Abstract
Pollen morphology of 58 species from 17 putative genera of the tribe Atripliceae (Chenopodiaceae) was investigated using light (LM) and scanning electron microscopy (SEM). Morphological variation was analyzed based on a dense sampling of the subtribes Atriplicinae and Eurotiinae, including many of the species in the two largest genera: Atriplex and Obione. The pantoporate pollen grains of Atripliceae are characterized by their spheroidal or subspheroidal shape, flat or moderately vaulted mesoporia with 21–120 pores, tectum with 1–8 spinules and 5–28(−38) puncta per µm2, and 1–13 ectexinous bodies bearing 1–7 spinules each. Taxonomic relevance of the most important pollen morphological characters is discussed (pollen diameter, pore number, pore diameter, interporal distance, spinule and puncta density and ratio, number of ectexinous bodies, and their spinules). Pollen morphological data support the exclusion of Suckleya from the tribe and the recognition of subtribe Eurotiinae, but suggest that it needs to be reviewed. Pollen does not support generic recognition of Atriplex, Neopreissia and Obione and infrageneric subdivisions as currently recognized, and suggests the need to review them. Smaller or monotypic genera, such as Axyris, Ceratocarpus, Endolepis, Krascheninnikovia, Microgynoecium, Proatriplex and Spinacia have distinctive pollen morphological characters that support their generic status. Grayia needs to be reevaluated; although its two species are distinct from all the other species in the study, there are notable differences between each of them, and this suggests they may not form a natural group. Multivariate techniques were employed to investigate if there are discrete patterns of variation within Atripliceae. Principal Component Analyses (PCA) weakly differentiates four groups based on variation in pore number, puncta density per µm2, and ratio between spinule and puncta density per µm2; species of Ceratocarpus, Haloxanthium, Krascheninnikovia, Manochlamys, Microgynoecium, Spinacia, and some species of Atriplex and Obione are isolated. Preliminary results indicate that pollen data are potentially useful in the classification of the tribe, and further studies will be of taxonomic value.
The Chenopodiaceae are one of the largest families of Caryophyllales (Cuénoud et al., Citation2002) and comprise ca. 100 genera and 1 400 species of nearly global distribution (Kühn et al., Citation1993). Although molecular phylogenetic studies have shown that Amaranthaceae and Chenopodiaceae are closely related, relationships between these families remain unclear (Downie et al., Citation1997; Kadereit et al., Citation2003; Müller & Borsch, Citation2005). The most critical clade in this respect is the Polycnemoideae (Chenopodiaceae), which is inferred as sister to the Amaranthaceae using results from rbcL analyses (Kadereit et al., Citation2003) or as sister to all other Amaranthaceae plus Chenopodiaceae using matK/trnK (Müller & Borsch, Citation2005). However, statistical support for either hypothesis is low. Unless relationships are resolved and justify a change in family concepts, we prefer to use Chenopodiaceae boundaries in the traditional sense.
Another long‐lasting debate in Chenopodiaceae concerns subfamilial and tribal delimitations. Based on morphological (Flores Olvera & Davis, Citation2001) and sequence data (Kadereit et al., Citation2003; Müller & Borsch, Citation2005), Atripliceae appears nested within a paraphyletic tribe Chenopodieae with a high confidence level, and both form the monophyletic subfamily Chenopodioideae. Although current data do not allow us to make a final decision regarding the relationships of major clades within Chenopodioideae, the Atripliceae, in the traditional circumscription of Kühn et al. Citation(1993), appears monophyletic. We therefore follow this tribal classification here.
Atripliceae is the most diverse tribe of the subfamily Chenopodioideae, and includes 19 genera and ca. 330 herbaceous and shrubby species. The tribe is distinguished by the perianth in staminate flowers, and fruits with accrescent bracteoles (Ulbrich, 1934; Kühn et al., Citation1993; Judd & Ferguson, Citation1999). Subtribal subdivisions of Atripliceae were proposed by Volkens Citation(1893) and Aellen Citation(1938), but abandoned by Kühn et al. Citation(1993). However, Ulbrich Citation(1960) accepted Volkens subtribes Atriplicinae and Eurotiinae on the basis of hair types and seed position, and these are the subdivisions we use in the present study.
Whereas pollen of several families of the Caryophyllales (e.g. Amaranthaceae, Caryophyllaceae, Dysphaniaceae, Nyctaginaceae, Phytolaccaceae, Portulacaceae) has been extensively studied (Nowicke, Citation1994), the Chenopodiaceae are still in need of detailed pollen studies. Both Amaranthaceae and Chenopodiaceae possess exclusively pantoporate pollen that is characterized by a spinulose and punctate tectum, with tectum perforations often being minute (Nowicke, Citation1975; Skvarla & Nowicke, Citation1976).
Extensive pollen studies revealed a high morphological diversity in Amaranthaceae (e.g. Borsch, Citation1998) and led to the recognition of 17 pollen types, largely defined by a combination of qualitative characters including pollen shape, appearance of mesoporia, and pore membrane type (i.e. variations in ectexinous bodies covering pore membranes). However, pollen of Chenopodiaceae appears to vary most in the number of apertures and number, size, and frequency of spinules on, and punctae in, the ectexine. In a few genera, including Anabasis L., Halocharis Moq., Nitrophila S. Watson and Traganum Delile, the pollen was described as having few, and slightly sunken pores, with a convex mesoporial exine (Nowicke & Skvarla, Citation1979). Pollen grains for which morphology was considered intermediate between these two types were described for the pollen of Beta and Camphorosma (Nowicke, Citation1975).
Pollen morphology of members of the Atripliceae, especially species of Atriplex L., is poorly documented. Nevertheless, a few data, for example on pollen diameter, pore number, spinule and punctae densities, are scattered in the literature (e.g. Wodehouse, Citation1965; Nair & Rastogi, Citation1966–67; Tsukada, Citation1967; Nowicke, Citation1975; Frankton & Bassett, Citation1970; Bassett et al., Citation1983; Chu, Citation1987; Hao et al.,Citation1989; Rosas, Citation1989; Flores Olvera, Citation1992). Several authors have suggested the potential taxonomic value of pollen in Chenopodiaceae. For example, Tsukada Citation(1967) hinted at the importance of spinule and puncta density, and spinule: puncta ratios in the identification of Chenopodiaceae at generic and at species level. Frankton and Bassett Citation(1970) suggested that pore size, pore number, and spinule shape are useful characters in Atriplex.
The aims of this study are to describe pollen morphology of Atripliceae, to explore the potential taxonomic utility of pollen characters at different taxonomic levels in Atripliceae, and to identify pollen morphological characters that might be useful for species and genus identification.
Material and methods
Pollen samples were collected from herbarium specimens deposited at BH, ENCB, GH, K, MO, NY, and RSA (herbarium citation follows Holmgren et al., Citation1990). At least five floral buds per specimen were dissected for anthers. The anthers were then acetolyzed according to Erdtman Citation(1960) for eight to nine minutes at 80–90°C; depending on the condition of the anthers the duration and temperature were modified as necessary. The pollen grains were subsequently examined using light (LM) and scanning electron microscopy (SEM).
Samples for light microscopy were mounted on slides using glycerol jelly, then sealed with nail polish, and observed under a Zeiss Axioscope microscope. Samples for SEM were dehydrated in an ethanol series and dried with an Emitech K850 critical point dryer.
