Abstract
Pollen morphology in ten plants of F2 progeny of an interspecific tree hybrid, Chorisia insignis H. B.& K.×C. speciosa St. Hil (Bombacoideae, Malvaceae) has been studied with a view to have an insight in to the effect of hybridization on the pollen morphological features in F2 generation. The study is a sequel to an earlier study on the pollen morphology of Chorisia species and their F1 hybrid, in which case the hybrid pollen uniformly exhibited the apertural features of the male parent and exine features of the female parent. In the F2 progeny the pollen grains display the apertural feature of the male parent in all the plants. However, with regard to exine ornamentation, variability has been observed. Of the ten plants, two plants exhibit the exine features of the male parent showing empty lumina (without bacula), five plants have their pollen exactly like that of the F1 plant (showing prominent columellar heads in the lumina of the apocolpium region) and the remaining three plants showing columellae in lumina of both apocolpium and mesocolpium region being different from the above types. It has been inferred that the variability in the exine pattern in F2 pollen indicates that the pollen exine pattern in hybrids, perhaps, is not unequivocally controlled by sporophytic or gametophytic genomes.
Keywords:
Effect of hybridization of plants has been convincingly expressed on pollen features in several studies, and like other plant characters the variability in the pollen morphology of the F1 hybrid depicts the combination of pollen features contributed by both the parents. Among the earlier studies, Henderson Citation(1972) observed that in respect of spine size, the pollens of the hybrids Meconopsis×cookei G. Taylor were intermediate between their parents. Qurios (Citation1975) studied pollen exine ultrastructure of Lycospersicon esculentum Mill., its wild relative Solanum pennellii Correll and their hybrid and found that with regard to large pollen size and shape and exine density, the genes of S. pennellii (male) were dominant while in pollen grain shape and size of spine, the genes of L. esculantum dominated. Ravi Kumar and Nair Citation(1986) conducted studies in three species of Gloriosa L. and their three hybrids, and observed that the pollen exine patterns in the hybrids were different from the patterns of the parent plants. None of the above‐mentioned investigations showed segregation of exine ornamentation in the hybrids, and the absence of segregation suggests sporophytic control in determination of pollen exine pattern. Segregation of pollen exine characters in hybrids has been reported by Goetz Citation(1982) where he found segregation in five of nine interspecific F1 hybrids of Hibiscus and in one F2 progeny. Godward and Pell Citation(1994) found two distinct types of exine patterns in plants of F1 generation of Nicotiana×sanderae (Solanaceae) and assumed that the plants of F2 generations raised from self and cross pollination displayed one pattern as recessive and the other as dominant possibly controlled by two different genes. He contended the existence of a plant heterozygous for exine pattern. Working in the same line, Chaturvedi et al. Citation(1993) made pollen morphological observation in two species of Chorisia – an arboreal genus of the subfamily Bombacoideae, e.g. C. insignis H.B. & K. and C. speciosa St. Hil. and their F1 hybrid, where they found differences in apertural configuration and pollen exine features in the parent species. The hybrid showed apertural configuration of the male parent and exine pattern of the female. Morphologically the hybrid pollen was uniform without any segregation. The hybrid plants were maintained in the garden of the National Botanical Research Institute and several F2 plants were raised by selfing and grown to full maturity. In continuation of the above study, the pollen morphology in ten plants of F2 generation has been studied with both light microscope and scanning electron microscope.
Material and methods
The F2 plants of Chorisia insignis×C. speciosa were raised by selfing and are being maintained in the botanical garden of the National Botanical Research Institute, Lucknow. For scanning electron‐ and light microscope (SEM and LM) studies, pollen material was collected from fresh flowers from ten plants (numbered as 1–10) for three consecutive years for comparison and to avoid stray results. Pollen grains were prepared for morphological studies following acetolysis method (Erdtman, Citation1952) for both SEM and LM. For LM examination the acetolysed grains were mounted in glycerol jelly and studied under an Olympus binocular microscope. Measurements of pollen grains, colpi and exine thickness are based on 50 grains per plant. In the cases of size dimorphism, at least 200 grains were sampled. For determining exine ornamentation pattern a minimum of 500 grains per plant were examined.
For SEM, the acetolysed grains were transferred to 100% ethanol, mounted on stubs, coated with gold and photographed in a 10 KV Philips×L20 Scanning Electron Microscope. SEM micrographs of the pollen of parent species and those of the F1 hybrid are produced from earlier study (Chaturvedi et al., Citation1993).
