Abstract
The pollen content of 31 honey samples from 19 different apiaries of El Hierro (Canary Islands) were subject to qualitative and quantitative melissopalynological analysis. The quantitative analysis demonstrated that 13% of the honey belonged to Maurizio Class I (<2 000 grains), 68% to Class II (2 000–10 000 grains) and 19% to Class III (10 000–50 000 grains). The pollen density ranges from 1 042 grains/g of honey to 24 478 grains/g with an average of 7 471 grains/g. According to the qualitative analysis, six honeys were typified as unifloral and 25 as multifloral. The unifloral honey samples were broken down as follows: two of heather (Erica arborea L.), two of Chamaecytisus proliferus (L. f.) Link‐type (“tagasaste”), one of Fabaceae (Genisteae sp.) and one of Lamiaceae Origanum vulgare L. ssp. virens (Hoffmanns. & Link) Ietsw.‐type (thyme: Micromeria hyssopifolia Webb & Berthel.). Honeydew elements were practically absent. Sixty‐nine pollen types were identified belonging to 42 families. The number of pollen types range between 18 and 39 (mean of 27.42). Foeniculum vulgare Mill.‐type pollen is present in all the samples. Galactites tomentosa Moench‐type, Echium plantagineum L., Echium L. sp., Bituminaria bituminosa (L.) C. H. Stirt., Chamaecytisus proliferus ‐type and Origanum vulgare ssp. virens ‐type pollen were found in 96.8% of the samples. The sensorial analysis indicated that honey types are generally of good quality, because 62% were evaluated as very high (16%), high (23%), and good (23%).
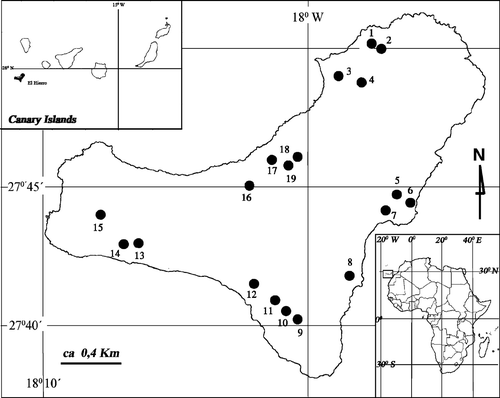
The island of El Hierro is the smallest of the Canarian Archipelago (Fig. ), with a surface area of 287 km2. It is located in the western end of the Canary Islands, crossed by the 18°W meridian, and its highest altitude is Tinganar or Malpaso at 1 501 m.
Figure 1 Map of El Hierro Island with location of the apiaries investigated(in parentheses altitude a.s.l.): 1 – El Mocanal, El Cernícalo (300 m); 2 – El Mocanal, El Cernícalo (350 m); 3 – Guarazoca, La Peña (550 m); 4 – Hoya de Lemus (800 m)+Los Valles (300 m); 5 – Isora, La Fuente (550 m); 6 – Isora, Las Playecillas (50 m); 7 – Isora, El Risco (600 m); 8 – El Pinar, Campos Viejos (500 m); 9 – El Pinar, Hoya de los Roques (300 m); 10 – El Pinar, Hoya de los Roques (320 m); 11 – El Pinar, Montaña Quemada (400 m); 12 – El Pinar, El Julan, El Pinito (500 m); 13 – La Dehesa, Hoya de los Cariles (700 m); 14 – La Dehesa: Hoya de los Cariles (600 m); 15 – La Dehesa, Santuario de la Virgen (675 m); 16 – Las Toscas (400 m); 17 – Los Mocanes (200 m); 18 – El Unchón, Pié Risco (400 m); 19 – Las Lapas (400m).
El Hierro is the most recently formed island in the archipelago (González Morales et al. Citation2000). The oldest formations are no more than three million years old. It was formed in three eruptive series, identified as ancient, intermediate and recent, associated with three great Y‐shaped fissure lines aligned south‐west, north‐east‐south‐east and north‐south.
Despite its small size a wide variety of honeys with unique aroma and taste are produced on this island. This variety is partly due to the geographic and great floristic diversity of the island. The floristic diversity is reflected in 608 taxa of vascular plants: 30 ferns, 4 gymnosperms, 484 dicot angiosperms and 90 monocot angiosperms, of which 177 are endemic (19 from El Hierro, 119 Canarian, 39 Macaronesian), 278 are of Mediterranean distribution and 153 of various origins. The bioclimatic diversity also affects the variety in honey due to the elevation range and the influence of the trade winds. Several Canarian climatic (zonal) communities are controlled by these variables. From the coast to the summit, they are: “tabaibal‐cardonal” (lower Inframediterranean arid and semiarid level) with a potential area surrounding the island from the coast to an altitude of 300 m; “sabinar” (upper infra‐Thermomediterranean semiarid and dry Thermomediterranean level without mist or with occasional mist) a thermophilic woodland of middle altitude between 300 and 600 m above sea level; “monteverde” (laurel wood and fayal heath: infra‐Mesomediterranean level with a dry, sub‐humid or humid ombroclimate) above 600 m in the windward slope with trade wind mist and “pinar” (thermo‐Mesomediterranean level) above 600 m in the southern slopes, outside the influence of the mist or subject to its overflow (Del Arco Aguilar & Rodríguez Delgado Citation2000).