Dry pollen grains were mounted on double‐sided carbon tape affixed to aluminum stubs. Grains were coated with gold at 20 nA for approximately 90 s with an Emitech K550 sputter coater. Observations were made using a Hitachi S‐2460N scanning electron microscope in the Instituto de Biología, UNAM. We note that there is an apparent distortion artifact in the SEM images (Figure ) causing the pollen grains to appear oblate rather than spheroidal as observed with LM (Figure ).
Figure 1 LM of pollen grains of selected Atripliceae taxa; focal level varies between images. A. Axyris amaranthoides. B. Ceratocarpus arenarius. C. Endolepis dioica. D. Exomis microphylla. E. Grayia spinosa. F. Krascheninnikovia lanata. G. Manochlamys albicans. H. Microgynoecium tibeticum. I. Proatriplex pleiantha. J. Spinacia oleracea. K. Suckleya suckleyana. L. Theleophyton billardierei. Scale bar – 10 µm.
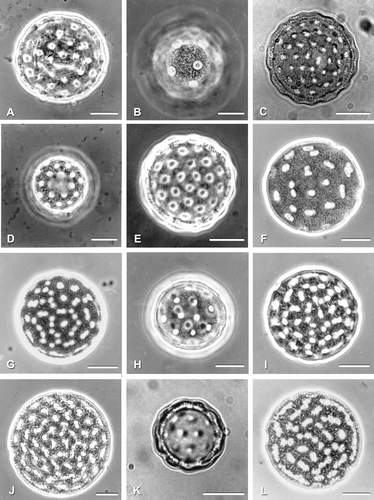
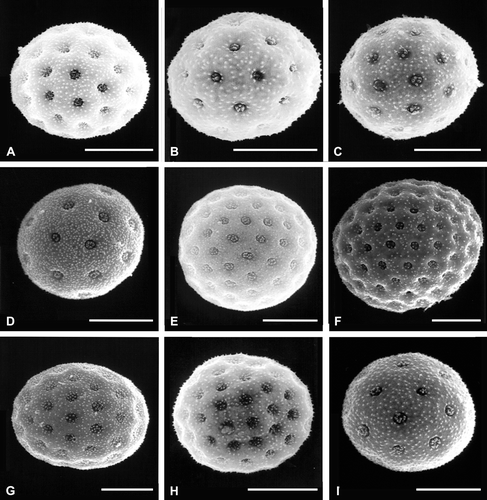
Figure 2 SEM micrographs of pollen grains: A. Atriplex calotheca. B. A. eardleyae. C. A. semibaccata. D. Ceratocarpus arenarius. E. Grayia spinosa. F. Microgynoecium tibeticum. G. Neopreissia isatidea. H. Obione lampa. I. O. pusilla. Scale bar – 10 µm.
A total of 58 representative species of Atripliceae were examined (Table ). The sampling comprises most of the taxonomic categories traditionally recognized for this tribe including its two subtribes, 19 of 22 putative genera, and an extensive account of Atriplex and Obione Gärtner sections and series (see Specimens Investigated). Availability of herbarium material, well‐preserved floral buds limited our sampling to a single specimen for each species (voucher list provided in Specimens Investigated). When possible, a minimum of 20 pollen grains per specimen were examined.
Table I. Pollen data for the examined species.
From light microscopy, we described and measured maximum‐minimum variation and/or average of pollen diameter, pore number per grain, pore diameter, interporal distance, exine thickness, and nexine: sexine ratio. SEM images were used to describe and measure the number of spinules and punctae per µm2 and their ratios, number, shape, and position of ectexinous bodies, and the number of spinules at a range of comparable magnifications (Table ). Spinule height was measured on SEM images at 20 k, using the corresponding scale to measure spinules that were sharply profiled at the edge of the grain and completely perpendicular. All spinules observed in this position on selected SEM images were measured to try and account for variation.
Quantitative data were analyzed using multivariate analyses in PC‐OR 4.25 software (McCune & Mefford, Citation1999). A total of 16 quantitative variables were included in the analyses (Table ). Ten of these correspond to minimum and maximum values for five characters. The remaining six quantitative variables correspond either to the maximum or minimum value for the five characters. Coding format was constrained to data availability (Table ). One data set was analyzed; it included all 58 species from the 17 genera of Atripliceae. Data partitioning allowed us to understand patterns of pollen morphological variation between and within genera of the tribe.
Table II. List of variable characters included in the Principal Component Analyses of Atripliceae.
Multivariate variation was investigated with Principal Component Analyses (PCA) using Euclidian distances. Measurements were standardized to eliminate the effect of different scales of measurement. Similarity matrices using correlation were then generated, and eigenvalue and eigenvector matrices were derived from the correlation matrix. The standardized data were projected onto the eigenvectors in two‐dimensional scatter plots.
Results
Pollen grains for all species of Atripliceae in this study are spheroidal or subspheroidal, pantoporate with numerous pores, and have flat or moderately vaulted mesoporia and numerous evenly distributed spinules, and a punctate tectum (Figures , ).
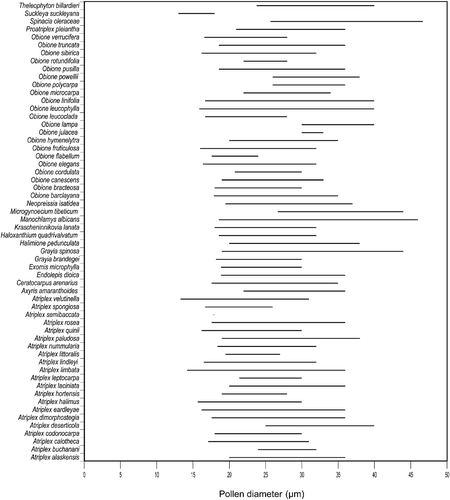
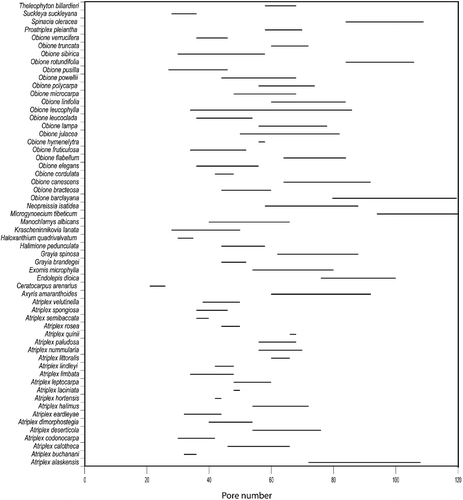
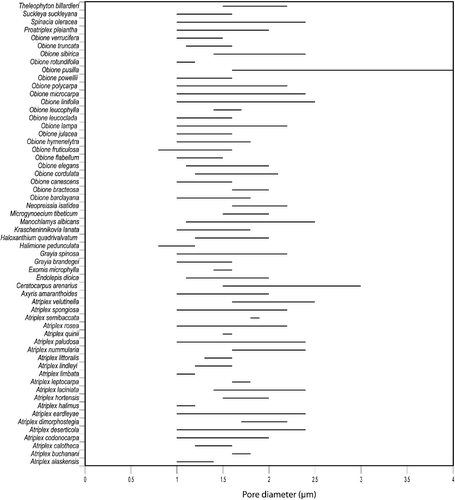
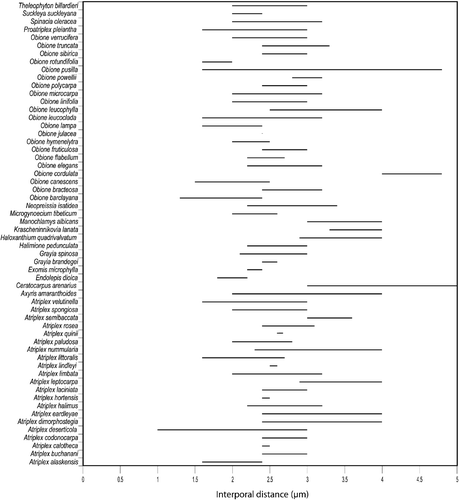
Pollen morphological variation within Atripliceae is largely quantitative (Table ). There is a large amount of variation in pollen diameter (Figure ), pore number (Figure ), pore diameter (Figure ), and interporal distance (Figure ). Variation in tectum characters, such as density of spinules (Figure ) and punctae (Figure ), and their ratio to each other (Figure ), size and number of spinules, exine thickness, and number of ectexinous bodies on the pore membrane (Figure ) is presented in Table .
