Abstract
The aim of this study is to analyse, describe and compare the spores of the two Adiantopsis species that grow in Argentina, A. chlorophylla (Sw.) Fée and A. radiata (L.) Fée. The study used herbarium material observed with light microscopy (LM), scanning electron microscopes (SEM) and transmission electron microscopes (TEM). The spores of both species are trilete with an echinate surface. The exospore is smooth, two‐layered in section; both layers with different thickness and contrast. Depending on the plane of sectioning, channels are seen running through the exospore and in the apertural region. The perispore strongly contrasted when seen with TEM, is two‐layered and bears ornamentation. The two layers have different thickness and structure. The inner layer P1 is the thickest layer and has three strata, which form the sculptural elements. The outer layer P2 covers all the surfaces of P1. Two levels of ornamentation are clearly distinguished: a basal level composed of fused ridges, and an upper level composed of echinae. The spores of A. chlorophylla are triangular‐globose in polar view, with convex sides, 25 – 50 µm in equatorial diameter and have more ornamental processes per surface unit than the spores of A. radiata. The spores of A. radiata are triangular in polar view and 30 – 40 µm in equatorial diameter. Size and ornamentation help to establish differences at species level, and together with the exomorphological characteristics of the sporophyte, contribute to the systematics of this genus.
The spores of Adiantopsis taxa that grow in the southern part of S. America are analysed in this work as part of the palynological study of the Pteridaceae in the area.
Adiantopsis Fée in tropical America is represented by seven species. Two of the species occur in Argentina: A. chlorophylla (Sw.) Fée in the north‐west (Jujuy, Salta and Tucumán provinces) and north‐east (Corrientes, Entre Ríos and Misiones provinces), Chaco and Buenos Aires; A. radiata (L.) Fée in the north‐east (Corrientes and Misiones provinces) and Chaco and Formosa (de la Sota & Ponce Citation1992; Ponce Citation1996).
Christensen Citation(1938) included Adiantopsis within the “cheilanthoid ferns”. Cheilanthoid ferns were studied by Tryon and Tryon Citation(1973) who showed a correlation between the geographic distributions of cheilanthoid ferns with morphological characteristics of their spores, and defined six sculptural patterns. They included Adiantopsis within the reticulate spore type and Pellaea paradoxa, P. rotundifolia and P. falcata within the echinate spore type (Tryon & Tryon, Citation1973). Later Tryon and Tryon Citation(1982) described the sculpture of Adiantopsis spores as echinate and a subsequent study by Tryon and Lugardon Citation(1991) described the sculpture as echinate‐reticulate with echinate elements apparently formed by strands that project from a basal reticulum. An echinate sculpture was also recorded for Adiantopsis chlorophylla spores by Michelena Citation(1989).
A palynological study of cheilanthoid ferns from north‐west Argentina proposed combined patterns of structure and sculpture for Cheilanthes species (Morbelli & Michelena, Citation1989). Morbelli et al. Citation(1994) analysed the spore characteristics of the “South American cheilanthoids” and found structural similarities within the group. Lellinger and Taylor Citation(1997) determined the spore ornamentation patterns and placed those produced by Adiantopsis within the basic “lacunate” pattern. According to Lugardon (Citation1972, Citation1974) and Lugardon and Brousmiche Delcambre Citation(1997), the structural characteristics of Adiantopsis exospore correspond to the basic type of the most derived ferns.
In 2003, Hickey et al. quoted the hybrid Adiantopsis × australopedata Hickey, Barker & Ponce for the first time. It was collected in Iguazú National Park, Misiones. The authors described abnormalities in spore number produced per sporangium and the presence of aborted spores.
In this work we studied spore morphology and ultrastructure of two species of Adiantopsis growing in Argentina in order to define and compare the spore wall ultrastructure within the genus and identify similarities with other cheilanthoid ferns. The main aim is to test whether morphological characteristics are diagnostic for the group and of use in studying relationships among cheilanthoids ferns as well as other ferns.
Material and methods
Spores were extracted from herbarium material from the Museo de La Plata (LP) and the Instituto de Botánica Darwinion (SI).