The distribution of the climatic vegetation zones of the islands is not continuous. The large number of recent lava flows and deposits of volcanic sand or lapilli, together with manmade landscape alteration, including the heavy impact of grazing and agriculture, have led to the systematic destruction of all forest formations and have favoured the spread of introduced and invasive plants into the landscape.
In the volcanic sands of low and medium altitude, “iramales” of Schizogyne sericea (L. f.) DC. is dominant, and recent badlands are colonised by lichens, bryophytes and the very invasive plants of the “cardonal” or its substitute communities, all of which have a high apicultural interest, such as Aeonium hierrense (R. P. Murray) Pit & Proust and Ae. valverdense (Praeger) Praeger (“bejeques”), Kleinia neriifolia Haw. (“verode”), or Rumex lunaria L. (“calcosa”). The shrublands of Echium aculeatum Poir. (“tajinaste”) and Micromeria hyssopifolia (“tomillo”) grow on volcanic sands at high altitude. The “tabaibales amargos” characterized by Euphorbia obtusifolia Poir. in Lam., are common in altered lands at low and medium elevation.
The present paper is part of the pollen characterization of Canarian honeys research program that was started about ten years ago (La‐Serna Ramos, Citation2001; La‐Serna Ramos et al. Citation1994; La‐Serna Ramos et al. Citation1998; La‐Serna Ramos et al. Citation1999a , Citation b ; La‐Serna Ramos et al. Citation2002). The purpose of the program is to improve the quality of Canarian honeys, stimulate marketing, establish one or more origins or quality and help determine possible adulteration of local honey by mixing it with foreign honey.
Material and methods
The qualitative and quantitative melissopalynological analysis of 31 honey samples from 19 different apiaries (Fig. ) on El Hierro (Canary Islands) were examined. “Perfection” hives containing either a hybrid of the black “criolla” or Canary bee+the Italian bee (Apis mellifera iberica x Apis mellifera ligustica) or the “bucfas” race (Apis mellifera bucfas) were used to collect the honey. Apiaries 8, 9, 12 and 15 contained the “bucfas” bees and the rest were hybrid bees.
The following data were recorded for each sample: site of origin, beekeeper, date of extraction, an identification number of the sample and the number of slides kept at the Melissopalynotheque of the Department of Plant Biology — University of La Laguna, MP‐TFC– (Table ). The honey was extracted by centrifugation.
Table I. List of honey samples examined.
For the quantitative analysis, natural honey (Vorwohl, Citation1967) was used, following the methodology proposed by Méndez Pérez et al. Citation1994; modified by La‐Serna et al. Citation1999a . For a suspension of 50 ml of acid water (3.5 ml of concentrate H2SO4 in 1 l of distilled water) was added to 30 g of honey. The suspension was homogenized with a magnetic thermostirrer at 40°C. Using a Pasteur pipette, the hemacytometer (Fusch‐Rosenthal counting chamber) was loaded and pollen grains were counted under an optical microscope with a 40× objective. The chamber is 0.2 mm high and its grid has 16 large squares of 1 mm2 each, which are in turn subdivided into 16 small squares of 0.0625 mm2 each; this means a volume of 0.0002 ml in each large square and 0.0000125 ml in each small one. Taking into account the higher or lower pollen content of the sample, several small or large squares are counted and the arithmetic mean is calculated. For the present paper we always counted the 16 large squares. Using the volume of the suspension (50 ml) with the pollen sediment contained in 30 g of honey and the volume of the square counted, the correction factor extrapolated to 1 g of honey was calculated. Thus, multiplying the value obtained (arithmetic mean) by the factor we obtain the number of pollen grains/g of honey. In this case, the value of the factor is 133 333 for the count in small squares and 8 333 in the large ones.
Natural honey was also used to calculate the honeydew index, a ratio of honeydew elements (fungal spores and hyphae, algae and wax particles), and the pollen grains of nectariferous plants (Louveaux et al. Citation1978). The samples were placed into one of five classes: I = <2 000 pollen grains; II = 2 000–10 000 pollen grains; III = 10 000–50 000 pollen grains; IV = 50 000–100 000 pollen grains; V = >100 000 pollen grains (Maurizio, Citation1939).
Acetolysed honey was used for qualitative analysis (Gadbin, Citation1979). The acetolysed slides were prepared according to the method described by Erdtman Citation(1969), but with the modification suggested by Hideux Citation(1972), which involved washing the acetolysed pollen sediment with acetic acid, prior to the distilled water rinse, in order to solubilise the compounds formed after acetolysis. Following “First Meeting of Spanish Melissopalynology” a minimum of 700 grains of pollen per sample were counted. The results are presented in five categories (Louveaux et al. Citation1978): dominant or predominant pollen (D: >45%); secondary pollen (S: 16–45%); important minor pollen (I: 3–15%); minor pollen (m: 1–3%); pollen present (p: <1%).
