Abstract
Allergic rhinitis is an increasing health problem and pollen allergens are amongst the main elicitors of hay fever symptoms. Allergenic pollen contains a set of differently allergenic proteins which are thought to play a role in the pollen germination and fertilisation process. They are released upon contact with the stigma or mucosa or upon pollen grain rupture. Although the determinants of allergenicity of these proteins are still largely undiscovered, accessibility and solubility are now thought to mainly influence allergenic potency. Pollen of 61 allergenic plants was investigated with scanning and transmission electron microscopy. Most of the minor allergenic plants like species of the families Salicaceae, Fagaceae or Ulmaceae show the typical pollen wall organisation with intine, compact endexine and ektexine whereas in the majority of the major allergenic plants like species of Betulaceae and Poaceae the endexine is not detectable. Ambrosia artemisiifolia exhibits a laminated endexine. In addition, pollen of these major allergenic plants does not have electron‐dense pollenkitt and starch is stored in a high proportion of the examined pollen. The question is raised whether pollen morphology and ultrastructure might contribute to the accessibility and therefore allergenicity of allergenic proteins.
Walls of allergenic pollen: Special reference to the endexine
Allergic rhinitis, or hay fever, is the most frequently occurring airway disease of our time. More than 25% of the population of industrialised countries (almost 500 million people) suffers from it and the incidence has more than doubled in the past three decades (Holgate & Church, Citation1995; Suck et al., Citation2000; Stumvoll et al., Citation2002; Colombo et al., Citation2003; Traidl‐Hoffmann et al., Citation2003; Swoboda et al., Citation2004). A type I allergy is a disorder of the immune system characterized by an overreaction to non‐hazardous proteins (Stumvoll et al., Citation2002). It is mainly due to inhalation of allergenic proteins from pollen, house dust mites or animal dander (Colombo et al., Citation2003). It occurs when allergens crosslink effector cell‐bound IgE antibodies in the mucosa of susceptible humans. This leads to the release of histamines, leukotrienes and other inflammatory mediators from the effector cells (mast cells, basophils, dentritic cells, eosinophils) that cause the typical symptoms of allergic rhinitis (Holgate & Church, Citation1995; Knox & Suphioglu, Citation1996a ; Venarske & de Shazo, Citation2003). These symptoms include vasodilatation (widening of the blood vessels) and hence resulting redness and swelling as well as increased mucus secretion (watery rhinorrhoea), itching and sneezing (Holgate & Church, Citation1995; Durham, Citation1998).
Allergenic plants are usually very common, widespread species that produce pollen in high quantity and they are mostly wind pollinated (Behrendt & Becker, Citation2001; Culley et al., Citation2002). The most important allergenic pollen in Europe belongs to members of the families of Betulaceae, Poaceae, Asteraceae, Oleaceae and Urticaceae.
Pollen allergens are water soluble, stable proteins or glycoproteins of molecular weight between 5 and 80 kDa (Knox & Suphioglu, Citation1996; Grote et al., Citation2000, Citation2005). Sequence comparison showed that they belong to only 29 of 2615 protein families found in seed plants including pathogenesis‐related proteins, profilins, expansins and calcium‐binding proteins (Radauer & Breiteneder, Citation2006; Chapman et al., Citation2007). As a consequence, many allergens show remarkable sequence similarities which cause a phenomenon known as cross‐reactivity. Cross‐reactivity leads to the development of allergic reactions to a source without previous exposure, due to the similarity of the IgE epitopes of the proteins (Wopfner et al., Citation2005). A quite common example is the oral‐allergy syndrome that occurs due to the similarity of pollen allergens with certain food proteins as found for birch pollen and Rosaceae fruit mainly caused by Bet v 1 related allergens and profilins (Valenta et al., Citation1996; Salcedo et al., Citation2004; Palacin et al., Citation2006). Grass pollen group 1 allergens also show extensive cross‐reactivity between grass species when they share more than 50% sequence identity (Chapman et al., Citation2007).
A single pollen type usually contains several allergens. In grass pollen, 11 groups of allergens have been described (Andersson & Lidholm, Citation2003; Petersen et al., Citation2006), eight to ten allergen groups were identified in Betula pendula Roth. (El‐Ghazaly et al., Citation1999; Mothes et al., Citation2004), ten in Olea europaea L. pollen (Alche et al., Citation2004), six in the pollen of Ambrosia artemisiifolia L. (Wopfner et al., Citation2005) and Parietaria judaica L. pollen was shown to contain at least nine allergenic proteins (Asturias et al., Citation2003). Some protein groups are thereby more frequently recognized by IgE of sensitised individuals than others. Major allergens are per definition recognized by IgE antibodies of at least 50% of patients allergic to the particular pollen type whereas less than 50% of IgE binding define minor allergens (Aalberse, Citation2000). The major group 1 and 5 allergens of grass pollen thus show prevalence of sensitisation of up to 90%, while minor group 12 allergens (profilins) are reported to only bind 15 to 30% of IgE (Andersson & Lidholm, Citation2003).