Spores for light microscopy (LM) and scanning electron microscope (SEM) were treated with hot 3% sodium carbonate for 2 minutes. For LM studies the spores were then acetolyzed (Erdtman, Citation1960), embedded in glycerine‐jelly and the slides sealed with paraffin.
The spores were observed with an LM Olympus CH‐2. For each palynological sample 25 spores were measured. The values were given as minimum, average and maximum.
For SEM analysis the treated spores were cleaned with distillate water and then transferred to ethanol. For observation the material was placed on acetate plates and sputter coated with gold‐palladium and studied using a JEOL, JSMT‐100.
For transmission electron microscope (TEM) analysis the material was prefixed with (1%) glutaraldehyde plus (1%) alcian blue and postfixed with (1%) osmium tetroxide in water solution and (1%) alcian blue. Dehydration was performed in an acetone series. The semithin sections were stained with toluidine blue and studied with LM. The thin sections were stained with uranil acetate for 15 minutes and followed by lead citrate for 3 minutes. The observations were made with a Zeiss T‐109 TEM.
In order to estimate the ornamentation density, estimations of the echinae number per square micrometer were obtained.
The slides corresponding to each specimen analysed are filed according to the collection number (MP), in the Cátedra de Palinología de la Facultad de Ciencias Naturales y Museo de La Plata, Universidad Nacional de La Plata.
Results
Adiantopsis chlorophylla (Sw.) Fée (Figures & )
Figure 2 SEM micrographs ofAdiantopsis chlorophylla (Sw.) Fée and A. radiata (L.) Fée. A–D. A. chlorophylla. A. A spore in proximal view. The ornamentation is echinate. The laesurae are short and are evident on the perispore surface. B. A spore in distal view. The ornamentation is echinate. Many processes are fused. The elements of the ornamentation are densely distributed. C. Detail with a higher magnification of the echinate distal surface; the echinae are robust, their tips show varieties of shapes, while bases are wide and uniform. The echinae bases are complex and consist of rodlets fused that form a net (arrow). D. Detail of a fracture through the perispore (P). Below the perispore the exospore (E) is evident. It has a slightly rugulate surface. The two levels of the perispore are clearly distinguished: a basal level formed of a thin layer adhered to the exospore with rodlets, and a high level composed of echinae. E–H. A. radiata. E. A spore in proximal view. The ornamentation is echinate and the surface between echinae has a complex net of rodlets. The laesurae are short. F. A spore in distal view with ornamentation similar to the proximal face. G & H. Details of the distal surface at a higher magnification. G. The echinae are thin and acute, their bases are formed of radial rodlets fused to those of contiguous processes. H. In the basal level between the rodlets there are spherical elements of varied sizes. Scale bar – 10 µm (A, B, E, F, H); 1 µm (C, D, G).

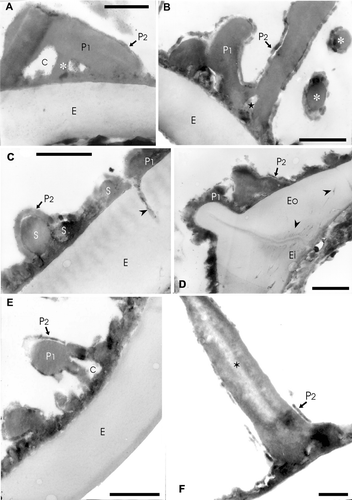
Figure 3 TEM ofAdiantopsis chlorophylla (Sw.) Fée. A–F. The exospore (E) is evident in A to E, its margin is smooth and in section its structure is homogenous. The exospore has uniform thickness and is less contrasted than the perispore. The perispore is two‐layered. The P1 layer is adhered to the exospore and forms the main part of this wall. The layer P2 is thin and covers the inner and outer surfaces of P1. A. In the middle part of layer P1 there are chambers (C) and radial elements (asterisk) of different thickness while this layer is compact towards the outside. The layer P2 is uniform in thickness and its margin is irregular. B. A section that shows spaced elements and those which form the bases of the complex processes of layer P1. Two processes (asterisks) in transversal section can be seen on the right. In both cases the layer P2 covers P1. Spaces of different width are evident in basal part of the processes (star). The spaces are covered with the P2 layer. In the contact between perispore and exospore, short superimposed plates are evident. C. This section crosses a zone with low elements, an interruption in the perispore is also evident that is continuous with a channel (arrowhead) in the exospore (E). This channel is larger in diameter than those at the inner part, and it is filled with helicoidal material of the same contrast as the perispore. (S) points spherules embodied between P1 and P2. D. Section at the laesura level, the exospore thickens and is continuous over the aperture. The perispore is also continuous over the aperture and its margin is irregular according to the sector sectioned. The perispore layer P2 covers P1 and is also continuous on the laesura. At the inner part of the laesura in the exospore, there are thin channels tangentially arranged (arrowheads); other channels are visible in the middle and inner part of the exospore with mainly radial orientation and apparently there exist others that connect each other. E. Detail of a section that shows the exospore and perispore. The layer P1 and P2 are clearly evident. There is a process with a chamber (C) in its base. The inner part of the chamber is covered by P2. F. An echina formed of P1 with a central region of low density (star). The process is covered by P2 which shows different degrees of development on the same spore. Scale bar – 1 µm (A–F).