Permanent slides were made (Louveaux et al. Citation1978) to confirm the presence/absence of honeydew elements and fragile grains that cannot withstand acetolysis. The pollen types were identified to species level whenever possible and otherwise to genus, family, or pollen type ranks. Pollen type (t.) includes species and/or genus (genera) present in the area, which have the same floral spectra and the same, or similar, pollen morphology. The classification of taxa follows Acebes Ginovés et al. Citation(2001). The organoleptic analysis was based on the methodology of Gonnet and Vache Citation(1985) using the visual, olfactory and gustative sensations.
For analysis with the scanning electron microscope (SEM), acetolysed pollen sediments were dehydrated in an ethanol series (70%, 90%, 95% and absolute) and transferred with Pasteur pipettes to the SEM stubs. Once air‐dry they were covered with a 15 nm gold film in a sputtering BAL‐TEC SCD. SEM micrographs were obtained on a JEOL JSM‐6300.
Results
The results of the pollen analysis are shown in Tables and . The honeydew index was omitted because in all the honey analysed it was very low, under 0.09%. This low value is considered nil or practically nil (Louveaux et al. Citation1978).
Table II. Pollen types and their frequency‐class distribution in the 31 honeys studied.
Table III. Synopsis of the melissopalynological analysis.
The quantitative analysis (Table ) of the honeys under study placed them in the following classes (Maurizio, Citation1939): four in Class I, 21 in Class II, and six in Class III. This shows that these honeys have low‐medium pollen content because 68% of the samples fell into Class II (Fig. ). The pollen density ranged from 1 042 grains/g of honey to 24 478 grains/g with an average of 7 471 grains/g. Six honey samples were typified as unifloral and 25 as multifloral (Table ; Fig. ). The unifloral honey samples were broken down as follows: two of Erica arborea (heather), two of Chamaecytisus proliferus‐type (“tagasaste”), one of Fabaceae (Genisteae sp.) and one of Lamiaceae Origanum vulgare L. ssp. virens‐type (thyme: Micromeria hyssopifolia). In all, 69 pollen types were identified with between 18 and 39 pollen types per honey sample (mean value 27.42). These pollen types are distributed among 42 different families. Apiaceae, Asteraceae, Boraginaceae, Brassicaceae, Fabaceae and Lamiaceae were present in all samples (Fig. ). Foeniculum vulgare‐type pollen was present in all samples. Galactites tomentosa‐type, Echium plantagineum L., Echium L. sp. (endemic Echium), Bituminaria bituminosa, Chamaecytisus proliferus‐type and Origanum vulgare ssp. virens‐type pollen were found in 96.8% of the samples. Taxa found in more than 75% of these honeys are presented in Fig. .
Figure 2 Distribution(%) of the honey samples according to Maurizio's classes: Class I = <2 000 grains/g of honey found in 4 samples (13%). Class II = 2 000–10 000 grains/g of honey found in 21 samples (68%). Class III = 10 000–50 000 grains/g of honey found in 6 samples (19%).
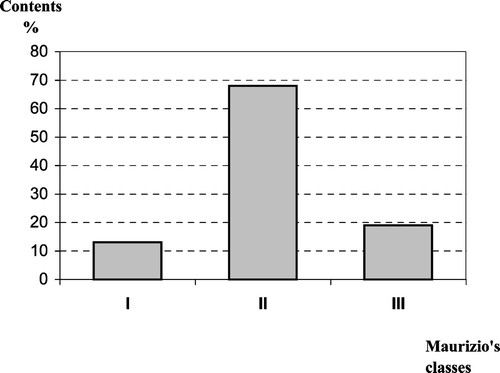
Figure 3 Percentage distribution of honey types: 25 honeys were multifloral (82%) and 6 were unifloral (18%): 2 of Erica arborea (6%), 2 of Chamaecytisus prolyferus‐type (6%), 1 of Genistea sp. (3%) and other of Origanum‐type, incl. Micromeria hyssopifolia (3%).
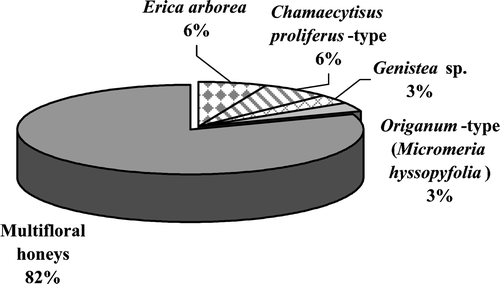
Figure 4 Families found in more than 50% of the samples: Apiaceae, Asteraceae, Boraginaceae, Brassicaceae, Fabaceae and Lamiaceae found in all samples (100%); Polygonaceae found in 29 samples (93.5%); Euphorbiaceae found in 28 samples (90.3%); Ericaceae found in 27 samples (87.1%); Cistaceae found in 24 samples (77.4%); Amaranthaceae/Chenopodiaceae found in 23 samples (74.2%); Aizoaceae and Fagaceae found in 22 samples (71%); Crassulaceae found in 21 samples (67.7%); Liliaceae found in 19 samples (61.3%); Myrtaceae and Rosaceae found in 18 samples (58.1%); Myricaceae and Resedaceae found in 16 samples (51.6%).