The extreme solubility and high accessibility, together with stability and size, are thought to determine the allergenicity of proteins since it enables them to cross the mucosal barriers (Vrtala et al., Citation1993; Aalberse, Citation2000; Castells et al., Citation2002). These properties and the resulting mobility and rapid dislocation upon hydration made it difficult to locate the allergens within the pollen grains using conventional aqueous fixation methods (Grote, Citation1999). More recent, strictly anhydrous fixation as well as cryofixation, however, demonstrated that a majority of major allergens mainly occur in the cytoplasm of dry pollen grains, in amyloplasts, sometimes associated with organelles and only occasionally in the pollen wall (Grote, Citation1999; Grote et al., 1994, Citation2000; Castells et al., Citation2002; Alche et al., Citation2004). Several studies, for example, showed that the major birch pollen allergen Bet v 1 is found almost exclusively in the cytoplasm (Grote et al., Citation1993; Vrtala et al., Citation1993; Taylor et al., Citation2004; Vinckier et al., Citation2006) whereas the minor allergen Bet v 7 additionally is located in the pollen wall (Vinckier et al., Citation2006). Upon hydration these proteins are released within minutes and can be found to a great extent in the pollen wall and the external exudates after only 0.5 to 1 minute (Vrtala et al., Citation1993; Castells et al., Citation2002; Grote et al., 1993, Citation2005; Suarez‐Cervera et al., Citation2005). Due to this rapid activation and release it is now generally agreed that most pollen allergens play a role in the fertilization process including pollen‐stigma recognition, adhesion between the pollen grain and a suitable stigma, transport, pollen tube formation or maternal tissue modification before pollen tube penetration (Cosgrove et al., Citation1997; El‐Ghazaly et al., Citation1999; Grote, Citation1999; Asturias et al., Citation2003; Vega‐Maray et al., Citation2004; Grote et al., Citation2005; Suarez‐Cervera et al., Citation2005). Release of these proteins also occurs when the pollen grain gets in contact with the moist and warm mucosa that imitates the stigma environment which then leads to the development of symptoms of allergic rhinitis (Casas et al., Citation1996; El‐Ghazaly et al., Citation1999; Grote, Citation1999; Castells et al., Citation2002; Grote et al., Citation2005). Another way of allergen release is via expulsion of the cytoplasmic content through the aperture or through rupture of the pollen grain upon contact with water (El‐Ghazaly et al., Citation1999; Grote et al., Citation2000, 2001, Citation2003; Taylor et al., Citation2004; Bacsi et al., Citation2006).
In this study, pollen of the most important Central European allergenic plants was examined using scanning (SEM) and transmission electron microscopy (TEM) especially focusing on pollen wall architecture to provide important morphological and ultrastructural information for further research focused on the possible role of the pollen wall in the release of allergens and on the mechanisms of protein storage and allergy induction.
Material and methods
For TEM, anthers of 61 minor and major allergenic plants collected during the pollen season 2004 in Austria were fixed for 6 hours in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and, after washing in buffer and distilled water, postfixed in 2% osmiumtetroxide (OsO4) and 0.8% potassium hexacyanoferrate (K4Fe(CN)6·3H2O) over night at 4°C. After fixation and washing in distilled water the material was dehydrated in 2,2‐dimethoxypropane (DMP) and acetone and embedded in Spurr's low‐viscosity epoxy resin (Spurr, Citation1969). Sections of about 70 – 90 nm were made on a Reichert‐Jung Ultracut S microtome with a diamond knife and transferred on copper and gold grids. Sections of all examined species were conventionally stained with uranyl acetate (U: Ultrostain 1, 705529, Leica, Vienna for 30 minutes) followed by lead citrate (Pb: Ultrostain 2, 705530, Leica, Vienna for 5 minutes) and a modified Thiéry‐test. For this short‐Thiéry test sections were treated with 1% periodic acid (PA) for 10 minutes, 0.2% thiocarbohydrazide (TCH) for 15 minutes and 1% silver proteinate (Sp) for 10 minutes (Weber & Frosch, Citation1995). Where those staining methods did not lead to satisfying results, additional Thiéry‐tests (PA for 30 minutes, TCH for 15 hours, Sp for 30 minutes) (Thiéry, Citation1967) and lipid‐tests (TCH for 15 hours, Sp for 30 minutes) (Rowley & Dahl, Citation1977) were applied. Furthermore 1% potassium permanganate (KMnO4, 7 minutes) showed good abilities to stain the endexine in particular. The sections were examined in a Zeiss EM 900 TEM at 50 kV.
For SEM, pollen was dehydrated in DMP and critical‐point dried in acetone (Halbritter, Citation1998). Samples were mounted on stubs and sputter‐coated with gold. For observations a Jeol T‐300 SEM was used at 10 kV.
Results
Observations included the most important Central European allergenic plants (species of Betulaceae, Poaceae, Oleaceae, Asteraceae, Urticaceae, Chenopodiaceae, Platanaceae, Brassicaceae) and many minor allergenic species (Salicaceae, Ulmaceae, Aceraceae, Tiliaceae, Polygonaceae, Fagaceae). From the 61 examined species only the most significant hay fever inducing plants were chosen for discussion. Scanning ‐ and transmission electron microscopic as well as light microscopic photographs of all 61 examined species, however, can be accessed through the Palynological Database of the Society for the Promotion of Palynological Research in Austria ( http://www.paldat.org ).