Trilete spores, triangular‐globose in polar view. The equatorial diameter is 30(42)64 µm, the polar diameter is 25(37)50 µm. The laesurae are 11(15)25 µm long. The exospore is 1 µm thick and smooth. The perispore has variable thickness (ca. 1–3 µm), with an irregular margin. It is ornamented with echinate processes with basal fused rodlets.
Comments: The colour of the spores changes to brown after acetolysis treatment.
SEM
The ornamentation has two clearly differentiated levels (Figure ): a basal level formed of fused rodlets and low ridges, and an upper echinate level with a total thickness including the two levels of 2–4 µm thick and two echinae per 15 µm2. The echinae are 1 µm wide and 4 µm high.
TEM
The exospore has uniform thickness, is less contrasted than the perispore and its margin is smooth. Two layers are distinguished, an outer (Eo) and an inner (Ei) layer (Figure ). The outer layer is thicker and less contrasted than the inner one. The thickness ratio of the inner‐outer exospore layers is 1:4. Thin radial channels, filled with a dark contrasted material (Figure ) run through the exospore. At the laesurae level, the exospore is continuous and thick. At the aperture base, fine channels perpendicular to the commissure and filled with a dark content are also observed (Figure ). The commissure margins are more contrasted than the centre (Figure ).
The perispore has two layers P1 and P2, which have different thickness and structure. The inner layer P1 is thicker, has a compact structure and forms the ornamentation elements (echinae). It forms a thin layer against the exospore and the framework of the hollow echinae of which the lower part is divided into a number of irregular basal elements showing very variable outlines in sections. Within this layer there are chambers at the echinae base (Figure ).
The outer layer P2 is thin (30 – 60 nm thick), variable in thickness and contrast, continuous and covers the inner and outer surface of P1 (Figure ; compare B and C with F). Embodied spherules between P1 and P2 can be observed (Figure ).
Adiantopsis radiata (L.) Fée (Figures & )
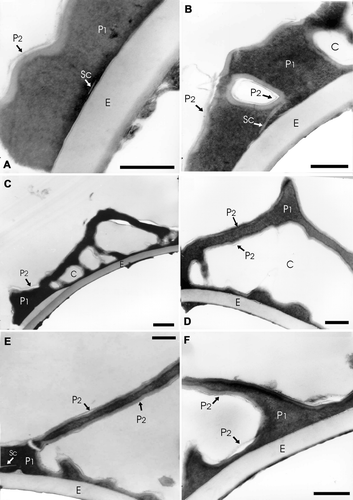
Figure 4 TEM ofA. radiata (L.) Fée A–F: In all the cases the exospore (E) margin is smooth and its structure is homogenous. The perispore is more contrasted than the exospore; it is composed of two layers: P1 and P2. P1 constitutes the main part of this wall. A. A scale (Sc) is evident in the inner part of P1. The layer P2 is very thin and covers P1. B. In this section chambers (C) within the layer P1 can be seen, they are surrounded by a less contrasted layer. A scale (Sc) is evident in the inner part of P1. C. In this case chambers (C) can be seen within P1. The (P2) layer is thin, less contrasted than layer P1 and only visible in some places. D. Spine with a basal chamber (C) in detail. The layer P2 covers the inner and outer surfaces of P1. E. The section shows a process of high length formed mainly by P1. A scale (Sc) is evident in the inner part of P1, near the left limit. F. In this section there are two perispore processes of different height, they are formed by P1 and covered by P2. A dark extensive lamina is visible between the exospore and perispore. Scale bar – 1 µm (A–E).
