Abstract
In the present study, the airborne concentrations of birch and oak pollen grains and birch pollen allergens have been recorded in samples from a common sampling station in Stockholm. The sampling period was between April 22nd and May 31st 2003. The objectives were to evaluate if analysis of allergen particles in parallel with pollen grains would be relevant to aid subjects suffering from pollinosis. Days with low birch pollen counts had relatively high nominal allergen concentrations in the beginning of the sampling period. The birch pollen grain concentration peaks, during the dry pollination culmination interval in the middle of the period, were associated with correspondingly lower nominal concentrations of allergens than grains. At the end of the sampling period very high nominal amounts of allergen appeared, as reflected by high concentrations of oak pollen grains. The high allergen concentrations were obtained as a result of inherent cross‐reactivity of anti‐ Bet v 1 antibodies with Que a antigens, which are immunologically analogous with Bet v 1. Allergen concentrations increased and decreased after light and heavy rain, respectively. Results obtained indicate that adding a pollen count forecast with allergen concentration data should aid pollen allergic subjects to avoid particulate allergens which might be expected to penetrate more easily than pollen grains into indoor environments.
Recording of atmospheric concentrations of pollen from various species is regularly performed at a number of stations in Sweden, for allergy‐service purposes. The allergenicity of pollen grains from birch and grasses, being the main sources for the development of pollinosis in Northwest Europe, is related to their properties to generate small protein‐containing particles (Spieksma et al. Citation1990, Citation1991; Pehkonen & Rantio‐Lehtimäki Citation1994, Rantio‐Lehtimäki et al. Citation1994, Yli‐Panula Citation1997). These are released from the pollen grains in contact with water under different meteorological conditions and can give very heterogeneous mixtures of submicronic respirable particles (Suphioglu et al. Citation1992, Schäppi et al. Citation1997). Vegetal fragments and orbicules are also regarded as potential aeroallergen carriers (D'Amato Citation2001). Weather‐dependent fractionation processes can lead to enrichment of the small particles in the air, even in the absence of detectable pollen grains (Schäppi et al. Citation1996, Pehkonen & Rantio‐Lehtimäki Citation1994). A direct on sampling filter analysis (DOSIS) technique (Holmquist & Vesterberg Citation1999 a, Holmquist et al. Citation2001) for quantification of airborne pollen allergen particles, has revealed that air in different indoor environments is often contaminated with substantial amounts of small allergenic pollen particles (Holmquist & Vesterberg Citation1999 a, Citation b ; Citation2001) originating from birch and grass pollen grains in the outdoor air. Thus, as a complement to the pollen counts and forecasts offered to allergic subjects for prediction of the risk of hay fever symptoms or asthmatic attacks, the simultaneous publishing of atmospheric pollen allergen concentrations would be valuable.
With the aim of evaluating the practicability of providing this service, we have analysed, in parallel, the birch and oak pollen grains and birch pollen allergen particle concentrations in the outdoor air at a common sampling station in Stockholm.
MATERIAL AND METHODS
Material
Water extracts of pollen from birch (Betula pendula) for skin prick testing; Soluprick SQ®, containing 100,000 SQ units/ml (manufacturer's specfication) was purchased from ALK‐Abelló A/S (Hoersholm, Denmark) and used as reference allergen for calibration curves. The birch pollen extract contained 23 μg Bet v 1 protein/ml (manufacturer's specfication) as major allergen (antigen) but did not contain oak (Quercus alba) antigen. Polyclonal primary antibodies, rabbit IgG anti‐Betula raised against oak antigen‐free water extracts of birch pollen, were from the same company. All other reagents and materials including the sample filter holders and 25 mm, 1.2 μm Teflon® filters were the same as previously described for the quantification of pollen allergens by DOSIS (Holmquist & Vesterberg Citation1999 a) as modified (Holmquist et al. Citation2001). AirChek® 2000 vacuum pumps for air sampling were purchased from SCK Inc. (Eighty Four, PA, USA).