Figure 7 SEM micrographs of exine surfaces and pores: A. Endolepis dioica. B. Atriplex lindleyi. C. Obione canescens. D. Krascheninnikovia lanata. Scale bar – 2 µm.
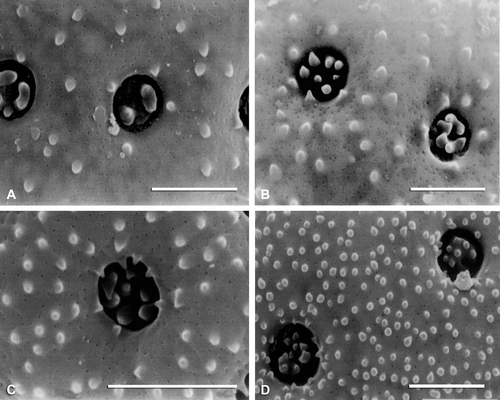
Figure 8 SEM micrographs of exine surfaces and pores: A. Manochlamys albicans. B. Theleophyton billardierei. C. Atriplex deserticola. D. A. eardleyae. E. A. calotheca. F. Atriplex limbata. Scale bar – 2 µm.
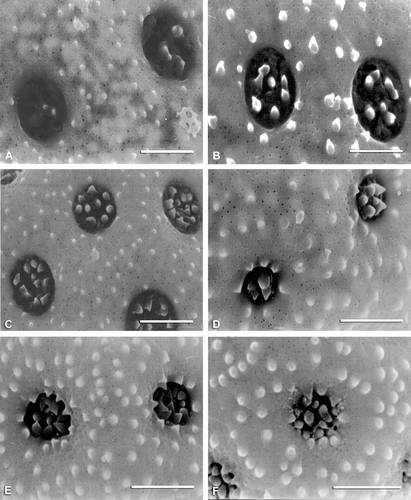
Figure 9 SEM micrographs of exine surfaces and pores: A. Proatriplex pleiantha. B. Spinacia oleracea. C. Obione pusilla. D. O. julacea. Scale bar – 2 µm.
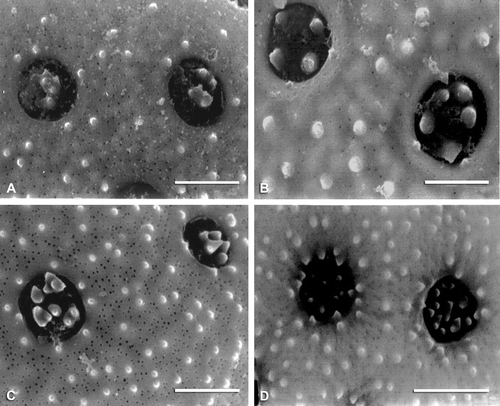
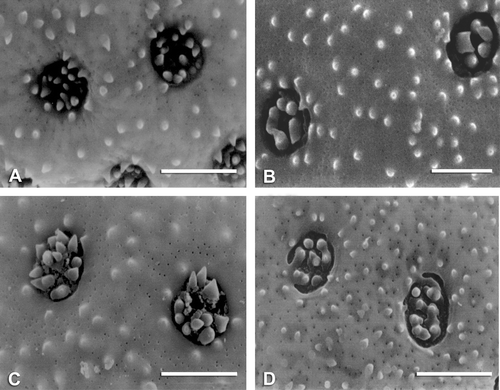
Figure 10 SEM micrographs of exine surfaces and pores: A. Obione lampa. B. Obione cordulata. C. A. codonocarpa. D. Grayia spinosa. Scale bar – 2 µm.
Pollen characters
Pollen diameter (Table , Figure ): [13(16–40)46.7] µm
Suckleya suckleyana has generally smaller pollen than the other genera examined in the tribe although the size range overlaps with some species of Atriplex and Obione. Grayia spinosa, Microgynoecium tibeticum, Manochlamys albicans and Spinacia oleracea have the largest grains. All the species of Atriplex have overlapping size ranges, but within Obione several species are distinguished from each other by their pollen diameter.
Pore number per grain (Table , Figure ): [(21)27–120]
Ceratocarpus arenarius has the lowest pore number while the highest pore numbers are found in Microgynoecium tibeticum, Endolepis dioica, Atriplex alaskensis, Obione barclayana, O. rotundifolia, and Spinacia oleracea. Difference in pore numbers between some species of Atriplex and Obione is a distinctive character.
Pore diameter (Table , Figure ): [0.8(1.0–2.5)4.0] µm
The smallest diameter pores occur in Halimione pedunculata and Obione fruticulosa, while the widest diameter pores are found in O. pusilla and Ceratocarpus arenarius.
Interporal distance (Table , Figure )
This is the breadth of the exine between pores (mesoporia) and has a range of [1.0(1.6–4.0)5.0] µm. The shortest distances were recorded in Atriplex deserticola, Obione barclayana, and O. canescens, while the greatest were recorded in Ceratocarpus arenarius, O. cordulata, and O. pusilla.
Spinule density (Table , Figures , )
Spinules are evenly distributed over the entire pollen surface, density ranges from 1–8 spinules per µm2. Approximately 98% of the species studied have 1–3 spinules per µm2, the remainder, which includes Obione canescens, Axyris amaranthoides, Ceratocarpus arenarius and Krascheninnikovia lanata, have 4, 5, 7, and 8 spinules per µm2, respectively.
Puncta density (Table , Figure )
Five to 28 (−38) puncta per µm2 although most species have 12–28 per µm2. Atriplex halimus has the highest puncta density, while the lowest densities occur in Manochlamys albicans, Spinacia oleracea, Ceratocarpus arenarius, Krascheninnikovia lanata, Theleophyton billardierei, and Exomis microphylla. Puncta diameter was not scored but, from our observations, small differences are noted at species level; compare for example, Figure with Figure .
Spinule/puncta ratio (Table , Figure )
This character is highly variable, ranging from 1:1 to 1:18. Ceratocarpus arenarius and Kraschennikovia lanata (Figure ) have the lowest ratio (1:1), Axyris amaranthoides has a ratio of 1:3, and Exomis microphylla, Manochlamys albicans (Figure ), Obione canescens, and O. flabellum have a ratio of 1:5. The highest ratio in the tribe was observed in Endolepis dioica (Figure ) followed by Grayia brandegei, and Proatriplex pleiantha (Figure ). The remaining species have a ratio of 1:6–13, these include, Spinacia oleracea (Figure ), Obione pusilla (Figure ) and O. julacea (Figure ).
Spinule height (Table ): 0.07–0.5 µm
Most species have spinules 0.21–0.42 µm high, although a few species including Obione elegans, Spinacia oleracea and Theleophyton billardierei exhibit spinules with extreme values.