Trilete spores, triangular with straight sides in polar view. The equatorial diameter is 35(42)54 µm, the polar diameter is 30(36)40 µm. The laesurae are 12(16)22 µm long. The exospore is 1 µm thick and is smooth; in section its structure is homogenous and compact. The perispore has variable thickness (ca. 1 – 3 µm) with irregular margin. It is ornamented with echinate processes with basal partially fused rodlets.
Comments: The spores change to a yellowish colour and compress easily after the acetolysis treatment.
SEM
The ornamentation has two clearly differentiated levels (Figure ): a basal level composed of rodlets and low partially fused ridges leaving spaces, and an upper echinate level with a total thickness, including the two levels, of 2 – 3 µm, with one echina per 15 µm2. The echinae have an average thickness of 580 nm and height of 2 µm (Figure ).
TEM
The exospore is less contrasted than the perispore. In section it has a homogenous structure and is two‐layered: the outer layer (Eo) is the thicker one and is less contrasted than the inner one (Ei). The inner‐outer exospore layers thickness ratio is 1:4.
The perispore in section has two differently contrasted layers: inner (P1) and outer (P2), both with different thickness and structure. The inner layer P1 is the thicker one, has a compact structure and it forms the ornamentation elements. This layer forms the chambers at the echinae bases and the irregular basal elements of varied shapes (Figure ).
The layer P2 is thin (50 – 60 nm thick), dark and continuous, and covers the inner and outer surface of P1. Spherules consisting of perisporal material can be observed (Figure ). In the inner area of the perispore some short white lines probably corresponding to “scales” can be seen (Figure ).
The morphological and palynological characteristics of the studied species are summarised in Table .
Table I. Diagnostic characteristics of Adiantopsis species that grow in Argentina.
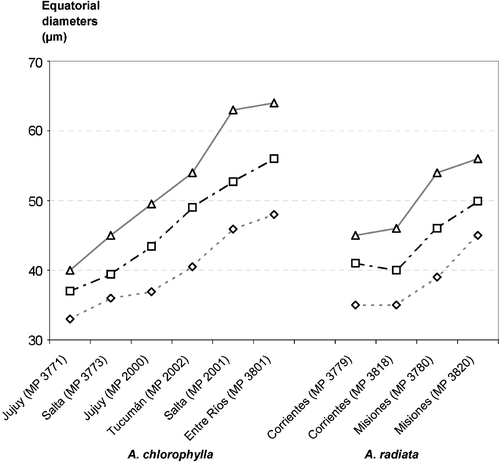
Graphs of the comparative measurements of the equatorial diameters in both species are shown in Figure . In general A. chlorophylla spores have a wider variability in their equatorial diameters than those of A. radiata.
Discussion and conclusions
Our observations confirm the surface pattern defined by Tryon and Lugardon (Citation1991, p. 155) as echinate with basal rodlets as well as the “lacunate” pattern proposed by Lellinger and Taylor (1977) for the spores of this genus.
All the specimens studied in this work produced normal spores. Nevertheless, Hickey et al. Citation(2003) found variations in shape and size of spores in the hybrid Adiantopsis x australopedata collected in Iguazú National Park, Misiones.
Even if the perispore structure was similar in both studied species, differences in spore size, shape and perispore characteristics allowed us to establish some variations. The largest spores were those of A. chlorophylla which were 64 µm.
Morphology and density of echinae also showed differences. The echinae in A. chlorophylla are thicker than in A. radiata. This characteristic gives a strong aspect to A. chlorophylla spores while echinae of A. radiata spores are tiny and slender.
Differences such as spore colour after acetolysis treatment, number of exospore layers in some spores, perispore thickness and structure ‐ especially when referred to P2 – could be related to stages in spore maturation.
