaughii, C. nashii, C. picardae, C. rubescens and C. spinosissima were collected from natural populations (see, list of Specimens Investigated). For light microscopy whole flowers were placed in plastic vials containing formalin‐acetic acid‐alcohol (FAA), and transported to the Bruce Fink laboratory, Miami University. Samples for sectioning were dehydrated in an ascending series of ethanol and embedded in paraffin. Blocks were sectioned at 7–12 µm and stained with safranin and fast‐green (D'Ambrogio de Argueso, Citation1986). Sections were viewed using an Olympus BH2 light microscope.
For electron microscopy, isolated anthers were pre‐fixed in the field with McDowell‐Trumps fixative (McDowell & Trump, Citation1976), kept refrigerated and transported to the EM facilities at Miami University. The fixed samples were rinsed with the same fixation buffer (Sodium phosphate (NaPO4), plus 5 mM CaCl, pH 7.2) and post‐fixed in 1.5% w/v Osmium tetroxide (OsO4) for 4 hrs at room temperature also in the same fixation buffer. After a second rinse with the fixation buffer, the samples were treated overnight at room temperature with 0.5% Uranyl acetate. The samples were then rinsed in double distilled water, dehydrated in an ascending acetone series and the anthers infiltrated and embedded in Spurr's resin (Spurr, Citation1969). Selected anthers were ultrathin sectioned (∼75 nm), collected on 200 mesh Cu grids, and stained with 2% uranyl acetate (10 min) and 0.5% lead citrate (10 min). The material was viewed using a Jeol 100 S transmission electron microscope, and photographed using TEM film (Kodak 4489).
Semithin (≈1–2 µm) sections were cut with glass knives, placed on glass slides, and stained with tolouidine blue (0.1% in 0.01% Na2CO3). Sections were examined and photographed with a Nikon Labophot 2 microscope with a Kodak 760C digital camera.
Results
Microsporogenesis and microgametogenesis in staminate flowers ‐ male fertile
Anther wall development, microsporogenesis, and microgametogenesis proceeded in a similar fashion in all studied species. Consolea picardae (below) serves as the standard to illustrate the four, well‐characterized stages:
Stage 1. Anther primordium
Young anthers are four sporangiate. The anther wall, composed of epidermis, endothecium, middle layer, and tapetum, surrounds the sporogenous mass (Figs , ). During this first stage the cells of the anther wall layers are similar in size, their nuclei occupy most of the cellular volume, and only small vacuoles are present (Fig. ). As the anther matures the tapetal cells enlarge radially, their cytoplasm becomes denser and only very small vacuoles are present (Fig. ). Cells of the anther wall and sporogenous tissue are similar in mitochondria, ER, Golgi, and ribosome content (Fig. ).
Figure 1 A–H. Bright field micrographs of Consolea picardae anther development. A–E. Male‐fertile anther (staminate flower) cross sections. F–H. Male‐sterile anther (pistillate flower) cross sections. A. Stage 1, anther primordium with four anther wall layers: epidermis (e), endothecium (en), middle layer (ml) and tapetum (tp) surrounding the sporogenous tissue (sp). B. Early stage 2, microspore mother cell (mmc) differentiated and surrounded by four anther wall layers, e, en, ml, and binucleate tp. C. Late stage 2, meiosis is complete and a tetrahedral microspore tetrad is surrounded by callose (c); the anther wall is composed of three layers, e, en, and very active tp; ml is flattened and crushed. D. Stage 3, released and enlarged microspores (m), with large central vacuole and peripheral nucleus against the cell wall; the anther wall is still composed by three layers, e, en and tp. E. Stage 4, mature pollen filled with starch grains, the anther wall has only two remaining layers, e and en with fibrous thickenings. F. Early stage 2, mmc differentiated and anther wall with four layers, e, en, ml and radially enlarged tp. G. Stage 2, mmc degenerating and oddly shaped due to tp invasion of the anther locule; the tp layer is greatly enlarged and its cells have a single large vacuole; e, en and ml are not enlarged. H. Degenerate anther, mmc's no longer visible; the anther wall is composed of e, somewhat enlarged en, and greatly radially enlarged ml; the tp has already been crushed. Scale bars – 50 µm.
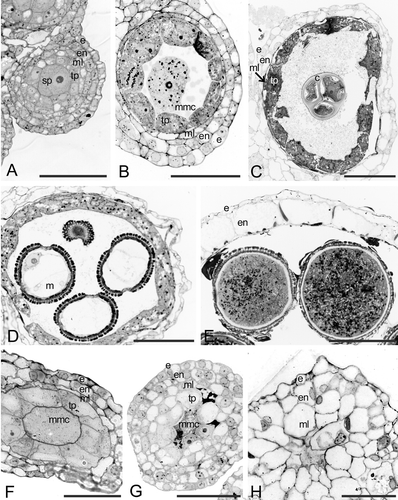
Figure 2 A–G. Transmission electron micrographs (TEM) of Consolea picardae male‐fertile anther development. A–B. Stage 1, anther primordium. A. Four young anther wall layers: epidermis (e), endothecium (en), middle layer (ml) and tapetum (tp) surrounding the sporogenous (sp) cell. B. Detail of sp cell: note very dense cytoplasm, small vacuoles (v), and abundant mitochondria and rER. C–E. Stage 2. C. Anther wall composed of three layers, e, en, flattened, crushed ml, and metabolically active tp. D. Detail of tp cell fused nuclei, showing the characteristic lobed nucleus (ln). E. Detail of tp cell cytoplasm with rER (solid arrow) and pro Ubisch bodies (hollow arrow). F–G. Stage 3: microspore. F. Detail of tp cell with dense cytoplasm and lipid bodies (lp), in which only the rER cisternae matrix is discernible (solid arrow); young Ubisch bodies are attached to the plasma membrane (hollow arrow). G. Microspore with a well‐developed exine. Scale bar – 2 µm (A, C, D, G); 1 µm (B); 0.4 µm (E, F).
Stage 2. Meiosis
At the onset of meiosis, each microspore mother cell (mmc) secretes a callose wall around itself, the tapetal cells have a much denser cytoplasm than the other anther wall layers cells, the middle layer starts to shrink, and the endothecium and epidermis have increased in size (Figs , ). During meiosis, most tapetal cells become binucleate (Fig. ). By the end of meiosis, when the tetrahedral microspore tetrads are formed, the two tapetal nuclei have fused together to form a large, lobed nucleus with several nucleoli (Fig. ). At the tetrad stage, the tapetal cytoplasm is very dense and stains darker than the cytoplasm of the other anther wall layer cells (Figs ; ). The tapetal cells have numerous mitochondria, short stalks of rER, numerous free ribosomes and polysomes, and lipidic globules (Fig. ). The inner tangential wall of the tapetum is disorganized and lax, and numerous pro‐Ubish bodies are seen transversing it (Fig. ).
Stage 3. Microspores
After each microspore is released from the tetrad they enlarge considerably by means of a large central vacuole that pushes the nucleus towards the cell wall (Figs , ). The microspore wall possesses a thick endexine and thin foot layer; the thick tectum is supported by columellae, but the initine is not yet formed. At this stage the anther wall is composed of three remnant layers: epidermis, radially enlarged endothecial cells, and tapetum (Fig. ). The middle layer is almost completely flattened and reabsorbed. The tapetum is the most metabolically active layer of the anther wall (Figs , ); its cells have dense cytoplasm full of free ribosomes, rER, mitochondria, and lipidic globules. The inner tangential wall of the tapetum is almost disintegrated and young Ubisch bodies are seen attached to the plasmalema (Fig. ). However, the tapetal cells have not radially enlarged further since the onset of meiosis, and by this stage the endothecium is slightly larger than any of the anther layers.
Stage 4. Mature pollen grains
After one mitotic division, each microspore has a vegetative (pollen tube) cell that occupies most of the pollen grain volume and a generative cell situated close to the pollen grain wall. The generative cell subsequently migrates to the center of the pollen grain and divides once more to form two sperm cells that will remain close to the vegetative cell nucleus to form the male germ unit. The vegetative cell cytoplasm is very dense and full of starch grains, lipid globules and micro vesicles (Figs , ). The sperm cell cytoplasm also has some lipidic globules, mitochondria and rER, but lacks starch grains (Fig. ). The pollen grain wall is now composed of a thick exine with basal layer, columella, tectum and a well developed intine; the intine is the only layer present at the apertures (Fig. ). Ultimately, the anther wall is composed of only two layers, epidermis and radially enlarged endothecium (Fig. ). These endothecial cells possess fibrous thickenings (Fig. ) that ultimately facilitate anther dehiscence. No remnants of the middle layer can be seen, and the only vestige of the tapetum are the Ubsich bodies situated close to the inner tangential wall of the endothecial cells (Fig. ). At this time the intersporangial septum cells have disintegrated, and the anther is bilocular. The anther dehisces longitudinally and releases the three‐celled pollen grains.