Exine thickness and sexine/nexine ratio: [1.0(1.2–2.5)2.8] µm
Grayia spinosa has the thinnest exine (1.0–1.2 µm) and Obione cordulata the thickest (2.5–2.8 µm). The sexine/nexine ratio in Atripliceae is 4–5:1.
Ectexinous bodies on the pore membrane (Table , Figure )
The number of ectexinous bodies on the pore membrane varies from 1–11(−13) discrete bodies; each of these bears 1–5 (−7) distinct spinules which have either rounded or acute apices. Individual ectexinous bodies with 1–3 spinules may be small and conical, elongate, or triangular in shape. However, those with more than three spinules are notably larger and irregular in shape. Pollen grains of most species have 2–11 ectexinous bodies, each with 1–3 (−4) distinct spinules. The bodies, distinctly separate from each other, are deep‐set within the pore and either at a distance from, or close to the pore margo. Overall the pore type in Atripliceae closely resembles ‘Pore type II’ in Amaranthaceae (Borsch, Citation1998).
A high number of ectexinous bodies (5–13) distinguishes eight species of Atriplex (Figure ) and Obione (Figures , ), whereas Axyris amaranthoides, Endolepis dioica (Figure ), Grayia brandegei, Krascheninnikovia lanata (Figure ), Microgynoecium tibeticum, O. microcarpa, and Proatriplex pleiantha (Figure ) have the smallest number (2–4). The highest numbers of spinules on individual ectexinous bodies are observed in A. leptocarpa (Figure ), Ceratocarpus arenarius, G. brandegei, K. lanata (Figure ), O. cordulata (Figure ), and P. pleiantha (Figure ) (see Table ).
A few species have atypical pore morphology; for example in Atriplex codonocarpa the ectexinous bodies are closely adpressed to each other, exerted in the pore and close to the pore margo (Figure ) while in Grayia spinosa the tectum has small projections to the pore (Figure ).
Principal Component Analysis (PCA)
In the Principal Component Analysis of pollen characters for Atripliceae nearly 50% of the patterns of pollen morphological variation are explained by the first three principal components (PC I–III). PC I explains 20.47% of the total variation, while PC II and PC III account for another 14.45% and 11.35% respectively. Eigenvectors resulting from the ordination analysis indicate that the minimum and maximum number of pores, puncta density, and spinule/puncta ratio are strongly correlated with PC I and PC II.
The scatter plot for the components PC I and PC II shows four distinct groups of species and 19 ungrouped species (Figure ). Group I (solid squares) is distinguished by a wider range of pore number, puncta density and spinule/puncta ratio, which overlap with the other groups, but reaches the highest values for puncta density (13–28) and spinule/puncta ratio (1:8–1:17). Groups II (solid triangles) and IV (open squares) have more similarity to each other, while Group II has a smaller number, and narrower range, for pores (54–88 vs 54–120) and a wider range for spinule/puncta ratio (1:3–1:10 vs 1:6–1:9). Group III (solid circles) overlaps considerably with Group IV in characteristics of puncta density (12–20 vs 12–18) and spinule/puncta ratio (1:6–1:10 vs 1:6–1:9), but differs by having a lower pore number and range (32–60 vs 54–120).
Figure 11 Principal Component Analysis of 58 species of Atripliceae. Solid squares– Species Group I; solid triangles – Species Group II; solid circles – Species Group III; open squares – Species Group IV; open numbered triangles – isolated species [see Table : column 1 for species numbers used in study, column 2 for corresponding species, and column 14 for corresponding Species Group].
![Figure 11 Principal Component Analysis of 58 species of Atripliceae. Solid squares– Species Group I; solid triangles – Species Group II; solid circles – Species Group III; open squares – Species Group IV; open numbered triangles – isolated species [see Table I: column 1 for species numbers used in study, column 2 for corresponding species, and column 14 for corresponding Species Group].](/cms/asset/1df5fe4e-60f8-4dd9-9a36-f2c10c522069/sgra_a_187321_o_f0011g.gif)
Species groups
Group I: Atriplex calotheca, A. codonocarpa, A. dimorphostegia, A. laciniata, A. limbata, A. lindleyi, A. nummularia, A. spongiosa, Grayia brandegei, Halimione pedunculata, Obione fruticulosa, O. leucophylla, O. powellii, O. sibirica, O. truncata.
Group II: Atriplex littoralis, A. paludosa, Axyris amaranthoides, Exomis microphylla, Grayia spinosa, Neopreissia isatidea, Obione flabellum, O. hymenelytra, O. linifolia, O. microcarpa, O. polycarpa, Proatriplex pleiantha, Theleophyton billardierei.
Group III: Atriplex eardleyae, A. hortensis, A. leptocarpa, A. rosea, Obione elegans, O. leucoclada.
Group IV: Atriplex deserticola, Endolepis dioica, Obione barclayana, O. lampa, O. rotundifolia.
Remaining species (open triangles) isolated in multivariate space from Groups I–IV: Atriplex alaskensis, A. buchanani, A. halimus, A. quinii, A. semibacata, A. velutinella, Ceratocarpus arenarius, Haloxanthium quadrivalvatum, Krascheninnikovia lanata, Manochlamys albicans, Microgynoecium tibeticum, Obione bracteosa, O. canescens, O. cordulata, O. julacea, O. pusilla, O. verrucifera, Spinacia oleracea, and Suckleya suckleyana.
Discussion
Taxonomic significance of pollen characters
The results of our study highlight some valuable pollen characters for taxonomic re‐assessment in tribe Atripliceae. Some characters proved to have value at supra‐generic level, others at generic level, while others have more value at species level. Overall, the most significant characters are: pollen diameter, pore number, puncta density, spinule density and spinule/puncta ratio.
Variation at suprageneric level
Certain pollen characters corroborate some of the proposals relating to supra‐generic classification. For example, the remarkably small diameter and low pore number of Suckleya pollen support its exclusion from tribe Atripliceae. This is also supported by the PCA analysis (Figure ). This genus was classified within the monotypic subtribe Suckleyinae (tribe Chenopodieae) by Chu et al. Citation(1991), but this proposal was not accepted by all authors (e.g. Kühn et al., Citation1993).
The lowest spinule/puncta density ratio characterizes Axyris (1:3), and Ceratocarpus and Kraschennikovia (1:1), classified in subtribe Eurotiinae (Ulbrich, Citation1960). However, Grayia, also classified in this subtribe, differs from these genera by a much higher spinule/puncta ratio (1:7–1:17). Interestingly, Axyris, Ceratocarpus and Krascheninnikovia have grains with the highest spinule density (5–8 per µm2) whereas Grayia has low density (1–2 per µm2). The PCA analysis supports a closer relationship between Ceratocarpus and Krascheninnikovia, nevertheless, Axyris (Group II) is not grouped near these genera (Figure : 23 & 30). This is in agreement with previous results (Flores & Davis, Citation2001). Pollen data support the recognition of subtribe Eurotiinae and suggest that Grayia, and perhaps Axyris, should be excluded. Further sampling within Axyris, Ceratocarpus and Krascheninnikovia is needed to corroborate the constancy of pollen characters at generic level and consequently their supra‐generic value.