Spheroids embodied within the perispore were present in A. chlorophylla. According to our interpretation those spheroids would be the “spherules” defined by Tryon and Lugardon Citation(1991) as typical of the spore surface of the homosporic Filicophyta.
The echinate pattern is also present among the Cheilanthoid ferns in Cheilanthes distans, C. sieberi and C. tenuifolia that grow in Australia (Tryon & Lugardon, Citation1991, p. 155, Figure 47.30, Figure 47.32 and Figure 47.35), Pellaea paradoxa, (l.c., p. 165, Figure 50.12 and 50.13), Doryopteris papuana from Papua (l.c., p. 167, Figure 51.11)), in Pellaea rotundifolia and P. falcata (Tryon & Tryon, Citation1973, p. 152). According to our survey this particular ornamentation type is only present a few times in Filicophyta spores. Consequently, it has allowed us to distinguish the spores of these species from those gathered in the cheilanthoid group, which mainly produce cristate and rugulate spores.
It is interesting to point out the strong similarity in ornamentation and structure found between the Adiantopsis spores studied here and the monolete spores of Cystopteris fragilis (Dryopteridaceae) from Costa Rica, France and Alaska, according to illustrations given by Tryon & Lugardon (Citation1991, p. 487 Figure 189.13–15 and p. 488 Figure 189.21–23).
It can be concluded that the spore characteristics, together with those related to blade architecture, could be useful for systematics and allow for differentiation at species level. They would enable relationships to be investigated between Adiantopsis and other cheilanthoid ferns to some genera within the Dryopteridaceae.
Specimens examined
Adiantopsis chlorophylla
Prov. Entre Ríos: Delta del Paraná, Burkart 5048 (SI), MP 3801. Prov. Misiones: Aristóbulo del Valle, Reserva UNLP, Marquez 40 (LP). Dpto Concepción, Estancia de Elsa Prates, Biganzolli et al. 1636 (SI), 28‐I‐2004. Prov. Jujuy: Dpto Ledesma, camino Mesada de las Colmenas a Abra de las Cañas, De la Sota 4405 (LP), 17‐III‐1966, MP 3771; Dpto Santa Bárbara, San Pedro, Sierra de Santa Bárbara, Venturi 9578 (LP), MP 2000; Dpto Capital, El Cucho, Quebrada A° Tacanas, De la Sota 4355 (LP), 10‐III‐1966, MP 3775; Prov. Salta: Dpto Sta. Victoria, Los Toldos, Fabris & Crisci 7364 (LP), 22‐IV‐1968, MP 2001; Dpto La Caldera, camino de cornisa de Salta a Jujuy, Palací et al. 869 (LP), 1‐II‐1987, MP 3772; Dpto Capital, cerros detrás Ciudad Universitaria, Bonavía 66, 16‐IX‐1985 (LP), MP 3773; Prov. Tucumán: Dpto Monteros, Quebrada Pueblo Viejo, en matorral cerca del camino, De la Sota 4065 (LP), 01‐1965, MP 2002.
Adiantopsis radiata
Prov. Corrientes: Dpto Santo Tomé, Estancia Garruchos, Krapovickas et al., 7‐II‐1972 (LP), MP 3779; Dpto Ituzaingó, Arroyo Garapé y Río Paraná, 45 km al E. de Ituzaingó, Tressens et al. 409 (LP), 23/24‐X‐1974, MP 3818; Puerto Aguirre, Frenguelli 40 (LP), VII‐1938, MP 3776; Prov. Misiones: Dpto Libertador Gral. San Martín, Puerto Leoni, Crisci 146 (LP), XI‐1967, MP 3777; Idem, El Dorado, Ruta 12, Arroyo Aguaray Miní, Fernández et al. 57 (LP), 8‐I‐1972, MP 3820; Idem, Aristóbulo del Valle, Reserva UNLP, picada del campito 1 al campito 2, Marquez 19 (LP), 8‐I‐2003 Dpto. Iguazú, Parque Nac. Iguazú, sendero Macuco, Liliana Katinas 31 (LP), 20‐V‐1991, MP 3780; Dpto Candelaria, Yabebyry, Montes 832 (LP), 23‐IV‐45, MP 3781; Dpto Cainguas, predio UNLP, Reserva Valle del arroyo Cuña Pirú, alrededores del balneario, Biganzolli et al. 1362 (SI), 23‐I‐2004.