Figure 3 A–C. TEM micrographs of Consolea picardae male‐fertile mature anther and pollen grain. A. Anther wall composed of epidermis (e) and endothecium (en) with fibrous thickenings (arrows), note Ubisch bodies along lower right. B. Detail of mature pollen grain, the cytoplasm is filled with starch grains (sg) and the pollen grain wall is formed by intine (in) and exine (ex); the ex is interrupted at the colpus. C. Detail of sperm cell, showing a wavy cell wall, a cytoplasm with rER (arrow), mitochondria, nucleus (n), and lipid globules but no sg. Scale bar – 5 µm (A); 2.5 µm (B); 0.5 µm (C).
Microsporogenesis in pistillate flowers ‐ male‐sterile
In all studied Consolea species anthers of pistillate flowers proceeded through a similar abortion pattern. Microsporogenesis never proceeded beyond prophase I, and no microspore tetrads were found; thus all the anthers were empty at maturity. At the first signs of abnormal tapetal behavior there was no sign of nuclear degeneration, either in mmc or tapetal cells. In addition, at that stage, the mitochondria developed normally and showed no signs of degeneration. Within this framework, minor variability in the way abortion proceeded was noted (Table ). We described the most common pattern observed, using C. picardae as the standard organism, and then discuss the differences observed (Table ). Stages 3 and 4 are approximate since the mmc are aborted and no microspores or pollen grains are formed.
Table I. Summary of developmental variations in the anther abortive pattern of Consolea pistillate flowers at stage 2 (early prophase I).
Stage 1
This stage is indistinguishable from Stage 1 of staminate flowers (Figs ; ).
Stage 2
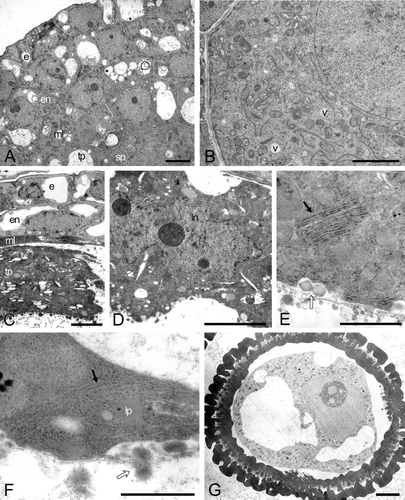
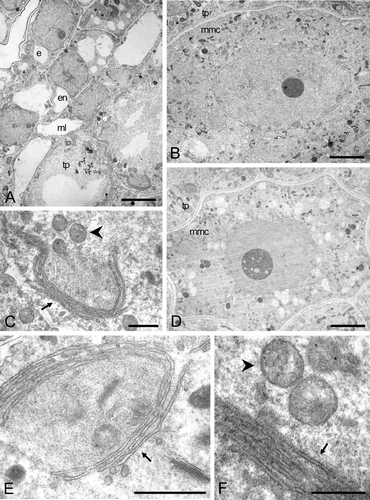
When the first signs of chromatin condensation are evident in the mmc's, the anther wall already shows differences. The epidermal, endothecial, and middle layers are equivalent in size but the tapetal layer is radially enlarged, possess a large vacuole and presses upon the sporogenous cells (Figs ; ). The tapetal cell cytoplasm is less dense than its counterpart in fertile anthers, has some lipid globules, normal looking mitochondria, and Golgi (Fig. ). However, the most striking ultrastructural feature is the presence of compactly clustered and elongated rER cisternae. These rER arrangements can be nearly round, and encircle regions of tapetal cell cytoplasm (Fig. ). Such rER arrangements were absent in the tapetal cell cytoplasm of fertile anthers (Fig. ). At this stage the mmc's are indistinguishable from the mmc's of fertile anthers (Fig. ), except in the cases in which the tapetal cells were pressing against them (Fig. ). At this stage, synaptonemal complexes were visible in the nucleus, and their cytoplasm was very dense with only small vacuoles present. The mitochondria, rER, Golgi, proplastids, and free ribosomes are normal in appearance (Fig. ).
Figure 4 A–F. TEM micrographs of Consolea picardae male‐sterile anther at Stage 2. A. Anther wall showing four layers: epidermis (e), endothecium (en), middle layer (ml) and a radially enlarged tapetum (tp). B. Microspore mother cell (mmc) at an early stage 2, before the tapetal cells start invading the locule, note the occasional vacuoles and the normal appearance of the cytoplasm and its contents. C. Tapetal cell showing peculiar rER configuration (arrow), and normal looking mitochondria (arrow head). D. mmc after tapetal cells begin radial elongation and compress the mmc; note the presence of numerous small vacuoles. E. Tapetal cell with almost circular rER configuration (arrow) encircling a portion of cytoplasm, a mitochondrium, and two Golgi. F. Tapetal cytoplasm with highly compressed and elongate rER cisternae (arrow) and normal looking mitochondria (arrow head). Scale bar – 2 µm (A, B, D); 1 µm (C); 0.5 µm (E, F).
Stage 3
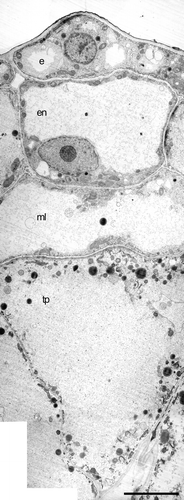
The tapetal cells continue to enlarge radially, and become hypertrophied. The vacuole enlarges to occupy the whole cellular volume, and only a thin layer of cytoplasm is left adjacent to the cell wall (Fig. ). The cytoplasmic contents are sparse at this stage, with almost no free ribosomes present, very few mitochondria, some Golgi, and very little rER; lipid globules are the most abundant cytoplasmic feature (Fig. ). These hypertrophied tapetal cells, almost devoid of cytoplasm, are completely different from male‐fertile tapetal cells at any stage. The mmc's completely degenerate and are visible only as dark masses. The anther wall is still composed of four layers: epidermis, enlarged endothecium, enlarged middle layer, and enlarged tapetum (Fig. ). The anther locule becomes completely filled with the intruding tapetal and middle layers (Fig. ). As the anther increases in volume, the middle layer keeps enlarging radially into the locule and the tapetal cells are eventually crushed (Fig. ).
Figure 5 TEM micrograph montage ofConsolea picardae male‐sterile aborted anther. The anther wall is composed of epidermis (e), endothecium (en), middle layer (ml) and radially enlarged tapetum (tp). Both ml and tp cells are devoid of cytoplasm except for a thin layer adjacent to the cell wall; some lipid globules, rER remnants, and Golgi can be recognized. Scale bar – 2.5 µm.
Stage 4
At anthesis the anther wall is composed of either only epidermis or epidermis and endothecium. The endothecium only occasionally develops fibrous thickenings. The anther remains four sporangiate and there is no sign of intersporangial septum disintegration. Individual locules disappear as the remaining anther wall layers collapse as the entire anther shrivels. In samples where fibrous thickenings are present in the endothecium, the integrity of the anther locule is preserved.
Abortion pattern variation
Consolea picardae
At stage 2 in some anthers, the tapetal cells become highly vacuolated but do not enlarge radially; the remaining cytoplasm is very dense and stains darkly; the rER stalks remain a major component of the cytoplasm (Fig. ). The tapetal cells disintegrate without invading the anther locule (Fig. ).