Variation at generic level
Within Atripliceae, many genera have only a few species, several are monotypic, and there are few large genera. The degree of taxonomic value from pollen characters, as well as macro morphological characters, at generic level reflects this, for example genera with one or only a few species, are likely to show less variation than genera with many species. Furthermore, generic level variation for some of the small or monotypic genera often overlaps the range of pollen variation found in genera with more diverse pollen. Nevertheless, there are pollen characters which are useful for distinguishing some of the small or monotypic genera. Pollen diameter is a good example: in most genera the diameter ranges overlap the ranges observed in Atriplex and Obione. However, in Manochlamys and Microgynoecium, which have the largest pollen grains, there is no overlap with diameter ranges in other genera. In addition, large pore number and few ectexinous bodies further distinguish Microgynoecium. In the PCA analysis this genus is quite distinct (32 in Figure ). Low puncta density and spinule/puncta ratio are characteristic of the pollen of Exomis and Manochlamys, although the low puncta density is not exactly the same for both genera. This is highlighted by the PCA analysis where Manochlamys is not grouped with Exomis. Our results support the generic distinction of Exomis and of Manochlamys which is accepted by some authors, for example, Aellen Citation(1939) and Dyer Citation(1975), rather than the congeneric status suggested by others authors such as Kühn et al. Citation(1993).
In Grayia (sensu Kühn et al., Citation1993), our results reveal notable pollen morphological differences between the two species which comprise the genus, G. brandegei and G. spinosa, especially pore number (44–52 vs 62–88), spinule/puncta density (1:17 vs 1:7), exine thickness (2.2–2.4 vs 1.0–2.0), and number of ectexinous bodies (2–4 vs 5–6). This, combined with the PCA analysis, which clusters G. brandegei in Group I, and G. spinosa in Group II (Figure ) supports their taxonomic separation. Welsh Citation(1984) transferred G. brandegei to the genus Zuckia Standley, a member of Atripliceae not included in this study. Zuckia was sister to both species of Grayia in a morphological phylogenetic analysis of the tribe which did not use pollen characters (Flores & Davis, Citation2001). It would be useful to undertake a further phylogenetic analysis which includes pollen of Zuckia to ascertain whether pollen data corroborate this proposal.
Only one species was sampled for some of the small genera such as Axyris, Ceratocarpus, Endolepis, Krascheninnikovia, and Spinacia, although ideally pollen of more species should be examined to establish the generic value of some of the apparently distinctive pollen characters, even though it would appear, provisionally, that the generic status of these taxa is supported by pollen morphology. Apart from the unique spinule densities and spinule/puncta ratios which distinguish Axyris, Ceratocarpus, and Krascheninnikovia (see comments in section above: ‘Variation at suprageneric level’), these genera have pollen with only a small number of ectexinous bodies and, furthermore, Ceratocarpus and Krascheninnikovia have a high number of spinules on each of the ectexinous bodies (Figure ). Endolepis pollen has the highest spinule/puncta density, a high pore number and only a few ectexinous bodies (Figure ). Nevertheless, the PCA analysis groups Endolepis with other species of Atriplex and Obione (Figure ). Spinacia pollen is distinguished by its large pollen diameter and high pore number, low puncta density, high spinule number and greatest spinule height; it is also shown to be distinct in the PCA analysis (56 in Figure ).
We studied the pollen of a number of representative species from the two largest genera Atriplex and Obione including most of the infrageneric groups recognized by Ulbrich Citation(1960). From these data we have tentatively extrapolated the taxonomic value for pollen characters at generic level, although our study does not provide any pollen characters which distinguish between Atriplex, Neopreissia or Obione. These three genera have been highly controversial and are not accepted by some authors who recognize only Atriplex (e.g. Hall & Clements, Citation1923; Wilson, Citation1984; Kühn et al., Citation1993). They are not well‐defined by the PCA analysis either (Figure , Table ). The fact that the PCA analysis places different species of Atriplex and Obione in Groups I, II, III and IV, probably also reflects that these genera are still in need of more critical revision. The possibilities of either a paraphyletic Atriplex with the potential need to transfer several other genera into the genus or, a narrower re‐circumscription of Atriplex, have both been suggested by Flores and Davis Citation(2001).
In contrast, some putatively segregate genera such as Halimione, Haloxanthium, Proatriplex and Theleophyton, sometimes included within Atriplex, can be distinguished by pollen morphological characters. For example, the relatively diverse genera Halimione and Haloxanthium, for which we have only sampled pollen for one species in each genus, have pollen differences that support generic status. Halimione has extreme values for pore diameter, while Haloxanthium has a high pore number and larger spinules (Table ). Nevertheless, only Haloxanthium is clearly distinguished by the PCA analysis (29 in Figure ). Further sampling within these genera will be needed to evaluate the generic constancy of these characters. The pollen of the monotypic genus Proatriplex (Stutz et al., Citation1990) has a higher spinule/puncta ratio than the Atriplex‐Obione complex and a notably low number of ectexinous bodies each with a high number of spinules (Figure ), which distinguish it from the pollen of most other species in the complex. Pollen of the monotypic Theleophyton (Moquin‐Tandon, Citation1840) has a lower puncta density and a higher spinule height than the Atriplex‐Obione complex. Nevertheless, none of these genera is distinguished by the PCA analysis where Proatriplex and Theleophyton both fall within Group II.
Variation at the interspecific level
At interspecific level the discussion is focused on the two large genera in the tribe: Atriplex and Obione where pollen has been studied from 21 species in each genus. There are several pollen polymorphic characters with overlapping values; this limits their use for the distinction of species. Nevertheless results so far suggest that for some pollen characters a larger infraspecific sampling, using appropriate statistical tools, such as simple box diagrams or significance tests, would be of value in the re‐examination of some of the species complexes within both Atriplex and Obione. A few pollen characters do, however, permit recognition of species groups within these genera. Notably, within Obione, pollen diameter (Figure , Table : column 3), for example, the larger pollen of O. julacea and O. lampa does not overlap with the smaller pollen of O. flabellum, O. leucoclada, O. rotundifolia, and O. verrucifera. This is not the case within Atriplex where pore number has been found to be more useful (Figure , Table : column 4). For example, A. alaskensis has larger pore number than A. buchanani, A. codonocarpa, A. eardleyae, A. limbata, A. semibaccata or A. spongiosa. Three of these species are distinct in the PCA analysis: A. alaskensis, A. buchanani, and A. semibaccata (1, 2, 19 in Figure ).
Pore diameter, interporal distance (Table : columns 5, 6), and the number of ectexinous bodies (Table : column 12) are not such useful characters in species delimitation within Atriplex and Obione although there is a small margin of usefulness for the recognition of a few species. For instance, pore diameter may be useful to distinguish between some species classified within some series (Ulbrich, Citation1960): A. leptocarpa from A. limbata and O. fruticulosa from O. pusilla (Figure ). Extreme values of interporal distance, for example, allow the distinction of O. cordulata from O. rotundifolia (Figure , Table : column 6) and these two species are also distinguished by the PCA analysis (37 in Figure and Group 5). Atriplex alaskensis, O. barclayana, and O. rotundifolia have short interporal distances combined with high pore numbers; A. deserticola and O. lampa have short interporal distance, which is combined with large pollen diameter while A. velutinella has a short interporal distance combined with a high pore diameter.
Within Atriplex and Obione some exceptions to uniformity are also found in tectum characters. For example, while a spinule density of 2 is fairly constant among most species, some exceptions exist: Obione leucophylla (spinule density 1), Atriplex halimus, O. flabellum, and O. powellii (spinule density 3), and O. canescens (spinule density 4) (Table : column 7). Atriplex halimus and Obione canescens are distinguished by the PCA analysis (8 in Figure and 36). Puncta density (Table : column 8) has a wide range of variation although the frequency most commonly ranges from 18 to 20 represented by nine species in Atriplex and seven species in Obione. However some species are distinguished by having infrequently occurring puncta density mean values, these include O. leucophylla (13) and O. powellii (28). The species with extreme values of spinule/puncta ratio also have less frequently occurring values of mean puncta density, although no correlation test was performed between these two characters.