Acknowledgements
The authors thank Lic. Rafael Urrejola of the Servicio de Microscopía Electrónica de Barrido de la Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata and Ing. Luis Zimmerman of Servicio de Microscopía Electrónica del Instituto de Biología Celular, Facultad de Medicina, Universidad de Buenos Aires. This contribution was granted with PIP 5044 (CONICET) and projects 274 and 363 of the Universidad Nacional de La Plata.
References
- Christensen , C. 1938 . “ Filicinae. ” . In Manual of Pteridology , Edited by: Verdoorn , F . 522 – 550 . The Hague : Nijhoff .
- Erdtman , G. 1960 . The acetolysis method. A revised description. . Sv. Bot. Tidsskr , 54 : 561 – 564 .
- Hickey , R. J. , Barker , M. S. and Ponce , M. M. 2003 . An Adiantopsis hybrid from Northeastern Argentina and vicinity. . Am. Fern J , 93 : 42 – 44 .
- Lellinger , D. B. and Taylor , W. C. 1997 . “ A classification of spore ornamentation in the Pteridophyta. ” . In Holttum Memorial Volume , Edited by: Johns , R. J . 33 – 42 . Kew : R. Bot. Gards .
- Lugardon , B. 1972 . La structure fine de l'exospore et de la périspore des Filicinées isosporées. I. Généralités. Eusporangiées et Osmundales. . Pollen & Spores , 14 : 227 – 261 .
- Lugardon , B. 1974 . La structure fine de l'exospore et de la périspore des Filicinées isosporées. II, Filicales. Commentaires. . Pollen & Spores , 16 : 161 – 226 .
- Lugardon , B. and Brousmiche Delcambre , C. 1997 . “ Exospore ultrastructure in Carboniferous sphenopsids. ” . In Ultrastructure of fossil spores and pollen , Edited by: Kurmann , M. H and Doyle , J. A . 53 – 68 . Kew : R. Bot. Gards .
- Michelena , I. G. 1989 . Esporas de Adiantaceae (Pteridophyta) de La Provincia de Buenos Aires, Argentina. . Bol. Asoc. Latinoam. Paleobot. Palinol , 12 : 25 – 31 .
- Morbelli , M. A. and Michelena , I. G. 1989 . Palynological analysis of Cheilanthes species (Adiantaceae‐Pteridophyta) of northwestern Argentina. . Grana , 28 : 295 – 304 .
- Morbelli , M. A. , Ponce , M. M. and Michelena , I. G. 1994 . “ La morfología de las esporas y las relaciones de parentesco entre los “Cheilanthoideos” (Adiantaceae‐Pteridophyta) sudamericanos. ” . In 6e Congr. Latinoamericano Bot. (Mar del Plata 1994) , Edited by: Org. Comm . 309 Buenos Aires : INTA . L. Resúm
- Ponce , M. M. 1996 . “ Pteridophyta. ” . In Catálogo de las plantas vasculares de la República Argentina. I , Edited by: Zuloaga , F. O and Morrone , O . St. Louis : Mo. Bot. Gard . Monogr. Syst. Bot. 60
- Sota , E. R. de la. and Ponce , M. M. 1992 . Nuevas citas de Pteridofitas para la flora Argentina. . Darwiniana , 31 : 327 – 333 .
- Tryon , R. M. and Tryon , A. F. 1973 . “ Geography, spores and evolutionary relations in the cheilanthoid ferns. ” . In The phylogeny and classification of the ferns , Edited by: Jermy , A. C , Crabbe , J. A and Thomas , B. A . 145 – 153 . London : Linn. Soc. Bot. J. Linn. Soc. 67 Suppl. 1 .
- Tryon , R. M. and Tryon , A. F. 1982 . Ferns and allied plants with special reference to Tropical America , New York : Springer .
- Tryon , A. F. and Lugardon , B. 1991 . Spores of the Pteridophyta , New York : Springer .