Results
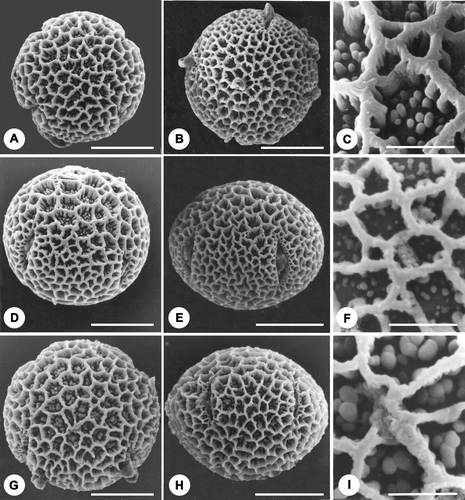
Details of pollen morphological features of the ten F2 plants of Chorisia insignis×C. speciosa based on observations made by light and scanning electron microscope are presented in Table . Basically the pollen grains are 4‐5‐zonocolporate, oblate to oblate spheroidal, brevicolpate with lolongate endocolpium. Dimorphism in size has been noted in two plants (no. 2 & 4). In all of the plants observed, the basic exine ornamentation type is reticulate but variation has been noted with regard to occurrence and density of the free columellae present within the lumina. In five plants (no. 1, 3, 7, 8 & 10, Figure ), in a majority of grains free columellar heads are discernible in the lumina of the apocolpium region only while the lumina of mesocolpial region remain empty. In three plants (nos. 2, 6 & 9, Figure ), 90–96% grains have free columella in all the lumina. In contrast to the above types, the free columellae are totally absent in the lumina of the remaining two plants (plant no. 4 & 5; Figure ). Much variation has also been observed with regard to the shape, size and density of the free columellae within the lumina, columellae being dense with broader heads in plant no. 6, 9 (Figure ), and short and sparse in plant no. 7, 8 & 10 (Figure ). However in some plants, e.g. plant no. 2 & 3, presence of medium to high density of free columellae has been observed (Figure ).
Table I. Pollen morphology in ten F2 plants of Chorisia insignis×Chorisia speciosa.
Figure 1A–I Scanning electron photomicrographs of pollen grains and exine ornamentation of F2 progeny of Chorisia insignis×C. speciosa. A, D. Pollen grains with free columellae in apocolpium region: (A) Polar view, pollen grain showing free columellae within lumina at polar region; (D) Oblique equatorial view, showing empty lumina at mesocolpium and with free columellae at apocolpium region. B, E. Pollen grains with empty lumina, free columellae lacking: (B) Polar view; (E) Oblique equatorial view showing both apocolpium and mesocolpium region lacking free columellae. G, H. Pollen grain having free columellae all over the exine: (G) Polar view, free columellae at the lumina of apocolpium region; (H) Equatorial view, showing free columellae at the lumina of mesocolpium area. C. Exine surface‐lumina having free columellae with medium density. F. Exine surface – lumina having short and scanty free columellae. I. Exine surface – lumina having broad and densely arranged free columellae. Scale bar – 20 µm (A, B, D, E, G & H); 5 µm (C, F & I).
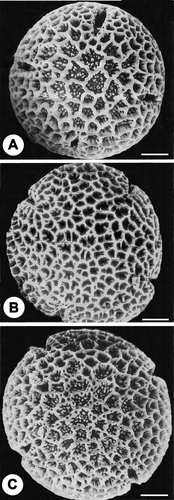
To facilitate comparison with the pollen of the parents and F1 hybrid as studied by Chaturvedi et al. Citation(1993) it may be mentioned that the pollen of C. insignis (female parent) are 4‐sub‐zonocolporate, oblate spheroidal with lolongate endocolpium. Grain surface is reticulate with dimorphic columellae; the longer ones supporting the muri and the shorter ones stand free in the lumen. The free columellae are discernable only in the lumina of the apocolpium region (Figure ). The male parent C. speciosa have 4‐zonocolporate, oblate spheroidal pollen with lolongate endocolpium, reticulate exine with empty lumina all over the surface (Figure ). The pollen of the F1 hybrid are 4‐5‐zonocolporate, oblate spheroidal with lolongate endocolpium resembling that of the male parent, but with respect to exine surface it contain reticulate exine the dimorphic columella as in C. insignis the female parent (Figure ).
Figure 2A–C SEM micrographs of pollen ofChorisia sp. and F1 hybrid‐ reproduced from Chaturvedi et al. Citation(1993): (A) C. insignis (female parent). (B) C. speciosa (male parent). (C) Pollen of F1 hybrid. Scale bar – 10 µm.