Results indicate that spinule height provides evidence for four groups of species (Table : 10): those with spinules of 0.14 µm (Atriplex nummularia, Obione elegans, O. julacea), those with spinules of 0.21 µm (Atriplex eardleyae, A. hortensis, A. leptocarpa, A. semibaccata, Obione bracteosa, O. cordulata, O. flabellum, O. leucophylla, O. powellii, O. rotundifolia), those with spinules of 0.28 µm (Atriplex laciniata, A. limbata, A. lindleyi, A. littoralis, A. paludosa, A. quinii, A. rosea, Obione hymenelytra, O. leucoclada, O. sibirica, O. truncata, O. verrucifera) and those with spinules of 0.35 µm (Atriplex alaskensis, A. calotheca, A. codonocarpa, A. dimorphostegia, A. spongiosa, A. velutinella, Obione linifolia, O. microcarpa).
Combinations of pollen morphological characters help to distinguish some groups of species within Atriplex and Obione, as shown the results of the PCA analysis (Figure ) where species of Atriplex and Obione are grouped in Group I or Group II.
Variation at the infraspecific level and ploidy
Correlations between ploidy and pollen morphological variation in the Caryophyllales were suggested by Borsch and Wilde Citation(2000). Previous to this a relationship between ploidy level and pollen diameter was noted in Atripliceae: Atriplex subspicata (Nutt.) Rydb. by Bassett et al. Citation(1983). In this species hexaploids have larger pollen grains (23–29 µm in diameter) than tetraploids (20–25 µm). There is no report for polyploids in O. powellii although the discrepancy between our pollen diameter measurements (26–38 µm) and those of Frankton and Bassett (Citation1970: 19.0–26.9 µm) might indicate potential polyploid populations for this species. The possible relationship between polyploidy and pollen diameter is certainly a topic worthy of further investigation; especially one involving a wider sampling of species where tetraploids have been reported (Stutz et al., Citation1987; Flores Olvera & Mercado‐Ruaro, Citation1997) such as Grayia brandegei (18.2–30 µm), Obione cordulata (20.8–30 µm), and O. leucophylla (15.9–40 µm).
In some species the pollen diameter ranges reported in this study vary in respect to those reported by other authors. In general our ranges are wider than those previously reported. For example, Bassett et al. Citation(1983) reported a pollen size of 23.5–30.0 µm for Endolepis dioica, while we recorded a size of 18.9–36 µm; for Atriplex hortensis the same authors reported a size range of 19.0–25.0 µm but we found a range of between 19.0–28.0 µm; for A. rosea these authors reported a size of 25.0 µm while we have recorded a size range of 17.6–36.0 µm. For Spinacia oleracea Nair & Rastogi Citation(1966–67) reported a pollen size range of 40–42 µm while we recorded a range between 25.7–46.7 µm. Similarly, elsewhere, the occurrences of polyploidy in species may result in larger pollen. For example, in hexaploids of Atriplex alaskensis (Bassett & Crompton, Citation1973; Bassett et al., Citation1983) a pollen diameter of 23.9–29.0 µm is recorded while we found a diameter range of 20–36 µm. In Obione canescens, where there are diploids, tetraploids, hexaploids, and dodecaploids (Stutz & Sanderson, Citation1979), Wodehouse Citation(1965) reported a pollen diameter range of 23.0–25.5 µm while we recorded a range of 19–33 µm. However, it should not be discounted that several other factors could account for pollen size differences; these include differing microscope techniques, sample size, or biological variants such as phenotypic plasticity and differences in distribution patterns.
Aperture number is another potentially interesting pollen character to compare with chromosome counts in Chenopodiaceae. It has already been correlated in other families of Caryophyllales (Borsch & Wilde, Citation2000). For instance, in our sampling the species in Atriplex with the highest pore number is A. alaskensis and, as commented above, it has a report of hexaploidy (Bassett & Crompton, Citation1973).
Conclusions
In Atripliceae there is no apparent correlation between taxonomic ranks and any of the main pollen characters: pollen diameter, pore number, pore diameter, number of ectexinous bodies, spinule size and density, puncta density, and spinule/puncta ratio. However, all the characters do have a degree of taxonomic value, either in isolation or in combination. Furthermore, a combination of several characters, notably pore number, spinule density, and spinule/puncta ratio, accounts for most of the variation within the tribe. Differences in the patterns of ectexinous bodies are less variable than in Amaranthaceae (Borsch, Citation1998) but, nevertheless, variations in their number, and the number of spinules on individual bodies is distinctive for some species.
In spite of the general similarity of the pollen throughout Atripliceae, our observations indicate that quantitative pollen morphology is sometimes a useful source of additional data in taxonomic re‐evaluations within the tribe, provide further insight into conflicting ideas based solely on macromorphology. Pollen characters have provided convincing evidence in support of the exclusion of Suckleya from the tribe. Pollen data also lend support to the recognition of subtribe Eurotiinae, but re‐circumscribed to include the genera Axyris, Ceratocarpus and Krascheninnikovia while excluding Grayia. However, pollen data for the other species of the first three genera are needed to corroborate this preliminary evidence. Pollen characters support the splitting of Grayia into two taxonomic entities, but whether G. brandegei should be transferred to Zuckia is uncertain without pollen data for Zuckia.
Pollen morphology supports the recognition of the controversial monotypic genera Proatriplex and Theleophyton as distinct from Atriplex and Obione, although pollen data for a much wider range of species in Atriplex and Obione is needed before reaching any conclusions. Pollen data also support the recognition of Endolepis and Haloxanthium, as well as the non‐controversial genera Exomis, Manochlamys, Microgynoecium and Spinacia.
There is no evidence from pollen morphology for the distinction of the largest genera Atriplex and Obione, or for the infrageneric classification of Ulbrich Citation(1960). The lack of useful pollen characters to distinguish these taxa mirrors weak macro morphological variation, and has so far resulted in a lack of consensus regarding taxonomic circumscription between and within these genera. However, there are a few species in both genera which can be distinguished by their pollen characteristics.
Principal Component Analysis of pollen data has resulted in the definition of four species groups within Atripliceae, although it will be necessary to expand species and population sampling to assess whether these four groups are robust, and to seek other, possibly novel, characters which could add further support to these groups.
If the distribution of some pollen characters proves to have strong phylogenetic signals, then the conflict between pollen groups and current ideas on classification highlight not only the need to review the classification of Atripliceae and perhaps the entire family (Flores & Davis, Citation2001) but also the importance of continuing to gather pollen data for the Chenopodiaceae.
Specimens Investigated
List of taxa examined and associated voucher data, following the modified classification of Ulbrich Citation(1960).
Subtribe: Atriplicinae
Atriplex
Sect. Dichospermum
A. hortensis L. British Isles: Tillingham. Redhual 6548 (K)
Sect. Halimoides
A. lindleyi Moq. Australia: “The Gorge” of Wittabrenna Creek (E of Tibooburra). Greuter 18466 (NY)
Sect. Hymenotheca
A. quinii F. Muell. Australia: Wittabrenna Creek. Greuter 18468 (NY)
Sect. Nummularia
A. nummularia Lindl. Unknown. Bethelo s.n. (NY)
Sect. Podiceps.