Figure 6 A–J. Bright field micrographs of Consolea millspaughii, C. moniliformis, C. nashii, C. rubescens and C. spinosissima male‐sterile anthers at stage 2 (left column) and at abortion (right column). A–B. C. millspaughii. A. Anther wall composed of epidermis (e), endothecium (en), middle layer (ml), and radially enlarged tapetum (tp); notice the single large vacuole in the tp cells. B. Remaining anther wall layers: e, en and radially enlarged ml; within the anther locule; some mmc and tp cell remmants are visible. C–D. C. moniliformis. C. e, en, ml and radially enlarged tp with single large vacuoles; the mmc's show some signs of degeneration. D. All anther layers except e, invading the locule, and in the center, a dark mass representing all that is left of the mmc's. E–F. C. nashii. E. e, en, ml, and radially enlarged tp with single large vacuoles. F. tp cells enlarged and invading the locule. G–H. C. rubescens. G. e, en, ml and somewhat enlarged tp cells with numerous small vacuoles. H. en and ml layers greatly enlarged and invading the locule; in the center, remnants of mmc's and tp cells are visible as dark masses. I–J. C. spinosissima. I. e, en, ml and somewhat radially enlarged tp with numerous small vacuoles. J. Enlarged en and ml invading the locule, and in the center, remnants of aborted tp cells and mmc's. Scale bars – 25 µm (A–J).
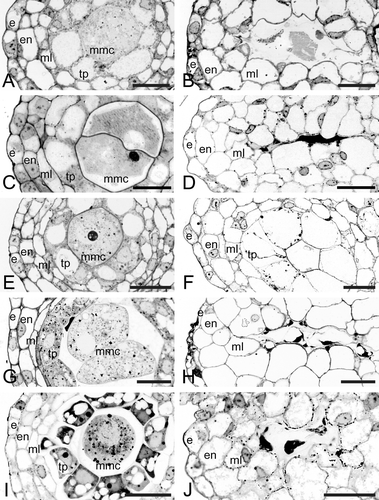
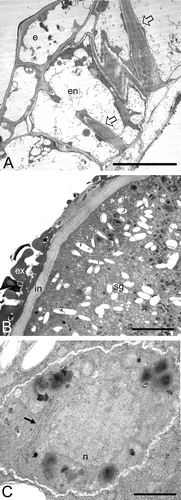
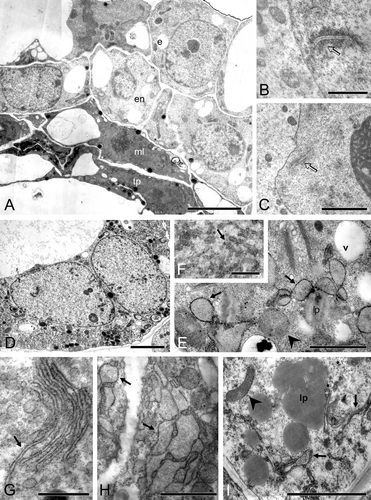
Figure 7 A–I. TEM micrographs of Consolea picardae, C. millspaughii, C. nashii, and C. rubescens male‐sterile anthers at Stage 2. A. Montage of C. picardae anther with a variant of the standard type of abortion; the middle layer (ml) and tapetal (tp) layers show a dense, dark cytoplasm in somewhat flattened cells while the epidermis (e) and endothecium (en) look healthy. B. C. millspaughii detail of microspore mother cell (mmc) nucleus and cytoplasm, a synaptonemal complex (arrow) is clearly visible inside the nucleus. C–F. C. rubescens. C. Detail of mmc nucleus with nucleolus; a synaptonemal complex (arrow) can be seen close to the nuclear membrane. D. Detail of tapetal cell with two nuclei. E–F. Detail of tapetal cell cytoplasm. E. rER with dilated cisternae that look almost like vescicles (solid arrow), also small vacuoles (v) and healthy looking mitochondria (arrowhead). F. Net‐like configuration of rER. G–H. C. nashii, tapetal cell cytoplasm. G. Clustered and elongated rER cisternae (solid arrow). H. Dilated rER cisternae (arrow). I. C. millspaughii tapetal cell with net‐like rER (arrow), abundant lipid globules (lp) and healthy looking mitochondria (arrowhead). Scale bar – 2 µm (A, D); 1 µm (C, I); 0.5 µm (B); 0.4 µm (E, H); 0.3 µm (G); 0.25 µm (F).
Consolea rubescens
Tapetal cells reach a binucleate stage before degenerating (Fig. ), though no nuclear fusion was observed. Although the tapetal cells enlarge radially, the enlargement is less pronounced than in C. picardae (Fig. ). As in C. picardae synaptonemal complexes are present in the nucleus of mmc's (Fig. ) at stage 2. The tapetal cytoplasm contains large amounts of rER but with a different configuration than previously seen; instead of elongated cisternae, the rER is net‐like and shows dilated rER cisternae and vescicles (Fig. ).
Consolea millspaughii
Again as in C. picardae, synaptonemal complexes in the nucleus of mmc' are present at Stage 2 (Fig. ). However, in the tapetum there is a noticeable increase in the size and abundance of lipid globules and net‐like rather than the elongated rER seen in C. picardae (Fig. ).
Consolea nashii
At Stage 2, the tapetum contains large amounts of rER, some in the form of elongated cisternae as in C. picardae, but also as compact stacks (Fig. ) with greatly dilated cisternae (Fig. ).
Consolea moniliformis
Variability in tapetal cell abortion was observed even among anther locules. The prevalent abortion pattern, however, is slightly different from the one observed on C. picardae. Tapetal cells in C. moniliformis enlarge slightly in a radial direction instead of becoming hypertrophied; the tapetal cytoplasm stains densely (Fig. ), and develops short, compact rER cisternae and contains many small vacuoles (Fig. ), and lipid globules (Fig. ). This is the only species in which cytomixis (nucleus or nuclear material movement through a cytoplasmic channel) was noted (Fig. ).
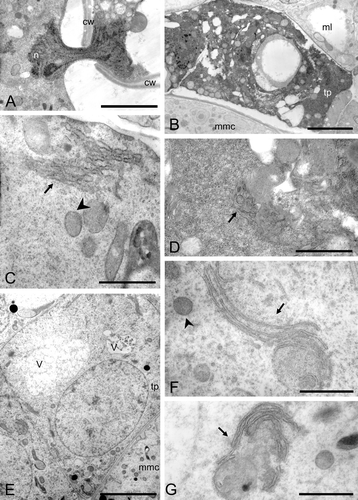
Figure 8 A–G. TEM micrographs of Consolea moniliformis and C. spinosissima male‐sterile anthers at Stage 2. A–D. C. moniliformis. A. Detail of a nucleus (n) traversing a cytoplasmic channel between two mmc's cell walls (cw). B–D. Tapetal cell. B. Somewhat enlarged tapetal cell (tp) with very dense cytoplasm and small vacuoles. C. Cytoplasm detail showing short compact rER cisternae forming dense stacks (solid arrow) and healthy looking mitochondria (arrowhead). D. Dense cytoplasm with some small vacuoles, some rER (solid arrow) and abundant free ribosomes. E–G. C. spinosissima tapetal cell (tp). E. Enlarged tp with vacuoles (v) and single nucleus. F. Cytoplasm detail showing elongated rER cisternae (solid arrow) encircling regions of cytoplasm, and healthy looking mitochondria (arrowhead). G. Cytoplasm detail showing nearly circular rER configuration (solid arrow). Scale bar – 2 µm (B, E); 1 µm (A); 0.5 µm (D, F, G); 0.4 µm (C).
Consolea spinosissima
In this species, tapetal cells also show slight radial enlargement at the onset of meiosis, and numerous small vacuoles become apparent (rather than a single large one as in C. picardae) (Figs ; ). The elongate, compact rER arrangements characteristic of C. picardae were also noted (Fig. ).
Discussion
Anther wall development and pollen development are uniform in staminate flowers of the six species of Consolea studied, and are characteristic of Cactaceae (Johri et al. Citation1992; Watson & Dallwitz, Citation1992). All six species possess tetrasporangiate anthers that dehisce longitudinally. The anther walls are composed of epidermis, endothecium with fibrous thickenings, a single ephemeral middle layer, and a secretory tapetum with lobed, polyploid nuclei.
The breakdown of microsporogenesis in anthers of pistillate flowers is similar in all the investigated species with only minor variations. Consolea male‐sterile anthers behave differently from their male‐fertile counterparts from Stage 2 onward. Specifically, a) mmc's reach early stages of prophase I but degenerate afterwards; b) uninucleated tapetal cells (except in C. rubescens) enlarge radially but are almost completely devoid of cytoplasm; c) middle layer cells persist and radially enlarge; d) endothecial cells lack fibrous thickenings (with some exceptions); e) intersporangial septum and stomium cells do not degenerate. As a consequence, the mature anther is indehiscent, empty, and shriveled with only epidermis and endothecium layers remaining. The tapetal layer in male‐sterile anthers is the first anther layer to deviate from what is observed in male‐fertile anthers, therefore we assume that male‐sterility in Consolea spp. pistillate flowers is associated with anomalies in tapetal development.