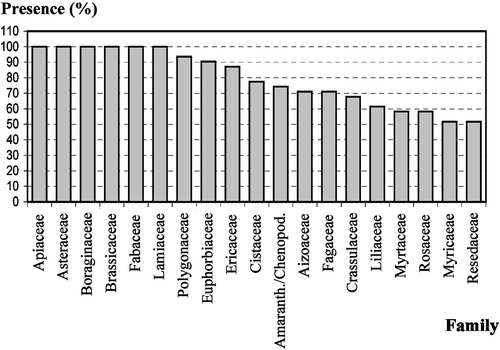
Figure 5 Pollen types found in more than 75% of the samples: Foeniculum vulgare‐type – found in all samples (100%); Galactites tomentosa‐type, Echium plantagineum, Echium sp., Bituminaria bituminosa, Chamaecytisus proliferus‐type and Origanum vulgare ssp. virens‐type – found in 30 samples (96.8%); Brassica‐type and Rumex sp. – found in 29 samples (93.5%); Sonchus‐type – found in 28 samples (90.3%); Erica arborea and Euphorbia obtusifolia – found in 27 samples (87.1%); Raphanus raphanistrum‐type – found in 26 samples (83.9%); Cistus sp. and Lotus glaucus – found in 24 samples (77.4%).
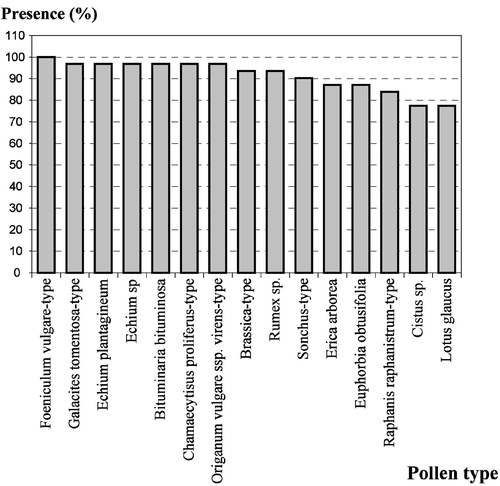
Phoenix canariensis Chabaud (9.7%), Ageratina adenophora (Spreng.) R. M. King & H. Rob.‐type (9.7%), Wahlenbergia lobelioides (L. f.) Link (9.7%), Cucurbita maxima Dcne. (9.7%), Liliaceae sp. 3 (9.7%), Malva L.‐type (9.7%), Lonicera caprifolium L. (6.5%), Ricinus communis L. (6.5%), Fumaria L. sp. (6.5%), Globularia salicina Lam. (6.5%), Lavandula canariensis Mill. (6.5%), Sideritis L. sp. (6.5%), Liliaceae sp. 2 (6.5%), Schinus molle L. (3.2%), Asteraceae sp. (3.2%), Croton L.‐type (3.2%), Geranium L. sp. (3.2), Allium L. sp. (3.2%), Acacia Miller 2‐type (3.2%), Passiflora edulis Sims (3.2%) and Datura inoxia Mill. (3.2%) were the least represented taxa. They were found in less than 10% of the samples.
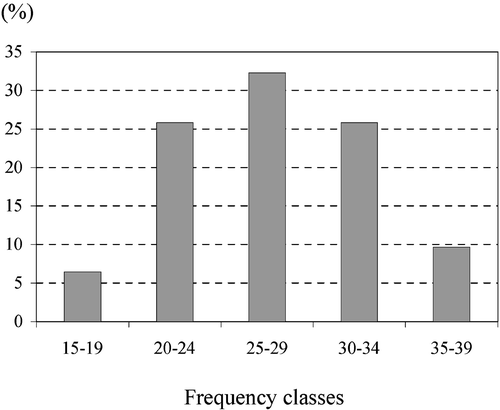
Frequency intervals for the pollen types in regard to the number of honey samples in which they occur showed that the 32% contained between 25–29 pollen types. No honey sample contained fewer than 20 types (Fig. ).
Figure 6 Distribution of the samples in frequency classes according to the number of the pollen types they contain: 15–19 pollen types – in two samples (6.15%); 20–24 pollen types – in eight samples (25.81%); 25–29 pollen types – in then samples (32.26%); 30–34 pollen types – in eight samples (25.81%); 35–39 pollen types – in three samples (9.68%).
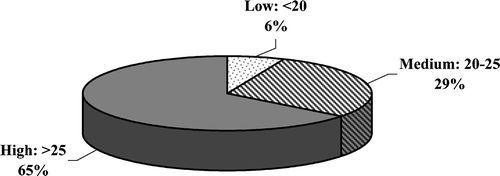
Over 50% of the samples proved to be very rich in pollen types (Fig. ).
Figure 7 Percentage of samples with low, medium and high pollen abundance. Low: <20 pollen types found in two samples (6%). Medium: 20–25 pollen types found in nine samples (29%). High: >25 pollen types found in 20 samples (65%).