Analytical methods
Quantification of pollen grains was performed using a Burkard Seven Day Pollen trap (Hirst Citation1952) from Burkard Manufacturing Ltd (Rickmansworth, Hertfordshire). The sampling tapes were cut into daily segments and the microscopic analyses were carried out applying 400 times magnification. Twelve transects were counted, one for every second hour. Pollen concentration is expressed as the average number of pollen grains per m3 air over periods of 24 hr (00:00–24:00). For this study also the pollen concentrations over 8 hr (08:00–16:00) were calculated.
Quantification of airborne pollen allergen (antigen) was performed with the DOSIS techniques as previously described in detail (Holmquist & Vesterberg Citation1999 a, Holmquist et al. Citation2001). DOSIS depends on the use of antibodies that bind specifically to the proteins in the calibration standard of the assay, which are the antigens but also the allergens in common prick tests. Thus allergens are quantified in the assay. Briefly, the allergens are adsorbed to an air sampling porous polytetrafluoroethylene (Teflon® ) filter, the hydrophobic character of which allows the unhindered passage of moist air without blocking the filter. The matrix bound allergens are subsequently reacted with allergen‐specific antibodies conjugated with alkaline phosphatase. This generates a filter‐bound allergen‐antibody‐phosphatase complex which is allowed to float on a chemiluminiscent phosphatase substrate solution. Aliquots of the reaction mixture are withdrawn at defined time intervals and the light emitted by the product of the enzyme activity, as recorded by a luminometer, is linearly related to the amount of allergen.
Note: As the pollen allergen and grain concentrations are defined as different units, SQ units and number (n) per m3 of air, respectively, the denomination of “high” and “low” concentrations of airborne allergens, in relation to those of the pollen grains in this study, merely refers to nominal values of the two parameters and does not express real differences in allergenicity or allergen masses.
Study Design
The pollen grain and allergen sampling was performed at the regular pollen count station used for allergy service, about 15 m above the ground of the roof of the Arrhenius Laboratory, Stockholm University (co‐ordinates: WGS 84: 59°21′57″ North and 18°03′46″ East). The pump for sampling of airborne birch pollen allergens was equipped with a 25 mm sampling filter as previously described (Holmquist & Vesterberg Citation1999 a, Holmquist et al. Citation2001). Each day of the sampling period a pump was placed on top of a 20 cm high box, centered under an 80×80 cm large table with 70 cm high legs. This was to protect the sampling device from rain showers. The table was firmly anchored to the floor of the station. The pump with the sampling filter was started at eight o'clock in the morning and ran for eight hours. Collected sample filters were wrapped in Parafilm® (American National Can, IL, USA) and transported to the analytical laboratory where they were kept at −20°C prior to analysis. The air sampling flow was one liter per minute (1 l/min).
The Burkard trap, with an air sampling flow of 10 l/min, was placed adjacent to the pollen allergen sampling pump and was run in parallel for 24 hour periods. The sampling tape was transported to the analytical laboratory and analysed as described above. The pollen and allergen sampling period ranged from April 22nd to May 31st 2003. No allergen sampling was performed between April 30th and May 3rd.
Meteorological parameters
The amount of precipitation was recorded each day at Stockholm (WGS 84: 59°20′32″ North and 18°03′30″ East) between 07:00 and 19:00 on the pollen and allergen sampling day, and from 19:00 to 07:00 on the following morning (). Precipitation measurements were performed by the Swedish Meteorological and Hydrological Institute.
Table I. Amount of precipitation during pollen and allergen sampling period, 07:00–19:00, and days from 07:00 in the morning of the sampling day to 07:00 on the following day.