Male‐sterility: Tapetum
The tapetum, a polyfunctional tissue that surrounds the microspore mother cells, ensures effective meiosis and normal microspore and pollen grain development in male‐fertile anthers (Kamelina, Citation2002). Considering the developmental importance of this tissue, its extended persistence or premature breakdown can, therefore, lead to male‐sterility, as evidenced by the high number of male‐sterile plants with anomalous tapetal form, function, or behavior (Kaul, Citation1988). For example, in three male‐sterile lines of flax, tapetum persistence is associated with pollen abortion (Dubey & Singh, Citation1965). Thus, Dubey and Singh Citation(1965) hypothesized that the failure of tapetal disintegration at the proper time jeopardizes microspore/pollen grain nutrition, either causing their abortion or rendering them sterile.
In all investigated Consolea spp., male‐sterile tapetal cells show ultrastructural differences relative to their male‐fertile counterparts during the earliest stages of prophase I. Not only are there small to medium size vacuoles (C. rubescens and C. spinosissima) or a single large vacuole (C. millspaughii, C. moniliformis, C. nashii and C. picardae), but there is also variation in rER abundance and atypical configurations (Table ). In all species except C. rubescens, male‐sterile tapetal cells remain uninucleated. These aspects contrast sharply with the almost total absence of vacuoles and the polyploid nature of the nuclei of male‐fertile tapetal cells. Even though the male‐sterile tapetal cell cytoplasm degenerates, the cell wall persists until other expanding anther wall layers crush it. Thus, Consolea spp. have a persistent, though physiologically inactive, tapetum that seems to be directly related to mmc's death. As previously discussed, minor variations in the abortion pathway are exhibited by male‐sterile anthers in both C. moniliformis and C. picardae (Table ). In some anther locules of these two species, necrotic and collapsed tapetal cells die without expanding and are crushed before the mmc's. In these cases, the complete absence of tapetal tissue establishes the destiny of otherwise normal looking mmc's that initiate, but do not complete, meiosis.
Abundant rER is present in tapetal cells of male‐fertile Consolea anthers, especially at late Stage 2 and Stage 3. This is expected, because in pollen producing anthers, stalks of rER are very common in metabolically active tapetal cells at the tetrad and early microspore stages (Owen & Makaroff, Citation1995; Platt et al. Citation1998). Thus, the presence of rER in the tapetal cells at those stages is related to its function. The endoplasmic reticulum is the most important organelle of the endomembranous system (Vitale et al. Citation1993). It functions in the synthesis and maturation of secretory and plasma membrane proteins and also of proteins involved in transport to various organelles in the endocytic and exocytic pathways (Vitale et al. Citation1993). According to Harris Citation(1986), ER is a key element in lipid and protein synthesis and in Ca++ regulation. In tapetal cells of male‐fertile anthers the rER has also been related to sporopollenin and Ubisch body production (Risueño et al. Citation1969; Suárez‐Cervera & Seoane‐Camba, Citation1986; El‐Ghazaly & Nilsson, Citation1991; Fernando & Cass, Citation1994; Galati & Rosenfeldt, Citation1998; Galati & Strittmatter, Citation1999; Amela García et al. Citation2002). In Consolea male‐fertile anthers, short and compact stalks of rER are abundant at late Stage 2 and Stage 3 (microspore tetrad and microspore respectively, Fig. ) in concordance with its active role as a sporopollenin producer. In contrast, in Consolea male‐sterile anthers rER is most abundant at early Stage 2 and acquires various unusual formations (Figs ; ). In all Consolea male‐sterile anthers, the presence of these various types of rER arrangements precedes and is predictive of tapetal cell and mmc's abortion.
Abnormal rER arrangements have also been found preceding tapetal cell degeneration in a number of genera (Brassica napus L. CMS line, Polowick & Sawhney, Citation1990 and Grant, Beversdorf & Peterson, Citation1986; Beta vulgaris L. CMS line, Majewska‐Sawka et al. Citation1993; male sterile soybean, Smith et al. Citation2002; snap bean, Suzuki et al. Citation2001). Some authors were able to relate rER abundance and unusual configurations in the cytoplasm of tapetal cells with tapetum and/or mmc abortion. For example, Fei and Sawhney Citation(1999) related the presence of extensive stacks of rER in tapetal cells of Arabidopsis thaliana male sterile32 (ms32) with early callose dissolution and ultimately with mmc abortion. Similarly, Suzuki et al. Citation(2001) hypothesized that the abnormal arrangement of rER in tapetal cells under high temperatures negatively affects the function of those cells. However, neither early callose dissolution, nor high temperature can explain the ER abundance and unusual configuration leading to tapetum and/or mmc death in Consolea male‐sterile anthers. The nature of the relationship between rER morphology and degenerating tapetal cells in Consolea is unknown. However, it is clear that in Consolea male‐sterile anthers one of the first signs of abnormal tapetal behavior preceding this tissue death is an increase in atypical configurations of rER in tapetal cells' cytoplasm.
Tapetum abnormalities like hypertrophied and highly vacuolated cells, have been observed in the three types of male sterility: CMS (Overman & Warmke, Citation1972; Lee et al. Citation1979; Polowick & Sawhney, Citation1990; Worral et al. Citation1992; Hidalgo et al. Citation1999); GMS (Rick Citation1948; Childers, Citation1952; Kaul & Singh, Citation1966; Graybosch & Palmer, Citation1985; Loukides et al. Citation1995; Hernould et al. Citation1998; Engelke et al. Citation2002; Smith et al. Citation2002), and GCMS (Brooks et al. Citation1966; Colhoun & Steer, Citation1981). Some of the morphological features that Consolea aborting anthers exhibit are comparable to features of both CMS and GMS systems. Specifically, early mmc abortion, lack of thickenings in the endothecial cells, and hypertrophied tapetum and middle layer invading the anther locule, are common in both CMS and GMS systems. Similarly, these characters were observed in several Arabidopsis thaliana genic male‐sterile mutants. Arabidopsis thaliana ms3, ms4, ms5, and ms15 mutants have an early arrest of microsporogenesis, and none of them produce any normal microspore tetrads. The ms3 mutant, in particular, shows markedly hypertrophied middle and tapetal layers (Chaudhury et al. Citation1994). Pollenless mutant fat tapetum (similar phenotype as ms3) has an endothecium without fibrous thickenings and persistent and enlarged middle and tapetal layers that crush the mmc's (Sanders et al. Citation1999). In some CMS systems, the mitochondria of tapetal cells are either extremely modified and are the first signs of tapetal cell abortion (Horner, Citation1977; Warmke & Lee, Citation1977; Lee & Warmke, Citation1979; Majewska‐Sawka et al. Citation1993; Hernould et al. Citation1998; Smith et al. Citation2002) or no signs of degeneration are found (Lee et al. Citation1979; Colhoun & Steer, Citation1981; Lee et al. Citation1980; Hidalgo et al. Citation1999). In Consolea, normal looking mitochondria were seen in radially enlarged tapetal cells that exhibit rER atypical configurations. However, the lack of aberrant mitochondria in tapetal cells' cytoplasm does not exclude the possibility of CMS in Consolea. Based only on morphological/cytological studies it is difficult to determine the type of male‐sterility Consolea exhibits.
Early mmc degeneration has not been reported as frequently as late stage abortion (Sun & Ganders, Citation1987). In CMS, Laser and Lersten Citation(1972) found that out of 62 investigated taxa, six percent of abortions were observed at the sporogenous mass‐mmc stage and nine percent at meiosis I or II. The most common stage for abortion (32%) was the early non‐vacuolate microspore. In GMS and GCMS, Gottschalk and Kaul Citation(1974) remarked that most of the male‐sterility genes affect the final stages of meiosis between interphase II and pollen formation, while a smaller group acts upon the early to middle stages of prophase I. Chaubal et al. Citation(2000) report that two recessive male‐sterile Zea mays L. mutants abort mmc's after synaptonemal complexes (during early prophase I) are evident and that tapetal cells become highly vacuolated without ever reaching the binucleate stage characteristic of normal tapetal development. The authors remarked on the importance of the tapetum's early role in mmc's fate, a function not as widely accepted as its nutritive one during later microspore development (Chaubal et al. Citation2000). On the other hand, it has been questioned whether the aborting mmc/microspore stimulates tapetum hypertrophy or whether the proliferating tapetum is the one interfering with the mmc/microspore development (Worrall et al. Citation1992, Engelke et al. Citation2002). Experimental studies (such as tapetal cell ablation by different methodologies) give support to the idea of the tapetum failure as the cause for mmc, tetrad, microspore or pollen grain abortion initiation (Vasil, Citation1967; Horner, Citation1977; Mariani et al. Citation1990; Goldberg et al. Citation1993; Kriete et al. Citation1996; Goetz et al. Citation2001).