Unifloral honeys
The unifloral honeys in this study belong to Class II (Maurizio, Citation1939). The pollen density ranged from 2 083 grains/g of honey to 7 812 grains/g with an average of 5 125.50 grains/g in the unifloral honeys.
Fifty‐seven pollen types were identified in the qualitative analysis with 24–39 per sample and a mean of 29.17. Grains of Achyranthes aspera L., Foeniculum vulgare‐type, Echium sp., Brassica L.‐type, Cistus L. sp., Erica arborea, Chamaecytisus proliferus ‐type, Castanea sativa Mill., Origanum vulgare ssp. virens‐type and Rumex L. sp. were found in all samples. Other taxa found in more than 60% of these honeys are: other Amaranthaceae‐Chenopodiaceae (83.3%), Bidens pilosa L.‐type (83.3%), Galactites tomentosa‐type (83.3%), Sonchus L.‐type (83.3%), Echium plantagineum (83.3%), Aeonium Webb & Berthel.‐type (83.3%), Eucalyptus sp. (83.3%), Opuntia ficus‐indica (L.) Mill. (66.7%), Euphorbia obtusifolia (66.7%), other Poaceae (66.7%) and Prunus L.‐type (66.7%).
Two honeys were assigned as heather honeys ‐ Erica arborea (Fig. ).This honey was creamy or separated in phases and toffee brown or dark brown in colour (Table ). These honeys had a medium to low pollen content (6 250 grains/g and 2 865 grains/g: Class II of Maurizio, Table ). The number of pollen types identified in this unifloral type were 29 and 39 respectively. The nectariferous taxa (89% of the total pollen types) present were Achyranthes aspera L., Foeniculum vulgare‐type, Bidens pilosa‐type, Dittrichia viscosa (L.) Greuter‐type, Galactites tomentosa‐type, Sonchus‐type, Echium plantagineum, Echium sp., Brassica‐type, Opuntia ficus‐indica, Aeonium‐type, Bituminaria bituminosa, Chamaecytisus proliferus‐type, Castanea sativa, Liliaceae sp1, Myrica faya Ait., Eucalyptus L'Hér. sp. and Tropaeolum majus L. The polliniferous taxa (11% of the total pollen types) included Amaranthaceae‐Chenopodiaceae, Cistus sp. and Rumex sp. The pollen of Poaceae and Urtica L. sp. were present only in sample 7.01 (MP‐TFC 144).
Figure 8 A, B. SEM micrographs of pollen in heather(Erica arborea) honeys: (A) Sample 7.01; (B) Sample 13.01; C & D. SEM micrographs of pollen in “tagasaste” (Chamaecytisus proliferus‐type) honeys, sample 2.00; E, F. SEM micrographs of pollen in Fabaceae (Genistea sp.) honey, sample 8.01; G, H. SEM micrographs of pollen in Lamiaceae Origanum vulgare ssp. virens‐type (“thyme”: Micromeria hyssopifolia) honey, sample 5.02. (1) Erica arborea; (2) Raphanus raphanistrum‐type; (3) Brassica‐type; (4) Rumex sp.; (5) Chamaecytisus proliferus‐type; (6) Prunus‐type; (7) Aeonium‐type; (8) Genisteae sp.; (9) Others Amaranthaceae‐Chenopodiaceae; (10) Castanea sativa; (11) Ilex canariensis; (12) Origanum vulgare ssp. virens‐type: Micromeria hyssopifolia; (13) Euphorbia obtusifolia; (14) Plantago sp.; (15) Galactites tomentosa‐type; (16) Bituminaria bituminosa. Scale bars – 1 µm (F); 10 µm (A‐E & G, H).
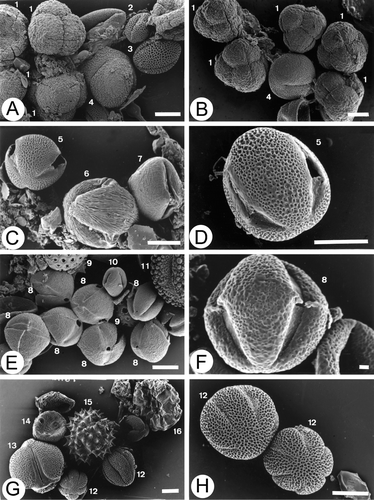
Table IV. Synopsis of the sensorial analysis.
“Tagasaste” honeys ‐ Chamaecytisus proliferus‐type (Fig. ) were crystallised honey of straw yellow colour (Table ) with a medium to low pollen content (2 083 grains/g and 6 511 grains/g: Class II of Maurizio, Table ). The number of pollen types identified was 24 in both samples. The nectariferous taxa (87% of the total pollen types) were Achyranthes aspera, Foeniculum vulgare‐type, Bidens pilosa‐type, Kleinia neriifolia, Sonchus‐type, Echium plantagineum, Echium sp., Brassica‐type, Aeonium‐type, Bryonia verrucosa Dryand., Erica arborea, Castanea sativa., Micromeria hyssopifolia., Eucalyptus sp. and Prunus‐type. The polliniferous taxa (13% of the pollen types) present in the two samples were Cistus sp. and Rumex sp. The pollen from Amaranthaceae‐Chenopodiaceae and Poaceae were present only in one of the samples (sample 12.01: MP‐TFC 243).