RESULTS AND DISCUSSION
At the beginning of the birch flowering season, April 22nd to May 4th, the birch pollen allergen concentrations in the air was generally higher, as estimated in SQ units, than the corresponding pollen grain concentrations. Both showed similar profiles of the concentration curves (). A high pollen concentration peak on April 30th coincided with an allergen peak. At the main birch pollen releasing period between May 5th and 13th, which had dry conditions, and when the trees had got small sticky leaves, lower concentrations of allergen as compared to those of pollen grains were recorded both when the eight hours allergen sampling period was compared with eight and 24 hours pollen counts (). Studies of the relation of airborne birch pollen grains and allergen concentrations in Australia (Schäppi et al. Citation1997) have revealed that the situation described above can occur as a result of the phenomenon that the surfaces of birch leaves under dry conditions become coated with pollen which only after light rainfall will release allergen molecules which can be dispersed in the air. A dry weather period with high birch pollen counts but low concentrations of allergens, analyzed in parallel in Finland, was suggested to be the result of production of pollen, empty of antigenic material (Rantio‐Lehtimäki et al. Citation1994). Dry days of the studied period between May 13th and 21st with low pollen counts yielded higher or similar concentrations of airborne allergen relative those of the pollen grains. On May 23rd the allergen concentration rose to a very high level although the birch pollen counts were low. Three large allergen peaks were recorded until May 31st (). Analysis of the Burkard tape showed that very high amounts of oak pollen were also present during this sampling period (). Like birch and grass pollen grains, the oak pollen has apertures where its inherent protein content can leak out and give rise to allergenic particles (Fernandez‐Caldas et al. Citation1989). The high allergen concentration values can be a result of the fact that rabbit polyclonal antibodies raised against Bet v 1 cross‐react with Que a 1 allergens (Ipsen & Hansen Citation1991). These are immunologically partial identical major allergens of pollen from birch and oak, respectively, genera belonging to different families of the order Fagales. This cross‐reactivity must also exist between the corresponding allergen originating from other Quercus species. In Sweden Quercus robur prevails. In addition, natural birch and oak pollen allergens share IgE epitopes with recombinant Bet v 1 (Niederberger et al. Citation1998) and Bet v 1‐specific human serum IgE and rabbit anti‐rBet v antibodies cross react with oak pollen allergen (Flicker et al. Citation2000). These findings justify the use of Bet v 1 as a calibration standard also for quantification of the oak allergen (Mari et al. Citation2003). The immunological analogy between birch and oak pollen allergens is reflected by the observation that oak pollen has a high sensitization potency causing more than 80 per cent of subjects allergic to birch pollen also to react to oak pollen extracts (Strandhede Citation2002). Similar findings have been reported by others (Mari et al. Citation2003, Ross et al. Citation1996).
Fig. 1. Upper panel. Concentrations of outdoor air birch pollen allergens (filled triangles) determined by the DOSIS technique after 8 hr air sampling (08–16) and of pollen grains obtained with the Burkard trap, reported as average values from 24 hr sampling periods (00–24) for birch (open squares) and 8 hr sampling periods (08–16) for oak pollen grains (dashed line), respectively. The horizontal bars mark days with rain ().
Lower panel. Same as upper panel but the birch pollen grain concentrations (open squares) represent average values obtained from the same 8 hr sampling period, 08 to 16, as that from sampling of birch pollen allergens.
It seems reasonable to assume that the high birch pollen allergen concentration peaks appearing from May 23rd to 31st result from immunological cross‐reactivity between anti‐Bet v antibodies with Que allergens released from oak pollen, which give a positive allergen concentration in the birch allergen assay (DOSIS). Note the close correlation between the birch pollen concentrations of the 8 hr and 24 hr sampling periods and that oak pollen (dashed line) were virtually absent from 2nd to 19th of May.