Male‐sterility: PCD
Programmed cell death is essential throughout a plants life cycle (Greenberg, Citation1996; Yu et al. Citation2002), specifically in the reproductive phase where failure to engage in various cell death programs can jeopardize reproduction (Wu & Cheung, Citation2000). PCD is a normal component of anther development (Greenberg, Citation1996; Ku et al. Citation2003). For instance, during male‐fertile anther wall development, the middle layer is ephemeral becoming flattened and crushed by early meiosis (Vasil, Citation1967). The tapetum disintegrates soon after pollen development and is not usually present at anthesis (Greenberg, Citation1996; Papini et al. Citation1999; Wang et al. Citation1999; Ku et al. Citation2003). During disintegration, tapetal cells release their lipid components which coat the pollen grain exine (Papini et al. Citation1999; Balk & Leaver, Citation2001). Intersporangial septum and stomium cells also go through PCD in order to allow anther dehiscence (Goldberg et al. Citation1993; Greenberg, Citation1996; Wang et al. Citation1999). Interestingly, in male‐sterile Consolea anthers, middle layer, intersporangial septum, and stomium cells have delayed PCD (i.e. middle layer) or do not have PCD (i.e. intersporangial septum and stomium cells). Thus, layers that during normal anther development are required to die remain intact or show only minor modifications and override PCD. The middle layer, which in male‐fertile Consolea anthers disintegrates by the end of meiosis persists and enlarges in male‐sterile anthers, pushing and crushing the radially enlarged tapetal cells to the center of the locule (Figs ; ). The middle layer cells eventually disintegrate leaving the epidermis and endothecium as the remaining anther wall layers. A similar process has been described in Macroptilium atropurpureus (Sessé and Moq. in DC) Urb. (as Phaseolus) male‐sterile plants (Pritchard & Hutton, Citation1972) and in the PET1‐CMS sunflower (Balk & Leaver, Citation2001). The death of intersporangial septum cells transforms the tetrasporangiate anther into a bilocular one, and the death of the stomium cells, in conjunction with the endothecial layer, allows anther dehiscence. Both of these sequences are a function of PCD (Beals & Goldberg, Citation1997; Greenberg, Citation1996; Wang et al. Citation1999; Wu & Cheung, Citation2000). As expected, the intersporangial septum and stomium cells disintegrate in Consolea male‐fertile anthers during the three‐celled pollen grain stage. However, in male‐sterile Consolea, the anther remains tetrasporangiate and does not dehisce, abnormalities that can be directly related to a disfunctional PCD. The abnormal behaviors of the middle layer, intersporangial septum and stomium cells, however, are merely correlated with and are not the direct cause for male‐sterility in Consolea pistillate flowers. The mutated gene or genes (nuclear or mitochondrial) affecting the tapetal layer are also affecting almost all the other anther layers as well. This pattern is not at all uncommon. Goldberg et al. Citation(1993) indicated that some mutations causing male sterility alter the function, or interfere with the differentiation, of many anther wall layers including the tapetum, middle layer and endothecium.
There are several lines of evidence to identify PCD. Structurally, the first signs include condensation and shrinkage of the cytoplasm (Danon et al. Citation2000, Coimbra et al. Citation2004), and enlarged ER cisternae circumscribing portions of the cytoplasm (Papini et al. Citation1999). PCD can also be detected by the TUNEL reaction which identifies nuclear fragmentation (Danon el al. Citation2000, Coimbra et al. Citation2004). This has been used successfully to show PCD of tapetal cells (Balk & Leaver, Citation2001; Ku et al. Citation2003). Unfortunately the TUNEL assay could not be performed in Consolea due to the lack of fresh material. All six Consolea spp., however, exhibit enlarged ER cisternae, and condensed and shrunken cytoplasm following high vacuolization of the tapetal cells. These morphological indicators suggest that PCD is responsible for early tapetal cell degeneration in Consolea.
Cytomixis, the migration of nuclei or nuclear material (i.e. chromosomes), can occur from one microspore mother cell to another through cytoplasmic channels (Sidorchuk et al. Citation2004). It can also occur in the vegetative tissue of anthers (Wang et al. Citation2004), leaf tissue (Tarkowska, Citation1960) and shoot apexes (Guzicka & Wozny, Citation2005). It is widespread and has been described for several plant species (Cheng et al. Citation1975; Gottschalk, Citation1970; Omara, Citation1976; Bellucci et al. Citation2003). In the past, cytomixis was viewed as an anomaly or as a fixation/tissue preparation artifact (Tarkowska, Citation1960; Heslop‐Harrison, Citation1966; Ross, Citation1981); more recently it is considered to be a normal cytological occurrence (Bellucci et al. Citation2003). It has often been associated with chromosomically unbalanced plants (i.e. haploids, triploids); hybrids and mutants (Bellucci et al. Citation2003; Sidorchuk et al. Citation2004), and polyploids (He et al. Citation2004); but it has also been described, to some extent, in diploid species (Pagliarini & Pereira, Citation1992; de Souza & Pagliarini, Citation1997; Malallah & Attia, Citation2003). In all these cases there is a certain percentage of pollen/spore abortion, since some of the mmc's are completely devoid of a nucleus; nevertheless, cytomixis is not related to full male‐sterility. In fact, it has been suggested that the presence of larger and smaller than normal pollen grains is a sign that cytomixis is occurring (Cheng et al. Citation1975). In Consolea, cytomixis occurs both in male‐fertile and male‐sterile anthers. In a male‐sterile anther of C. moniliformis, cytomixis was observed between two mmc's (Fig. ) and in a male‐fertile anther of C. corallicola between a tapetal cell and a mmc (unpublished data). At this point however, we do not know the fate of those binucleate cells derived from cytomixis nor do we fully understand the evolutionary consequences of this phenomenon in Consolea.
Male‐sterility: single origin?
Nuclear, cytoplasmic and genic‐cytoplasmic male sterility can follow different abortion pathways and/or have different timings. In general, CMS‐GCMS lines seem more prone to having several abortion pathways within a line, even in different locules of an anther, and pollen degeneration occurs at various stages (Chauhan & Singh, Citation1966; Chhabra et al. Citation1997; Smith et al. Citation2002). However, in Consolea, the timing of male sterility is identical for the six species studied and essentially identical in mode of action. In all six species, mmc's abort shortly after zygotene‐pachytene and tapetal abnormalities precede abortion. In all six, the middle layer is persistent and the endothecium does not develop the characteristic fibrous thickenings, with the exception in some anthers of all species. Overall, the whole abortive process is homogeneous, even considering the minor developmental variation. Therefore, we hypothesize that all of the studied Consolea species have a common origin and mechanism of male‐sterility in pistillate flowers. This is in agreement with the findings of Sun and Ganders Citation(1987) for gynodioecious Bidens L. Male‐sterility followed the same pathway in nine Hawaiian Bidens, with a complete pre‐meiotic degeneration of the mmc's following early, abnormal, and complete tapetum vacuolation (Sun & Ganders, Citation1987). Based on these findings and the nuclear nature of the male‐sterility causing genes, the authors suggested that gynodioecy originated only once in these Hawaiian Bidens (Sun & Ganders, Citation1987). Unfortunately, we could not determine the inheritance pattern of male‐sterility in Consolea. However, since a common pattern of male‐sterility was observed, a single origin for all the cryptically dioecious/subdioecious Consolea species, and a common mutation or gene involved in the process (regardless of its CMS or GMS nature), is a likely explanation for our findings.
Conclusions
In all six Consolea spp investigated, the abortion timing, anther layers affected, and events leading to male‐sterility in pistillate flowers are identical. The mmc are aborted after zygotene‐pachytene, an early abortive stage. As a consequence, no microspore tetrads or pollen grains are formed. Tapetal cells are the first to deviate from normal development. They remain uninucleate throughout, or if binucleate, as in C. rubescens, no nuclear fusion occurs. Abundant rER of unusual configurations is present concomitantly with the appearance of vacuole/s. Subsequently, the tapetal cells become highly vacuolated, enlarge radially and become hypertrophied and press against the mmc. Ultimately, these hypertrophied tapetal cells, almost devoid of cytoplasm invade the anther locule, crushing the mmc.