Only one unifloral honey was Fabaceae honey ‐ Genistea sp. (Fig. ). It was liquid and had a reddish colour (Table ). This honey had a medium to low pollen content (7 812 grains/g of honey) was placed into Class II (Maurizio, Citation1939). It contained 28 pollen types, the majority of which were nectariferous taxa (86%) with Castanea sativa accounting for 19.5% of the total pollen spectrum (Table ). Amaranthaceae‐Chenopodiaceae, Cistus sp., Poaceae and Rumex sp. were the only four polliniferous taxa (14% of the total pollen types) found in these honeys but with a low representation (1.7% Cistus sp., 1.0% Rumex sp. and less than 1% the other two).
Lamiaceae honey ‐ Origanum vulgare L. ssp. virens‐type: “thyme” honey ‐ Micromeria hyssopifolia (Fig. ) is crystallised and had a cream white colour (Table ). This honey had a medium to low pollen content (5 208 grains/g of honey) and was placed also in Class II (Maurizio, Citation1939). It contained 31 pollen types, the majority of which were nectariferous taxa (81%). Foeniculum vulgare‐type and Echium sp. accounted for 13.4% and 12.7% respectively of the total pollen spectrum (Table ). Amaranthaceae‐Chenopodiaceae, Cistus sp., Plantago L. sp., Zea mays L. other Poaceae, Rumex sp. and Urtica sp. were the only six polliniferous taxa (19% of the total pollen types) found in these honeys but with a low representation (2.0% Cistus sp. and less than 1% the other five).
Multifloral honeys (Fig. )
Figure 9 A–H. SEM micrographs of pollen in some multifloral honeys: (A) Sample 4.01; (B) Sample 4.01; (C) Sample 6.01; (D) Sample 6.01; (E) Sample 5.01; (F) Sample 10.01; (G) Sample 1.00; (H) Sample 9.02. Erica arborea; (3) Brassica‐type; (4) Rumex sp.; (5) Chamaecytisus proliferus‐type; (7) Aeonium‐type; (9) Others Amaranthaceae‐Chenopodiaceae; (10) Castanea sativa; (12) Origanum vulgare ssp. virens‐type: Micromeria hyssopifolia; (13) Euphorbia obtusifolia; (15) Galactites tomentosa‐type; (16) Bituminaria bituminosa; (17) Echium sp.: E. aculeatum, E. hierrense, E. strictum; (18) Echium plantagineum; (19) Cistus sp.; (20) Silene sp.; (21) Sonchus‐type; (22) Asphodelus aestivus; (23) Mesembrianthemum crystallinum; (24) Tropaeolum majus; (25) Bidens pilosa‐type. Scale bar – 10 µm (A–H).
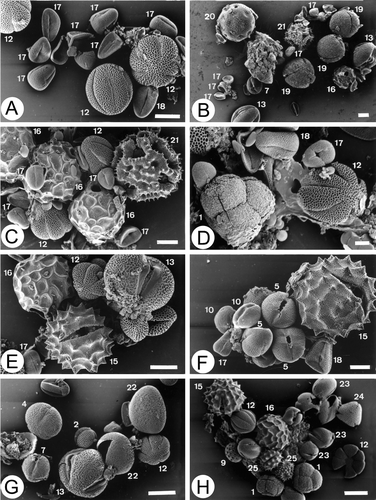
In this study the honeys have medium or medium‐low pollen content, 24% of the samples falling into Class III and 60% into Class II (Maurizio, Citation1939). The pollen density ranged from 1 042 grains/g of honey to 24 478 grains/g with an average of 8 034.52 grains/g.
Sixty‐four pollen types were identified in the qualitative analysis. Taxa diversity ranged between 18 and 36 per sample with a mean of 27.04. Foeniculum vulgare‐type, Galactites tomentosa‐type, Echium plantagineum and Bituminaria bituminosa C. H. Stirt. pollen were present in all samples. Echium sp., Chamaecytisus proliferus‐type and Origanum vulgare ssp. virens‐type grains were found in 96% of the honeys. Other taxa found in more than 60% of these honeys are: Sonchus‐type (92%), Raphanus raphanistrum‐type (92%), Brassica‐type (92%), Euphorbia obtusifolia (92%), Rumex sp. (92%), Lotus glaucus Dryand. in Aiton (88%), Erica arborea (84%), Mesembryanthemum crystallinum (80%), Bidens pilosa‐type (72%), Kleinia neriifolia (72%), Cistus sp. (72%), others Amaranthaceae‐Chenopodiaceae (64%), Aeonium‐type (64%) and Castanea sativa (64%).
According to abundance of pollen forms identified in each honey sample 64% of the samples proved to be very rich in the pollen forms (>25 pollen types).
These types belonged to 40 botanical families. Apiaceae, Asteraceae, Boraginaceae, Brassicaceae, Fabaceae and Lamiaceae are present in all samples, although the amounts differ from sample to sample.