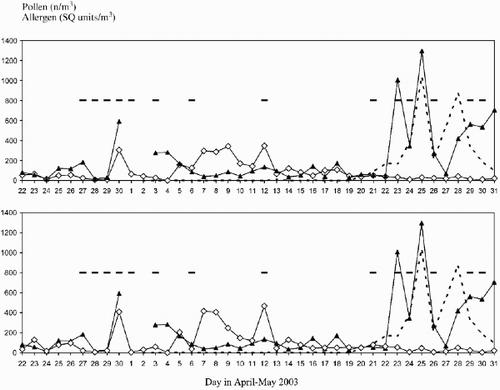
The influence of rain on pollen grain and allergen concentrations in the air is very complex (Berggren et al. Citation1995). Heavy rain is considered to reduce both airborne birch pollen and allergen concentrations whereas light rain, less than 1 mm, tends to result in a delayed increase of allergen amounts in the air, especially when followed by dry and windy weather (Schäppi et al. Citation1997, Holmquist & Vesterberg Citation1999 b). However, Berggren with co‐workers reported that heavy rain showers could result in an increase of airborne birch pollen concentrations, lasting for a few hours (Berggren et al. Citation1995).
In the present study both heavy and light rain occurred during the sampling period.
The influence of rain on allergen and pollen grain concentrations in the late sampling period are in agreement with these findings. Light rain was observed on May 21st (). A large increase of airborne pollen allergens from May 22nd to 23rd, but with a relatively low increase of pollen grains was recorded (). More rain on the latter day depressed this allergen concentration on May 24th with no rain during the sampling hours. It increased again in parallel with a large increase in airborne oak pollen concentration, which peaked on the dry May 25th. Heavy rain mixed with snow on the 26th during the sampling hours resulted in a large decrease of both allergen and oak pollen grains. The following two dry days resulted in a pollen grain concentration peak and an increasing, delayed allergen concentration, which was further increasing after two days with light rain. The influence of rain in the early sampling period with rather low birch pollen concentrations is inconsistent as no allergen sampling was performed between April 30th and May 3rd due to seasonal holidays.
CONCLUSIONS
The present study indicates that analysis of particulate airborne allergens released by birch and probably oak pollen gives complementary information to simultaneously recorded pollen counts, which could support subjects afflicted with pollinosis in their efforts to depress allergic symptoms. The study supports a previous proposal that pollen counts should be supplemented with information about allergen activities (Rantio‐Lehtimäki et al. Citation1994). Especially oak pollen counts and allergenic activities should be given adequate attention in regions with dominating birch trees due to the confirmed cross‐reactivity of birch and oak allergens.
ACKNOWLEDGEMENTS
The authors are indebted to AFA Trygghetsförsäkringar Stockholm for financial support.
References
REFERENCES
- Berggren , B , Nilsson , S and Boëthius , G . 1995 . Diurnal variation of airborne birch pollen in some sites in Sweden. . – Grana , 34 : 251 – 259 .
- D'Amato , G . 2001 . Airborne paucimicronic allergen‐carrying particles and seasonal respiratory allergy. . – Allergy , 56 : 1109 – 1111 .
- Fernandez‐Caldas , E , Swanson , MC , Pravda , P , Welsh , JW and Reed , CE . 1989 . Immunochemical demonstration of red oak pollen aeroallergens outside the oak pollination season. . – Grana , 28 : 205 – 209 .
- Flicker , S , Laffer , S , Steinberger , P , Alhani , B , Zhu , Y , Laukkanen , ML , Keinanen , K , Kraft , D and Valenta , R . 2000 . Engineering, purification and applications of His‐tagged recombinant antibody fragments with specificity for the major birch pollen allergens bet v 1. . – Biol. Chem. , 381 : 39 – 47 .
- Hirst , JM . 1952 . An automatic volumetric spore trap. . – Ann. Appl. Biol. , 39 : 257 – 265 .
- Holmquist , L and Vesterberg , O . 1999a . Luminescence immunoassay of pollen allergens on air sampling polyttetrafluoroethylene filters. . – J. Biophys. Biochem. Meth. , 41 : 49 – 60 .