Other anther layers are also affected, and in all six species the middle layer enlarges invading the anther locule, and eventually pushing and crushing the abnormal tapetal cells. The endothecium does not develop fibrous thickenings, and the intersporagial septum and stomium cells do not degenerate.
Only minor variations to this general pattern were observed. Given that in nature male‐sterility exhibits great diversity in timing and pattern, the presence of a conserved anther abortion pattern in Consolea strongly suggests a single evolutionary origin of male‐sterility for this group of species. In addition, the ovules' abortive pattern in staminate flowers of the six studied Consolea species is also homogenous (in prep.).
The parallelism between female‐sterility and male‐sterility gives further support for a single origin of cryptic dieocy/subdioecy breeding system in the studied Consolea species.
Acknowledgements
The authors thank Ethan Fried, Laurel Richey, Nathan Sammons, and Joel Timyan for invaluable help with fieldwork. We also thank the Jardín Botánico Nacional, Dr. Rafael M. Moscoso (Dominican Republic) and the Bahamas National Herbarium for their assistance with collecting permits. We also thank Dr. Beatriz G. Galati for reviewing this manuscript and for assistance in pollen descriptions used in this work, an anonymous reviewer for the useful comments to improve the manuscript, and the EM facility at Miami University for their helpful assistance. The Botany Department of Miami University, its Academic Challenge program, and the W. S. Turrell Herbarium Fund supported this dissertation research.
References
- Amela García , M. T. , Galati , B. G. and Anton , A. M. 2002 . Microsporogenesis, microgametogenesis and pollen morphology of Passiflora spp. (Passifloraceae). . Bot. J. Linn. Soc. , 139 : 383 – 394 .
- Anderson , G. J. and Symon , D. E. 1989 . Functional dioecy and andromonoecy in Solanum. . Evolution , 43 : 204 – 219 .
- Areces‐Mallea , A. E. 2001 . A new opuntioid cactus from the Cayman Islands, B. W. I., with a discussion and key to the genus Consolea Lemaire. . Brittonia , 53 : 96 – 107 .
- Balk , J. and Leaver , J. 2001 . The PET1‐CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. . Pl. Cell , 13 : 1803 – 1818 .
- Beals , T. P. and Goldberg , R. B. 1997 . A novel cell ablation strategy blocks tobacco anther dehiscence. . Pl. Cell , 9 : 1527 – 1545 .
- Bellucci , M. , Roscini , C. and Mariani , A. 2003 . Cytomixis in pollen mother cells of Medicago sativa L. . J. Hered. , 94 : 512 – 516 .
- Brooks , M. H. , Brooks , J. S. and Chien , L. 1966 . The anther tapetum in the cytoplasmic‐genetic male sterile sorghum. . Am. J. Bot. , 53 : 902 – 908 .
- Caporali , E. , Spada , A. , Marziani , G. , Failla , O. and Scienza , A. 2003 . The arrest of development of abortive reproductive organs in the unisexual flower of Vitis vinifera ssp. silvestris. . Sex. Pl. Repr., 15 , : 291 – 300 .
- Chaubal , R. , Zanella , C. , Trimnell , M. R. , Fox , T. W. , Albertsen , M. C. and Bedinger , P. 2000 . Two male‐sterile mutants of Zea mays (Poaceae) with an extra cell division in the anther wall. . Am. J. Bot. , 87 : 1193 – 1201 .
- Chaudhury , A. M. , Lavithis , M. , Taylor , P. E. , Craig , S. , Singh , M. B. , Signer , E. R. , Knox , R. B. and Dennis , E. S. 1994 . Genetic control of male fertility in Arabidopsis thaliana: Structural analysis of premeiotic developmental mutants. . Sex. Pl. Repr. , 7 : 17 – 28 .
- Chauhan , S. V. S. and Singh , S. P. 1966 . Pollen abortion in male‐sterile hexaploid wheat (“Norin”) having Aegilops ovata L. cytoplasm. . Crop Sci. , 6 : 532 – 535 .
- Cheng , K.‐C. , Nieh , H.‐W. , Yang , C.‐L. , Wang , I.‐M. , Chou , I.‐S. and Chen , J.‐S. 1975 . Light and electron microscopical observations on cytomixis and the study of its relation to variation and evolution (in Chinese). . Acta Bot. Sin. , 17 : 60 – 69 .
- Chhabra , A. K. , Khairwal , I. S. , Rai , K. N. , Hash , C. T. and Murthy , A. K. 1997 . Influence of cytoplsamic‐nuclear male‐sterility systems on microsporogenesis in pearl millet (Pennisetum glaucum (L.) R. Br.). . Euphytica , 98 : 1 – 10 .
- Childers , W. R. 1952 . Male sterility in Medicago sativa L. . Sci. Agricult. , 32 : 351 – 364 .
- Coimbra , S. , Torrão , L. and Abreu , I. 2004 . Programmed cell death induces male sterility in Actinidia delicosa female flowers. . Pl. Physiol. Biochem. , 42 : 537 – 541 .
- Colhoun , C. W. and Steer , M. W. 1981 . Microsporogenesis and the mechanism of cytoplasmic male sterility in maize. . Ann. Bot. , 48 : 417 – 424 .
- D'Ambrogio de Argüeso , A. 1986 . Manual de técnicas en histología vegetal , Buenos Aires : Hemisferio Sur S.A .
- Danon , A. , Delorme , V. , Mailhac , N. and Gallois , P. 2000 . Plant programmed cell death: A common way to die. . Pl. Physiol. Biochem. , 38 : 647 – 655 .
- Dawson , J. , Wilson , Z. A. , Aarts , M. G. M. , Braithwaite , A. F. , Briarty , L. G. and Mulligan , B. J. 1992 . Microspore and pollen development in six male‐sterile mutants of Arabidopsis thaliana. . Can. J. Bot. , 71 : 629 – 638 .
- Dubey , D. K. and Singh , S. P. 1965 . Mechanism of pollen abortion in three male sterile lines of flax (Linum usitatisimum L.). . Crop Sci. , 5 : 121 – 124 .
- El‐Ghazaly , G. and Nilsson , S. 1991 . “ Development of tapetum and orbicules of Catharanthus roseus (Apocynaceae). ” . In Pollen and spores , Edited by: Blackmore , S and Barnes , S. H . 317 – 329 . Oxford : Clarendon Press, Syst. Assoc. Sp. Vol. 44 .
- Engelke , T. , Hülsmann , S. and Tatlioglu , T. 2002 . A comparative study of microsporogenesis and anther wall development in different types of genic and cytoplasmic male sterilities in chives. . Pl. Breed. , 121 : 254 – 258 .
- Farbos , I. , Oliveira , M. , Negrutiu , I. and Mouras , A. 1997 . Sex organ determination and differentiation in the dioecious plant Melandrium album (Silene latifolia): A cytological and histological analysis. . Sex. Pl. Repr. , 10 : 155 – 167 .
- Fei , H. and Sawhney , V. K. 1999 . MS32‐regulated timing of callose degradation during microsporogenesis in Arabidopsis is associated with the accumulation of stacked rough ER in tapetal cells. . Sex. Pl. Repr. , 12 : 188 – 193 .
- Fernando , D. D. and Cass , D. D. 1994 . Plasmodial tapetum and pollen wall development in Butomus umbellatus (Butomaceae). . Am. J. Bot. , 81 : 1592 – 1600 .
- Galati , B. G. and Rosenfeldt , S. 1998 . The pollen development in Ceiba insignis (Kunth) Gibbs & Semir ex Chorisia speciosa St. Hil. (Bombacaceae). . Phytomorphology , 48 : 121 – 129 .
- Galati , B. G. and Strittmatter , L. I. 1999 . Correlation between pollen development and Übisch bodies ontogeny in Jacaranda mimosifolia (Bignoniaceae). . Beitr. Biol. Pflanz. , 71 : 249 – 260 .
- Goetz , M. , Godt , D. E. , Guivarc'h , A. , Kahmann , U. , Chriqui , D. and Roitsch , T. 2001 . Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. . Proc. Natl Acad. Sci. U. S. A. , 98 : 6522 – 6527 .
- Goldberg , R. B. , Beals , T. P. and Sanders , P. M. 1993 . Anther development: basic principles and practical applications. . Pl. Cell , 5 : 1217 – 1229 .