Honey colour varied from cream white to dark brown, through various shades of yellow and even some reddish colour (unifloral honey of Fabaceae: Genista sp.) (Table ). Although some samples stayed liquid longer, most crystallized quickly, due to the large nectariferous contribution of Echium sp. (“tajinastes”), Echium plantagineum (“sonaja”), Mesembryanthemum crystallinum (“barrilla”), and nectar from Asteraceae and Brassicaceae.
Discussion and conclusions
The lack of honeydew elements in all the honeys indicates their floral origin (Louveaux et al. Citation1978). The pollen spectrum of these honeys generally reflects the vegetation of the various areas where the apiaries were located.
The spectra of the unifloral honeys from El Hierro are not different to the other islands of the Canary archipelago (La Serna, Citation2001; La Serna et al. Citation1994, Citation1999b ).
The following combinations are proposed as characteristic for the multifloral honeys (Table ):
Multifloral I
Honeys containing 24–61% Echium sp. (nine honeys) with major abundance of the following pollen types: Origanum vulgare ssp. virens‐type – Micromeria hyssopifolia – (seven honeys), Dittrichia viscose (L.) Greuter‐type (one honey) and Echium plantagineum (one honey).
Multifloral II
Honeys containing 32–36% Echium plantagineum (three honeys) with considerable quantity of Reseda luteola L. (one honey) and the combination Echium sp. (endemic Echium) ‐ Origanum vulgare ssp. virens‐type (Micromeria hyssopifolia) in two honeys.
Multifloral III
Honeys containing 12–24% Origanum vulgare ssp. virens‐type –Micromeria hyssopifolia – (two honeys) with considerable quantity of Echium s.l. (one honey) and the combination Reseda luteola – Liliaceae sp.1 (one honey).
Multifloral IV
Honeys containing 26–45% Lotus glaucus (three honeys) with considerable quantities of the following pollen type combinations: Echium s.l. ‐ Origanum vulgare ssp. virens‐type – Micromeria hyssopifolia – (two honeys) and Chamaecytisus proliferus Link‐type – Echium s.l. (one honey).
Multifloral V
Honeys containing 23–32% Chamaecytisus proliferus Link‐type (two honeys) with considerable quantities of the pollen type combinations Echium s.l. – Prunus‐type (one honey) and Lotus glaucus – Echium s.l. (one honey).
Multifloral VI
Honeys containing 18–44% Castanea sativa (three honeys) with major abundance of the following pollen types: Chamaecytisus proliferus‐type (one honey), Galactites tomentosa‐type (one honey) and Mesembryanthemum crystallinum (one honey).
Multifloral VII
Honeys containing 18–44% Mesembryanthemum crystallinum (two honeys) with considerable quantity of Castanea sativa (one honey) and the combination Origanum vulgare ssp. virens‐type (Micromeria hyssopifolia) – Reseda luteola (one honey).
Multifloral VIII
One honey containing 36% Reseda luteola with 25% Origanum vulgare ssp. virens‐type (Micromeria hyssopifolia). In 14 of the 25 multifloral honeys (56%) the sum of the Echium and “thyme” pollen exceeded 45% and 11 of them (44%) may be regarded as mixed honey of Echium sp. (“tajinaste”), Echium plantagineum (“sonaja”) and Micromeria hyssopifolia (“thyme”). It may be stated that in most multifloral honeys produced in the island of El Hiero the dominant nectars are those of endemic Echium (“tajinaste”), Echium plantagineum (“sonaja”) and Micromeria hyssopifolia (“thyme”)
The presence in these honeys of the following species may be projected:
-
Ten endemic Canary species: Echium sp. (96.7% of the honeys), Micromeria hyssopifolia (93.5%), Euphorbia obtusifolia (87.1%), Cistus sp. (77.4%), Kleinia neriifolia (67.7%), Aeonium‐type (67.7), Artemisia thuscula Cav. (32.3%), Bryonia verrucosa (16.1%), Phoenix canariensis (9.7%) and Lavandula canariensis (6.5%).
-
Four endemic Macaronesian species: Lotus glaucus (77.4% of the honeys), Ilex canariensis Poir. (19.4%), Wahlenbergia lobelioides (9.7%) and Globularia salicina (6.5%).
As in the case of other Canary honeys, the following pollen types may be regarded as geographical markers:
-
Nectariferous and polliniferous taxa: Bituminaria bituminosa (96.8%), Euphorbia obtusifolia (87.1% of the samples), Aeonium‐type (67.7%), Kleinia neriifolia (67.7%), Opuntia ficus‐indica (L.) Mill. (41.8%), Ilex canariensis (19.4%) and Tropaeolum majus L. (38.7%).
-
Eminently polliniferous taxa: Myrica faya Aiton and Rumex sp. (R. lunaria L.), present in 51.6% and 93.5% of the honeys respectively.
Lastly, a differential characteristic of El Hierro honey in regard to other honeys from the archipelago is the absence of pollen from Cardiospermum grandiflorum Sw. (“farolito” or “farolillo de enredadera”) and the local palm tree (Phoenix canariensis).
In regard to sensory analysis, the honeys were generally of good quality, as 62% of them were evaluated as very high, high, and good (16%, 23% and 23% respectively). The only very low quality rating was probably due to incorrect manipulation.