- Holmquist , L and Vesterberg , O . 1999b . Quantification of birch and grass pollen allergens in indoor air. . – Indoor Air 9 , 85 : 91
- Holmquist L Vesterberg O 2001 Airborne birch and grass pollen allergens in driving compartments of coaches. – Arbete och Hälsa 2 1 13 (http:www.niwl.se/ah/)
- Holmquist , L , Weiner , J and Vesterberg , O . 2001 . Airborne birch and grass pollen allergens in street level shops. . – Indoor Air , 11 : 241 – 245 .
- Ipsen , H and Hansen , OC . 1991 . The NH2 ‐terminal amino acid sequence of the immunochemically partial identical major allergens of Alder (Alnus glutinosa) Aln g 1, Birch (Betula verrucosa) Bet v 1, hornbeam (Carpinus betulus) Car b 1 and oak (Quercus alba) Que a 1 pollens. . – Molec. Immunol , 28 : 1279 – 1288 .
- Mari , R , Wallner , M and Ferreira , F . 2003 . Fagales pollen sensitization in birch‐free area: a respiratory cohort survey using Fagales pollen extracts and birch recombinant allergens (rBet v1, rBet v 2, rBet v 4). . – Clin. Exp. Allergy , 33 : 1419 – 1428 .
- Niederberger , V , Pauli , G , Grönlund , H , Froschl , R , Rumpold , H , Kraft , D , Valenta , R and Spitzauer , S . 1998 . Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel and oak pollen: a quantitative IgE inhibition study with sera from different populations. . – J. Allergy Clin. Immunol. , 102 : 579 – 591 .
- Pehkonen , E and Rantio‐Lehtimäki , A . 1994 . Variations in airborne pollen antigenic particles caused by meteorologic factors. . – Allergy , 49 : 472 – 477 .
- Rantio – Lehtimäki , A , Viander , M and Koivikko , A . 1994 . Airborne birch pollen antigens in different particle sizes. . – Clin. Exp. Allergy , 24 : 23 – 28 .
- Ross , AM , Corden , JM and Fleming , DM . 1996 . The role of oak pollen in hay fever consultations in general practise and the factors influencing patient's decisions to consult. . – Br. J. Gen. Pract. , 46 : 451 – 455 .
- Schäppi , GF , Monn , C , Wüthrich , B and Wanner , HU . 1996 . Direct determination of allergens in ambient aerosols: methodological aspects. . – Int. Arch. Allergy Immunol. , 110 : 364 – 370 .
- Schäppi , GF , Suphioglu , C , Taylor , PE and Knox , RB . 1997 . Concentrations of the major birch tree allergen Bet v 1 in pollen and respirable fine particles in the atmosphere. . – J. Allergy Clin. Immunol. , 100 : 656 – 661 .
- Spieksma , FTM , Kramps , JA , Plomp , A and Koerten , HK . 1991 . Grass‐pollen allergen carried by smaller micronic aerosol fraction. . – Grana , 30 : 98 – 101 .
- Spieksma , FTM , Kramps , JA , Van der Linden , AC , Nikkels , BH , Plomp , A , Koerten , HK and Dijkman , JH . 1990 . Evidence of grass‐pollen allergenic activity in the smaller micronic atmospheric aerosol fraction. . – Clin. Exp. Allergy , 20 : 273 – 280 .
- Strandhede SO 2002 Farliga och ofarliga växter från A till Ö. – Bilda F., Stockholm (In Swedish)
- Suphioglu , C , Singh , MB , Taylor , P , Bellomo , R , Holmes , P , Puy , R and Knox , RB . 1992 . Mechanism of grass‐pollen‐induced asthma. . – Lancet , 339 : 569 – 572 .
- Yli‐Panula , E . 1997 . Allergenicity of grass pollen in settled dust in rural and urban homes in Finland. . – Grana , 36 : 306 – 310 .