- Gottschalk , W. 1970 . Chromosome and nucleus migration during microsporogenesis of Pisum sativum. . Nucleus , 13 : 1 – 9 .
- Gottschalk , W. and Kaul , M. L. H. 1974 . The genetic control of microsporogenesis in higher plants. . Nucleus , 17 : 133 – 166 .
- Grant , I. , Beversdorf , W. D. and Peterson , R. L. 1986 . A comparative light and electron microscopic study of microspore and tapetal development in male fertile and cytoplasmic male sterile oilseed rape (Brassica napus). . Can. J. Bot. , 64 : 1055 – 1068 .
- Graybosch , R. A. , Bernard , R. L. , Cremeens , C. R. and Palmer , R. G. 1984 . Genetic and cytological studies of a male‐sterile, female‐fertile soybean mutant: A new male‐sterile gene (ms2) in Glycine max (L.) Merr. . J, Hered. , 75 : 383 – 388 .
- Graybosch , R. A. and Palmer , R. G. 1985 . Male sterility in soybean (Glycine max). I. Phenotypic expression of the ms2 mutant. . Am. J. Bot. , 72 : 1738 – 1750 .
- Greenberg , J. T. 1996 . Programmed cell death: a way of life for plants. . Proc. Natl Acad. Sci. U. S. A. , 93 : 12094 – 12097 .
- Guzicka , M. and Wozny , A. 2005 . Cytomixis in the shoot apical meristem of the Norway spruce. . Trees , 18 : 722 – 724 .
- Hanson , M. R. and Conde , M. F. 1985 . Functioning and variation of cytoplasmic genomes: Lessons from cytoplasmic‐nuclear interactions affecting male fertility in plants. . Int. Rev. Cytol. , 94 : 213 – 267 .
- Harris , N. 1986 . Organization of the endomembrane system. . Ann. Rev. Pl. Physiol. , 37 : 73 – 92 .
- He , Z.‐C. , Wang , H.‐C. , Li , J.‐Q. , Ye , Q.‐G. and Taylor , W. C. 2004 . Chromosome behaviour during meiosis and development of spore mother cells in the Chinese quillwort Isoetes sinensis T. C. Palmer (Isoetaceae). . Am. Fern J. , 94 : 183 – 195 .
- Hernould , M. , Suharsono , Zabaleta , E. , Carde , J. P. , Litvak , S. , Araya , A. and Mouras , A. 1998 . Impairment of tapetum and mitochondria in engineered male‐sterile tobacco plants. . Pl. Molec. Biol. , 36 : 499 – 508 .
- Heslop‐Harrison , J. 1966 . Cytoplasmic connexions between angiosperm meiocytes. . Ann. Bot. , 30 : 221 – 234 .
- Hidalgo , P. J. , Hesse , M. , Ubera , J. L. and Frosch‐Radivo , A. 1999 . Microsporogenesis in male sterile Rosmarinus oficinalis L. (Lamiaceae), an ultrastructural study. . Grana , 38 : 343 – 355 .
- Hormaza , J. I. and Polito , V. S. 1996 . Pistillate and staminate flower development in dioecious Pistacia vera (Anacardiaceae). . Am. J. Bot. , 83 : 759 – 766 .
- Horner , H. T. 1977 . A comparative light‐ and electron‐microscopy study of microsporogenesis in male‐fertile and cytoplasmic male‐sterile sunflower (Helianthus annuus). . Am. J. Bot. , 64 : 745 – 759 .
- Jin , W. , Horner , H. T. and Palmer , R. G. 1997 . Genetics and cytology of a new genic male‐sterile soybean [Glycine max (L.) Merr.]. . Sex. Pl. Repr. , 10 : 13 – 21 .
- Johri , B. M. , Ambegaokar , K. B. and Srivastava , P. S. 1992 . Comparative embryology of angiosperms , Berlin : Springer .
- Joppa , L. R. , McNeal , F. H. and Welsh , J. R. 1966 . Pollen and anther development in cytoplasmic male sterile wheat, (Triticum aestivum L.). . Crop Sci. , 6 : 296 – 297 .
- Kamelina , O. P. 2002 . “ Tapetum. ” . In Embryology of flowering plants: terminology and concepts. Vol. I. Generative organs of flower , Edited by: Batygina , T. B . 19 Enfield NH : Sci. Publ .
- Kaul , M. L. H. 1988 . Male sterility in higher plants , Berlin/New York : Springer .
- Kaul , C. L. and Singh , S. P. 1966 . Studies in male‐sterile barley. II. Pollen abortion. . Crop Sci. , 6 : 539 – 541 .
- Kini , A. V. , Seetharam , A. and Joshi , S. S. 1994 . Mechanism of pollen abortion in cytoplasmic male sterile line of sunflower. . Cytologia , 59 : 121 – 124 .
- Kriete , G. , Niehaus , K. , Perlick , A. M. , Pühler , A. and Broer , I. 1996 . Male‐sterility in transgenic tobacco plants induced by tapetum‐specific deacetylation of the externally applied non‐toxic compound N‐acetyl‐L‐phosphinothricin. . Pl. J. , 9 : 809 – 818 .
- Ku , S.‐J. , Yoon , H.‐J. , Suh , H. S. and Chung , Y.‐Y. 2003 . Male‐sterility of the thermosensitive genic male‐sterile rice is associated with premature programmed cell death of the tapetum. . Planta , 217 : 559 – 565 .
- Kuriyama , H. and Fukuda , H. 2002 . Developmental programmed cell death in plants. . Curr. Opin. Pl. Biol. , 5 : 568 – 573 .
- Laser , K. D. and Lersten , N. R. 1972 . Anatomy and cytology of microsporogenesis in cytoplasmic male sterile angiosperms. . Bot. Rev. , 38 : 425 – 454 .
- Lee , S‐L. J. and Warmke , H. E. 1979 . Organelle size and number in fertile and T‐cytoplasmic male‐sterile corn. . Am. J. Bot. , 66 : 141 – 148 .
- Lee , S‐L. J. , Gracen , V. E. and Earle , E. D. 1979 . The cytology of pollen abortion in C‐cytoplasmic male‐sterile corn anthers. . Am. J. Bot. , 66 : 656 – 667 .
- Lee , S‐L. J. , Earle , E. D. and Gracen , V. E. 1980 . The cytology of pollen abortion in S cytoplasmic male‐sterile corn anthers. . Am. J. Bot. , 67 : 237 – 245 .
- Loukides , C. A. , Broadwater , A. H. and Bedinger , P. A. 1995 . Two new male‐sterile mutants of Zea mays (Poaceae) with abnormal tapetal cell morphology. . Am. J. Bot. , 82 : 1017 – 1023 .
- Majewska‐Sawka , A. , Rodriguez‐García , M. I. , Nakashima , H. and Jassen , B. 1993 . Ultrastrucutral expression of cytoplasmic male sterility in sugar beet (Beta vulgaris L.). . Sex. Pl. Repr. , 6 : 22 – 32 .
- Malallah , G. A. and Attia , T. A. 2003 . Cytomixis and its possible evolutionary role in a Kuwaiti population of Diplotaxis harra (Brassicaceae). . Bot. J. Linn. Soc. , 143 : 169 – 175 .
- Mariani , C. , Beuckeleer , M. D. , Truettner , J. , Leemans , J. and Goldberg , R. B. 1990 . Induction of male sterility in plants by a chimaeric ribonuclease gene. . Nature , 347 : 737 – 741 .
- McDowel , E. and Trump , B. 1976 . Histological fixatives for diagnostic light and electron microscopy. . Arch. Pathol. Lab. Med. , 100 : 405 – 414 .
- Negrón‐Ortiz , V. and Strittmatter , L. 2004 . Embryology of floral dimorphism and gender system in Consolea corallicola (Cactaceae), a rare species of the Florida Keys. . Haseltonia , 10 : 16 – 25 .
- Neumann , M. 1935 . Die Entwicklung des Pollens, der Samenanlage und des Embryosackes von Pereskia amapola var. argentina. . Österr. Bot. Zeitschr. , 84 : 1 – 30 .
- Omara , M. K. 1976 . Cytomixis in Lolium perenne. . Chromosoma , 55 : 267 – 271 .
- Overman , M. A. and Warmke , H. E. 1972 . Cytoplasmic male sterility in Sorghum. II. Tapetal behavior in fertile and sterile anthers. . J. Hered. , 63 : 227 – 234 .