Acknowledgements
We would like extend our gratitude to the General Directorate of Universities and Research (Education, Culture and Sports Dept. of the Canarian Government) for funding the PI 2001/057 Project, which has allowed us to carry out this work. We would also like to thank the beekeepers of El Hierro, particularly Mr. Emiliano Fernández Armas and Mr. Florencio Gutiérrez, for providing the honey samples and Mr. Juan Luis González Álvarez, SEM operator of the University of La Laguna, for his collaboration in the preparation of the scanning electron micrographs.
References
- Acebes Ginovés , J. R. , del Arco Aguilar , M. , Garcia Gallo , A. , León Arencibia , M. C. , Pérez de Paz , P. L. , Rodríguez Delgado , O. and Wildpret de la Torre , W. 2001 . “ División Pteridophyta y División Spermatophyta. ” . In Lista de especies silvestres de Canarias (hongos, plantas y animales terrestres) , Edited by: Izquierdo , I , Martín , J. L , Zurita , N and Arechevaleta , M . 98 – 140 . Tenerife, La Laguna (Canary Is.) : Cons. Polít. Territ. & Med. Amb. Gob. de Canarias .
- Del Arco Aguilar , M. , Rodríguez Delgado , O. and Centro Cult. Pop. Can. 2000 . “ El Hierro: Flora y Vegetación. ” . In Canarias Isla a Isla , 555 – 557 . Tenerife, (Canary Is.) : Centro Cult. Pop. Can .
- Erdtman , G. 1969 . Handbook of Palynology , Copenhagen : Munksgaard .
- Gadbin , C. 1979 . L'intérêt de l'acétolyse en mélissopalynologie. . Apidologie , 10 : 23 – 28 .
- Gonnet , M. and Vache , G. 1985 . L'analyse sensorielle et la promotion des miels de qualité. Le goÛt du miel , París : UNAF Ed .
- González Morales , A. , Hernández Luis , J. A. , Ramírez Torrecabota , P. and Centro de la Cultura Popular Canaria . 2000 . “ El Hierro: Geografía. ” . In Canarias Isla a Isla , 552 – 554 . Tenerife (Can. Is.) : Centro Cult. Pop. Can .
- Hideux , M. 1972 . Techniques d'étude du pollen an MEB: effects comparés des différents traitements physicochimiques. . Micron , 3 : 1 – 31 .
- La‐Serna Ramos , I. 2001 . “ Caracterización polínica de mieles canarias producidas en el año 1997. ” . In Libro de textos completos. 13th Simp. Asoc. Palinól. Leng. Esp. (A.P.L.E.), Cartagena 2000 , Edited by: Moreno Grau , S , Elvira Rendueles , B and Moreno Angosto , J. M . 173 – 198 . Cartagena (Spain) : Univ. Politécn. Cartagena .
- La‐Serna Ramos , I. , Méndez Pérez , B. and Gómez Ferreras , C. 1998 . The importance of Persea americana as a source of nectar in some honeys from La Palma (Canary Islands). . Grana , 37 : 102 – 109 .
- La‐Serna Ramos , I. , Méndez Pérez , B. and Gómez Ferreras , C. 1999a . Aplicación de nuevas tecnologías en mieles canarias para su tipificación y control de calidad , Tenerife : Ed. Confed. Cajas de Ahorros, Caja Can .
- La‐Serna Ramos , I. , Méndez Pérez , B. and Gómez Ferreras , C. 1999b . Pollen characterization of multifloral honeys from La Palma (Canary Islands). . Grana , 38 : 356 – 363 .
- La‐Serna Ramos , I. , Méndez Pérez , B. and Gómez Ferreras , C. 2002 . Pollen spectra of different unifloral honeys from La Palma (Canary Islands, Spain). . Grana , 41 : 48 – 57 .
- La‐Serna Ramos , I. , Méndez Pérez , B. , Cabrera Rodríguez , F. and Pérez de Paz , P. L. 1994 . Pollen spectrum of some honeys from the Santa Cruz and Valle de Aridane districts of La Palma (Canary Islands). . Apicoltura , 9 : 31 – 49 .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1978 . Methods of melissopalynology. . Bee World , 59 : 139 – 157 .
- Maurizio , A. 1939 . Untersuchungen zur quantitativen Pollenanalyse des Honigs. . Mitt. Geb. Lebensmittelunters. Hyg. , 30 : 27 – 69 .
- Méndez Pérez , B. , La‐Serna Ramos , I. , Cabrera Rodríguez , F. and Domínguez Santana , M. D. 1994 . “ Aportación de una nueva alternativa metodológica para la melitopalinología cuantitativa. ” . In Polen y esporas: contribución a su conocimiento , Edited by: La‐Serna , I . 289 – 294 . Tenerife : Serv. Publ. Univ. La Laguna, Ser. Inform. 35 .
- Vorwohl , G. 1967 . The microscopic analysis of honey, a comparison of its methods with those of the other branches of palynology. . Rev. Palaeobot. Palynol. , 3 : 287 – 290 .