Ser. Leptocarpa
A. leptocarpa F. Muell. Australia: Lake Eyre. Mungeranie Station. Waukatanna Water hole, Cooper Creek. Badman 5167 (NY)
A. limbata Benth. Australia: Along Woomera Road, vicinity of Port, Augusta Arid Land Botanical Garden. Nee 45700 (NY)
Ser. Stipitata
A. eardleyae Aellen. Australia: Merredin. Canning 6217 (MO)
Ser. Velutinella
A. velutinella F. Muell. Australia: Lake Eyre Basin. Badman 845 (MO)
Sect. Pseudochenopodium
A. buchanani Kirk ex Cheeseman. New Zealand: Chatham Island. Given & Williams 12873 (K)
Sect. Sclerocalymma
A. dimorphostegia Kar. & Kir. Israel: Negev Revivine. [Dacute]Angelis s.n. (BH)
A. laciniata L. Unknown. Fernald 7397 (BH)
A. rosea L. USA: California. Wiegard et al. 2909a (BH)
Sect. Semibaccata
A. semibaccata R. Br. Unknown. Bailey & Bailey 7950 (BH)
Sect. Spongiosa
A. spongiosa F. Muell. Australia: Broken Mill. Morriz 2010 (K)
A. codonocarpa Paul. G. Wilson. Unknown. Strid 20015 (MO)
Sect. Teutlioides
Ser. Euhalimus
A. halimus L. Belgium. Leonard 4698 (MO)
Ser. Austrohalimus
A. paludosa R. Br. Australia: Onxaparinza. Walter 40073 (K)
Sect. Teutliopsis
A. alaskensis S. Wats. USA: Alaska, Kadiak Island. Trelease & Sounderson 3623 (MO)
A. calotheca (Rafn.) Fries. Unknown. Nannfeldt 13459 (MO)
A. littoralis L. Unknown. Zabel s.n. (BH)
Endolepis dioica (Nutt.) Standl. USA. Stephens 66048 (NY)
Exomis microphylla (Thunb.) Aellen. Africa: Cape Region. Boucher 3991 (MO)
Halimione pedunculata (L.) Aellen. Sweden: Skåne, Hököpinge. Westling s.n. (NY)
Haloxanthium quadrivalvatum (Diels) Ulbr. Australia: Lake Eyre Basin. Ising s.n. (NY)
Manochlamys albicans (Soland. in Ait.) Aellen. Africa: Prov. Cape Region. Hanckon 2496 (K)
Microgynoecium tibeticum Hook. f. China: Karakorum and Kunlun Mountains. Wu et al. 3061 (MO)
Neopreissia isatidea (Moq.) Ulbr. Australia. Sauer 3466 (MO)
Obione
Subgen. Euobione
Sect. Atripliceae
Ser. Annuae
O. flabellum (Bunge ex Boiss.) Ulbr. Unknown. Roshevitz s.n. (GH)
O. sibirica (L.) Fisch. China: Inner Mongolia. Near Wulashan, Chungt'an. Hsia 3151 (NY)
Ser. Perennes
O. leucoclada (Boiss.) Ulbr. Africa: Egypt. Wissa & Nabih 3392 (MO)
O. verrucifera (M. Bief.) Moq. Iran: Lake Rezaiyeh. Furse 3519 (K)
Ser. Annuae neogaeae
O. cordulata (Jeps.) Ulbr. USA: California. Howell & True 44007 (MO)
O. elegans Moq. USA: Nevada, Clark Co. Moapa Valley. Pinzl 10950 (NY)
O. fruticulosa (Jeps.) Ulbr.USA: California. Howell 24286 (MO)
O. leucophylla Moq. USA: California, San Mateo County, Princeton. Rose 41460 (BH)
O. linifolia (Willd.) Moq. Mexico: Durango. Palmer 495 (NY)
O. microcarpa Benth. Mexico: Isla Cedros. Moran 18425 (ENCB)
O. powellii (S. Wats.) Ulbr. USA: New Mexico. 9 mile south of Gallup, route to Zuni. Fergunson & Ottley 5526 (NY)
O. pusilla Torr. ex S. Wats. USA: Nevada, Dixie Valley. Tiehm 6022 (MO)
O. bracteosa Durand et Hilg. USA: California. Wolf 3790 (RSA)
O. truncata Torr. ex S. Wats. USA: Nevada, Lahontan Valley. Tiehm 7521 (MO)
Sect. Deserticola
O. barclayana Benth. Mexico: Baja California Norte. Lewis s.n. (NY)
A. deserticola Phil. Chile: Atacama. Werderman 985 (GH)
O. hymenelytra Torr. USA. Martineau s.n. (BH)
O. julacea (S. Wats.) Ulbr.Mexico: Baja California. Thorne et al. 5865 (NY)
O. lampa (Gillies) Moq. Argentina: Prov. Mendoza. Half way from Alto Verde to Santa Rosa. Bartlett 20667 (NY)
O. polycarpa Torr. USA: California. Inyo Co. Peterson 691 (NY)
Sect. Lomobione
O. rotundifolia Moq. Bolivia. Beck 2360 (NY)
Subgen. Pterochiton
O. canescens (Pursh.) Moq. USA. Wiegand 473 (BH)
Proatriplex pleiantha (W.A. Weber) Stutz & Chu. USA: New Mexico, San Juan Co. Spellemberg 9497 (NY)
Spinacia oleracea L. China. Steward 1981 (MO)
Suckleya suckleyana (Torr.) Rydb. USA: Texas. Randall Co. Higgins 12376 (NY)
Theleophyton billardierei (Moq.) Moq. in DC. Australia: Tasmania, Bond Bay. Davis 1305 (GH)
Subtribe: Eurotiinae
Axyris amaranthoides L. Russia: Siberia. Mameev 451 (K)
Ceratocarpus arenarius L. Russia: Siberia. Kpachocopos N448 (MO)
Grayia brandegei Gray. USA: Utah. Garfield Co. Collotzi 413 (NY);
G. spinosa (Hook.) Moq. USA. Erther 1413 (NY)
Krascheninnikovia lanata (Pursh) A. Meeuse & A. Smit. USA: New Mexico. Crosby 14693 (MO)
Acknowledgements
We would like to express our gratitude to the curators of BH, ENCB, GH, K, MO, NY and RSA for permission to remove pollen from specimens in their collections. Berenit Mendoza also assisted with some SEM micrographs. Thomas Borsch, Zachary S. Rogers, Stefan Vinckier, Helga Ochoterena and Madeline Harley provided valuable comments, which greatly improved the paper. Helga Ochoterena, Julio César Montero Rojas, Pedro Mercado and Patricia Hernández helped with the preparation of Figures.
References
- Aellen , P. 1938 . Revision der australischen und neuseeländischen Chenopodiaceen. . Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. , 68 : 345 – 434 .
- Aellen , A. 1939 . Exomis und Manochlamys in Südafrika. . Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. , 70 : 373 – 381 .
- Bassett , I. J. and Crompton , C. W. 1973 . The genus Atriplex (Chenopodiaceae) in Canada and Alaska. III. Three hexaploid annuals: A. subspicata, A. gmelinii, and A. alaskensis. . Can. J. Bot. , 51 : 1715 – 1723 .
- Bassett , I. J. , Crompton , C. W. , McNeill , J. and Taschereau , P. M. 1983 . The genus Atriplex (Chenopodiaceae) in Canada , Ottawa : Com. Branch, Agricult . Canada, Monogr. 31
- Borsch , T. 1998 . Pollen types in the Amaranthaceae. Morphology and evolutionary significance. . Grana , 37 : 129 – 142 .