- Owen , H. A. and Makaroff , C. A. 1995 . Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. Ecotype Wassilewskija (Brassicaceae). . Protoplasma , 185 : 7 – 21 .
- Pagliarini , M. S. and Pereira , M. A. S. 1992 . Meiotic studies in Pilocarpus pennatifolius Lem. (Rutaceae). . Cytologia , 57 : 231 – 235 .
- Papini , A. , Mosti , S. and Brighigna , L. 1999 . Programmed‐cell‐death events during tapetum development of angiosperms. . Protoplasma , 207 : 213 – 221 .
- Platt , K. A. , Huang , A. H. C. and Thomson , W. W. 1998 . Ultrastructural study of lipid accumulation in tapetal cells of Brassica napus L. cv. Westar during microsporogenesis. . Int. J. Pl. Sci. , 159 : 724 – 737 .
- Pollak , P. E. 1992 . Cytological differences between a cytoplasmic male sterile tobacco cybrid and its fertile counterpart during early anther development. . Am. J. Bot. , 79 : 937 – 945 .
- Polowick , P. L. and Sawhney , V. K. 1990 . Microsporogenesis in a normal line and in the ogu cytoplasmic male‐sterile line of Brassica napus I. the influence of high temperatures. . Sex. Pl. Repr. , 3 : 263 – 276 .
- Pritchard , A. J. and Hutton , E. M. 1972 . Anther and pollen development in male‐sterile Phaseolus atropurpureus. . J. Hered. , 63 : 280 – 282 .
- Rick , C. M. 1948 . Genetics and development of nine male‐sterile tomato mutants. . Hilgardia , 18 : 599 – 633 .
- Risueño , M. C. , Giménez‐Martín , G. , López‐Sáez , J. F. and García , M. I. R. 1969 . Origin and development of sporopollenin bodies. . Protoplasma , 67 : 361 – 374 .
- Ross , R. 1981 . Chromosome counts, cytology and reproduction in the Cactaceae. . Am. J. Bot. , 68 : 463 – 470 .
- Sanders , P. M. , Bui , A. Q. , Weterings , K. , McIntire , K. N. , Hsu , Y‐C. , Lee , P. Y. , Truong , M. T. , Beals , T. P. and Goldberg , R. B. 1999 . Anther developmental defects in Arabidopsis thaliana male‐sterile mutants. . Sex. Pl. Repr. , 11 : 297 – 322 .
- Sawhney , V. K. and Bhadula , S. K. 1988 . Microsporogenesis in the normal and male‐sterile stamenless‐2 mutant of tomato (Lycopersicon esculentum). . Can. J. Bot. , 66 : 2013 – 2021 .
- Sawhney , V. K. and Shukla , A. 1994 . Male sterility in flowering plants: Are plant growing substances involved? . Am. J. Bot. , 81 : 1640 – 1647 .
- Schnable , P. S. and Wise , R. P. 1998 . The molecular basis of cytoplasmic male sterility and fertility restoration. . Trends Pl. Sci. Rev. , 3 : 175 – 180 .
- Sidorchuk , Y. V. , Deineko , E. V. and Shumnyi , V. K. 2004 . Cytomixis in mother pollen cells of transgenic tobacco (Nicotiana tabacum L.) plants. . Dokl. Biol. Sci. , 394 : 47 – 50 .
- Singh , S. P. and Rhodes , A. M. 1961 . A morphological and cytological study of male sterility in Cucurbita maxima. . Am. Soc. Horticult. Sci. Proc. , 78 : 375 – 378 .
- Smith , M. B. , Palmer , R. G. and Horner , H. T. 2002 . Microscopy of a cytoplasmic male‐sterile soybean from an interspecific cross between Glycine max and G. soja (Leguminosae). . Am. J. Bot. , 89 : 417 – 426 .
- Souza , A. de. and Pagliarini , M. 1997 . Cytomixis in Brassica napus var. oleifera and Brassica campestris var. oleifera (Brassicaceae). . Cytologia , 62 : 25 – 29 .
- Spurr , A. R. 1969 . A low viscosity epoxy resin embedding medium for electronmicroscopy. . J. Ultrastr. Res. , 26 : 31
- Strittmatter , L. I. , Negrón‐Ortiz , V. and Hickey , R. J. 2002 . Subdioecy in Consolea spinosissima (Cactaceae): breeding system and embryological studies. . Am. J. Bot. , 89 : 1373 – 1387 .
- Suárez‐Cervera , M. and Seoane‐Camba , J. A. 1986 . Ontogénese des grains de pollen de Lavandula dentata L. et évolution des cellules tapétales. . Pollen & Spores , 28 : 5 – 28 .
- Sun , M. and Ganders , F. R. 1987 . Microsporogenesis in male‐sterile and hermaphroditic plants of nine gynodioecious taxa of Hawaiian bidens (Asteraceae). . Am. J. Bot. , 74 : 209 – 217 .
- Suzuki , K. , Takeda , H. , Tsukaguchi , T. and Egawa , Y. 2001 . Ultrastructural study on degeneration of tapetum in anther of snap bean (Phaseolus vulgaris L.) under heat stress. . Sex. Pl. Repr. , 13 : 293 – 299 .
- Tarkowska , J. 1960 . Cytomixis in the epidermis of scales and leaves and in meristemes of root apex of Allium cepa L. (in Polish). . Acta Soc. Bot. Poloniae , 29 : 149 – 168 .
- Tiagi , Y. D. 1954 . Studies in the floral morphology of Opuntia dillenii Haworth. . Bot. Not. , 4 : 343 – 356 .
- Tiagi , Y. D. 1961 . Studies in floral morphology VII. The development of the anther and ovule of Mammillaria carnea Zucc. with a discussion on the systematic position of the family Cactacae. . Madhya Bharati P. II Sect. B, Nat. Sci. J. Univ. Saugar , B10 : 12 – 24 .
- Vasil , I. K. 1967 . Physiology and cytology of anther development. . Biol. Rev. & Biol. Proc. Cambridge Philos. Soc. , 42 : 327 – 373 .
- Vitale , A. , Ceriotti , A. and Denecke , J. 1993 . The role of the endoplasmic reticulum in protein synthesis, modification and intracellular transport. . J. Exp. Bot. , 44 : 1417 – 1444 .
- Wang , M. , Hoekstra , S. , van Bergen , S. , Lamers , G. E. M. , Oppedijk , B. I. , van der Heijden , M. W. , de Priester , W. and Schilperoort , R. A. 1999 . Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. . Pl. Mol. Biol. , 39 : 489 – 501 .
- Wang , X.‐Yu. , Yu , C.‐H. , Li , X. W. , Chong , Y. and Zheng , G. ‐C. 2004 . Ultrastructural aspects and possible origin of cytoplasmic channels providing intercellular connection in vegetative tissues of anthers. . Russ. J. Pl. Physiol. , 51 : 97 – 106 .
- Warmke , H. E. and Lee , S.‐L. J. 1977 . Mitochondrial degeneration in Texas cytoplasmic male‐sterile corn anthers. . J. Hered. , 68 : 213 – 222 .
- Watson L, Dallwitz MJ. 1992 onwards. The families of flowering plants: descriptions, illustrations, identification, and information retrieval. Vers. 13 th January 2005. http://delta‐intkey.com/angio/www/cactacea.htm (http://delta-intkey.com/angio/www/cactacea.htm)
- Worral , D. , Hird , D. L. , Hodge , R. , Paul , W. , Draper , J. and Scott , R. 1992 . Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. . Pl. Cell. , 4 : 759 – 771 .
- Wu , H.‐M. and Cheung , A. Y. 2000 . Programmed cell death in plant reproduction. . Pl. Molec. Biol. , 44 : 267 – 281 .
- Yonggen , L. and Rutger , J. N. 1984 . Cytological observations on induced genetic male sterile mutants in rice (Oryza sativa L.). . Sci. Sin. (B) , 27 : 487 – 493 .
- Yu , X‐H. , Perdue , T. D. , Heimer , Y. M. and Jones , A. M. 2002 . Mitochondrial involvement in tracheary element programmed cell death. . Cell Death & Different. , 9 : 189 – 198 .
- Zhang , C. , Guinel , F. C. and Moffatt , B. A. 2002 . A comparative ultrastrucutral study of pollen development in Arabidopsis thaliana ecotype Columbia and male‐sterile mutant apt1‐3. . Protoplasma , 219 : 59 – 71 .