Discussion
The understanding about the controlling factor that determines the sculpturing pattern of the pollen exine is not clear. Earlier views based on the ontogeny of pollen exine (Heslop‐Harrison, Citation1964; Skvarla & Larson, Citation1966; Echlin & Godwin, Citation1968) suggest that the architectural pattern of pollen exine is under genetic control of haploid microspore as the primexine is initiated in very young microspores when they remain within the tetrad enclosed in callose wall. Contrary to this view, studies based on inheritance pattern of pollen exine in hybrids with relation to those in their parents (Henderson, Citation1972; Quiros, Citation1975; Ravi Kumar & Nair, Citation1986; Chaturvedi et al. Citation1993) argue in favour of sporophytic control (pollen mother cell) of exine patterning and their conclusion is substantiated by the absence of segregation of exine pattern in hybrids they had studied.
The present investigation comprising pollen morphology of ten plants of F2 progeny displays remarkable variations in pollen exine pattern existing among the plants and within the same anther of the flower of the same plant. The observation shows that five of the ten plants possess the exine pattern of the female parent as studied by Chaturvedi et al. (Citation1993; exine having free columellae in the lumina of the apocolpial region; Figs & ) while the characteristic exine pattern of the male parent (absence of free columellae, Chaturvedi et al. Citation1993; Figs & ) is exhibited in two plants and the remaining three plants have new pattern (free columellae present all over the exine Figure ). Variation has also been noticed with regard to shape and density of free columellae occurring in the same plant and within the same anther of flower. It may be mentioned that the common type of exine ornamentation found in F2 plants is the characteristic type of the F1 hybrid, which also resembles that of the female parent (Chaturvedi et al. Citation1993; Figure ). Segregation of pollen exine pattern was reported by Goetz Citation(1982) where he found striking segregation in five of nine F1 interspecific hybrids of Hibiscus and the process continued in three F2 progenies displaying novel types. From this observation Goetz Citation(1982) concluded that at least in the segregated hybrids the haploid spore genome participated in controlling pollen exine pattern, he also maintained that appearance of new type of exine pattern in F2 pollen is the result of continuing genetic recombination. It may be mentioned here that new characteristic types of exine ornamentation pattern, different from the parental types was reported earlier in three interspecific F‐I hybrids of Gloriosa (Ravi Kumar & Nair, Citation1986). With reference to the cases where hybrids were intermediate in exine pattern between the parents, Godward and Pell (Citation1994) stated that the possible reason might be the influence of the two or more genes with varying types of interaction. The present study relates to exine patterns of F2 plants where the segregation of parental characters is expected only if there is any contribution of haploid genome. It is fairly well demonstrated in the observation that in F2 progeny the parental exine characteristics are expressed in variable frequencies among the pollen though any clear ratio of segregation could not be ascertained. Therefore it is concluded that participation of haploid genome in exine patterning can not be ruled out and perhaps none of gametophytic or sporophytic genome have absolute control over pollen exine pattern determination. Further studies in both F1 and F2 progeny involving greater population may help to fully understand the phenomenon.
Acknowledgements
The authors are thankful to the Director of the National Botanical Research Institute, Lucknow, for providing facilities.
References
- Chaturvedi , M. , Ram , T. and Pal , M. 1993 . Pollen morphology in Chorisia species and their hybrid. . Phytomorphology , 43 : 25 – 28 .
- Echlin , P. and Godwin , H. 1968 . The ultrastructure and ontogeny of pollen in Helleborous foetidus. II Pollen grain development through the callose special wall stage. . J. Cell Sci , 3 : 175 – 186 .
- Erdtman , G. 1952 . Pollen morphology and plant taxonomy, Angiosperms , Stockholm : A & W .
- Godward , M. B. E. and Pell , K. 1994 . Inheritance of exine pattern in Nicotiana×sanderae (Solanaceae). . Bot. J. Linn. Soc , 115 : 145 – 159 .
- Goetz , S. G. 1982 . Segregation of pollen exine features in Hibiscus section Furcaria interspecific hybrids. . Grana , 21 : 21 – 27 .
- Heslop‐Harrison , J. 1964 . “ Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. ” . In Pollen physiology and fertilization , Edited by: Linskens , H. F . 39 – 47 . Amsterdam : N. Holland Publ. Co .
- Henderson , D. M. 1972 . The hybrid pollen of Meconopsis×cookei. . Grana , 12 : 52 – 56 .
- Quiros , C. F. 1975 . Exine pattern of a hybrid between Lycopersicon esculantum and Solanum pennellii. . J. Heredity , 66 : 45 – 47 .
- Ravi Kumar , C. and Nair , P. K. K. 1986 . Inheritance of exine ornamentation and pollen shape in interspecific tetraploid hybrids of Gloriosa. . Can. J. Bot , 64 : 3134 – 3140 .
- Skvarla , J. J. and Larson , D. A. 1966 . Fine structural studies of Zea mays pollen I: Cell membranes and exine ontogeny. . Am. J. Bot , 53 : 1112 – 1125 .