- Borsch , T. and Wilde , V. 2000 . “ Pollen variability within species, populations, and individuals, with particular reference to Nelumbo. ” . In Pollen and spores: Morphology and biology , Edited by: Harley , M. M , Morton , C. M and Blackmore , S . 285 – 299 . London (Kew) : R. Bot. Gard .
- Chu , G. L. 1987 . Archiatriplex, a new Chenopodiaceous genus from China. . J. Arnold Arbor. , 68 : 461 – 469 .
- Chu , G. L. , Stutz , H. C. and Sanderson , S. C. 1991 . Morphology and taxonomic position of Suckleya suckleyana (Chenopodiaceae). . Am. J. Bot. , 78 : 63 – 68 .
- Cuénoud , P. , Savolainen , V. , Chatrou , L. W. , Powell , M. , Grayer , R. J. and Chase , M. W. 2002 . Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. . Am. J. Bot. , 89 : 132 – 144 .
- Downie , S. R. , Katz‐Downie , D. S. and Cho , K. 1997 . Relationships in the Caryophyllales as suggested by phylogenetic analyses of partial chloroplast DNA ORF2280 homolog sequences. . Am. J. Bot. , 84 : 253 – 273 .
- Dyer , R. A. 1975 . The genera of Southern African flowering plants , Vol. I , Pretoria : Techn. Serv. Dept Agricult .
- Erdtman , G. 1960 . The acetolysis method. A revised description. . Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Flores Olvera , H. 1992 . Taxonomía del grupo Atriplex pentandra (Chenopodiaceae). . An. Inst. Biol. Univ. N. Autón. México, Ser. Bot. , 63 : 155 – 194 .
- Flores Olvera , H. and Mercado‐Ruaro , P. 1997 . Chromosome numbers of species of Atriplex section Obione (Chenopodiaceae) and their relation to taxonomy. . Cytologia (Tokyo) , 62 : 157 – 162 .
- Flores Olvera , H. and Davis , J. I. 2001 . A cladistic analysis of Atripliceae (Chenopodiaceae) based on morphological data. . J. Torrey Bot. Soc. , 128 : 297 – 319 .
- Frankton , C. and Bassett , I. J. 1970 . The genus Atriplex (Chenopodiaceae) in Canada. II. Four native western annuals: A. argentea, A. truncata, A. powelliii, and A. dioica. . Can. J. Bot. , 48 : 981 – 989 .
- Hall , H. M. and Clements , F. C. 1923 . The phylogenetic method in taxonomy. The genus Atriplex. Carnegie Inst. . 326 : 235 – 355 . Washington Publ.
- Hao , H. ‐P. , Zhang , J. ‐T. and Yan , S. 1989 . Scanning electron microscope observation on the pollen grains of Chenopodiaceae. . Acta Bot. Sin. , 31 : 650 – 652 .
- Holmgren , P. K , Holmgren , N. H and Barnett , L. C , eds. 1990 . “ Index Herbariorum. P. 1. ” . Bronx, NY : N.Y. Bot. Gard .
- Judd , W. S. and Ferguson , I. K. 1999 . The genera of Chenopodiaceae in the southeastern United States. . Harvard Pap. Bot. , 4 : 365 – 416 .
- Judd , W. S. , Campbell , C. S. , Kellogg , E. A. and Stevens , P. F. 1999 . Plant systematics: A phylogenetic approach , Sunderland : Sinauer Ass. Inc .
- Kadereit , G. , Borsch , T. , Weising , K. and Freitag , H. 2003 . Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. . Int. J. Pl. Sci. , 164 : 959 – 986 .
- Kühn , U. , Bittrich , V. , Carolin , R. , Freitag , H. , Hedge , I. C. , Uotila , P. and Wilson , P. G. 1993 . “ Chenopodiaceae. ” . In Families and genera of vascular plants , Edited by: Kubitzki , K , Rohwer , J. G and Bittrich , V . 253 – 281 . Berlin : Springer . Vol. II, Flowering plants, Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families
- McCune , B. and Mefford , M. J. 1999 . PC‐ORD. Multivariate analysis of ecological data , Gleneden Beach, OR : MjM Software Design . Vers. 4
- Moquin‐Tandon , A. 1840 . Chenopodearum monographica enumeratio , Paris : J.‐P. Loss .
- Müller , K. and Borsch , T. 2005 . Phylogenetics of Amaranthaceae based on matK/trnK sequence data – evidence from parsimony, likelihood, and Bayesian analyses. . Ann. Mo. Bot. Gard. , 92 : 66 – 102 .
- Nair , P. K. K. and Rastogi , K. 1966–67 . Pollen morphology of Indian Chenopodiaceae. . Palynol. Bull. (India) , 2–3 : 50 – 56 .
- Nowicke , J. W. 1975 . Pollen morphology in the order Centrospermae. . Grana , 15 : 51 – 77 .
- Nowicke , J. W. 1994 . “ Pollen morphology and exine ultrastructure. ” . In Caryophyllales. Evolution and systematics , Edited by: Behnke , H. D and Mabry , T. J . 167 – 221 . Berlin : Springer .
- Nowicke , J. W. and Skvarla , J. J. 1979 . Pollen morphology: The potential influence in higher order systematics. . Ann. Mo. Bot. Gard. , 66 : 633 – 700 .
- Rosas , M. R. 1989 . El genero Atriplex (Chenopodiaceae) en Chile. . Gayana, Bot. , 46 : 3 – 82 .
- Skvarla , J. J. and Nowicke , J. W. 1976 . Ultrastructure of pollen exine in centrospermous families. . Pl. Syst. Evol. , 126 : 55 – 78 .
- Stutz , H. C. , Sanderson , E. D. , McArthur , S. C. and Chu , G. ‐L. 1987 . Chromosome races of Grayia brandegei (Chenopodiaceae). . Madroño , 34 : 142 – 149 .
- Stutz , H. C. , Chu , G.‐L. and Sanderson , S. C. 1990 . Evolutionary studies of Atriplex: Phylogenetic relationships of Atriplex pleiantha. . Am. J. Bot. , 77 : 364 – 369 .
- Stutz , H. C. and Sanderson , S. C. 1979 . “ The role of polyploidy in the evolution of Atriplex canescens. ” . In Arid land plant resources , Edited by: Goodin , J. R and Northington , D. K . 615 – 621 . Lubbock, TX : Int. Cent. Arid Semi‐Arid Land Studs Tex. Tech. Univ .
- Tsukada , M. 1967 . Chenopod and amaranth Pollen: Electron‐Microscopic Identification. . Science , 157 : 80 – 82 .
- Ulbrich , E. 1960 . “ Chenopodiaceae. ” . In Die natürlichen Pflanzenfamilien , Edited by: Engler , A and Prantl , K . 379 – 584 . Berlin : Duncker & Humblot . 2nd ed. A. Engler & H. Harms Bd 16c
- Volkens , G. 1893 . “ Chenopodiaceae. ” . In Die natürlichen Pflanzenfamilien , Edited by: Engler , A and Prantl , K . 36 – 91 . Leipzig : W. Engelmann . Bd 3.1a
- Welsh , S. L. 1984 . Utah flora: Chenopodiaceae. . Great Basin Nat. , 44 : 183 – 209 .
- Wilson , P. G. 1984 . “ Chenopodiaceae. ” . In Flora of Australia. Vol. 4. Phytolaccaceae to Chenopodiaceae , Edited by: George , A. S . 81 – 330 . Canberra : Austral. Gov. Publ. Serv .
- Wodehouse , R. P. 1965 . Pollen grains , New York & London : Hafner Publ Co . (3rd print.)