The pollen wall of many minor allergenic plants, like Salicaceae, Fagaceae or Ulmaceae, shows the typical organisation with intine, compact endexine and ektexine (Figure ). Also some of the most important allergenic families like Oleaceae (Figure ) and Platanaceae (Figure ), possess the same wall type. The endexine of Fraxinus excelsior L. and Platanus×hispanica Münchh. is thereby fragmented and laminated/granulose, respectively in the apertural region (Figure ). Another pollen wall type is characterised by the absence of a compact endexine (Figure ). A great proportion of the major allergenic plants belongs to this type with a discontinuous, hardly or not detectable endexine (Table ), including Betulaceae (Figure ), Poaceae (Figure ), Chenopodiaceae (Figure ) as well as Brassica napus L. emend. Metzg (Figure ). In Chenopodium album L. a discontinuous endexine might be distinguished (Figure ) but none of the staining methods applied gave a clear differentiation of this wall layer. The endexine of the observed Urticaceae is extremely thin and hardly detectable in TEM (not shown). Overall, in more than 50% of the examined 61 species the endexine is absent or discontinuous. The endexine of Ambrosia artemisiifolia appears laminated in TEM (Figure ). Microchannels are visible in the exine of the investigated Betulaceae (Figures and ) and Poaceae (Figure ).
The pollen grains of the examined species are usually quite small (20 – 40 µm) whereas the Urticaceae with a diameter of 15 µm produce smaller pollen than the average. Pollen grains of crop species like Triticum aestivum L. emend. Fiori et Paol., Secale cereale L., Zea mays L. and Avena sativa L. are the largest within the group of allergenic pollen (50 – 70 µm). Pollen of the examined allergenic plants mostly has round apertures (Figures and ) and the exine is simply sculptured. Ornamentation is more or less uniform within related taxa and comprises rugulate (Figure ), granulate (Figure ), microechinate (Figures and ) and more rarely echinate (Figure ) or reticulate (Figures and ) sculpture elements. Pollen coatings like pollenkitt are not as abundant in anemophilous species as in insect pollinated plants (Table ) where they often almost seal the pollen grain surface (Figure ). Neither the examined Betulaceae (Figure ), Poaceae (Figure ), Fagaceae nor Urticaceae have any kind of obvious pollen coating although small amounts of electron‐lucent pollenkitt can occasionally be observed. Notable amounts of electron‐dense pollenkitt can be found in the observed Oleaceae, Salicaceae and Chenopodiaceae, the last also storing primexine‐matrix in the exine cavities (Figure ). Brassica napus is the only species examined that has tryphine (Figure ). A very high proportion of the examined species (75%) stores starch within the pollen grains (Table ).
Figure 1 Pollen grains representing different wall characteristics.A. Salix fragilis. Typical pollen wall organisation with intine, compact endexine and columellate ektexine. U+Pb. B. Corylus avellana. Pollen wall with intine and ektexine. Endexine not detectable, microchannels in ektexine, TCH+Sp (lipid‐test). C. Fraxinus excelsior. Microchannels within the endexine and intine of the apertural region. TCH+Sp (lipid‐test). D. Platanus×hispanica. Endexine laminated/granulose in apertural region. PA+TCH+Sp (short). E. Syringa vulgaris. Ektexine cavities completely sealed by electron‐dense pollenkitt. Endexine compact. PA+TCH+Sp (short). F. Quercus robur. Little electron‐lucent pollenkitt within exine. Endexine compact. TCH+Sp (lipid‐test). A–F. TEM (ekt = ektexine, end = endexine, i = intine, p = pollenkitt).
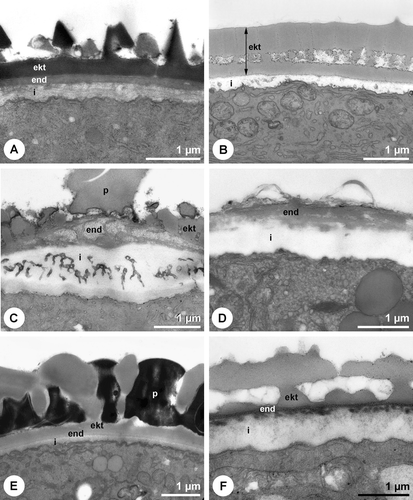
Figure 2 Pollen walls without compact or detectable endexine.A. Betula sp. Overall view of the birch pollen grain. B. Betula sp. Cross section of pollen grain wall. Microchannels within the ektexine. TCH+Sp (lipid‐test). C. Secale cereale. Overall view of the rye pollen grain. D. Secale cereale. Cross section of pollen grain wall. Microchannels within the ektexine. PA+TCH+Sp (short). E. Phleum pratense. Overall view of the grass pollen grain. F. Phleum pratense. Cross section of pollen grain wall. Microchannels within the ektexine. PA+TCH+Sp (short). A, C & E. SEM. B, D & F. TEM (ekt = ektexine, i = intine).
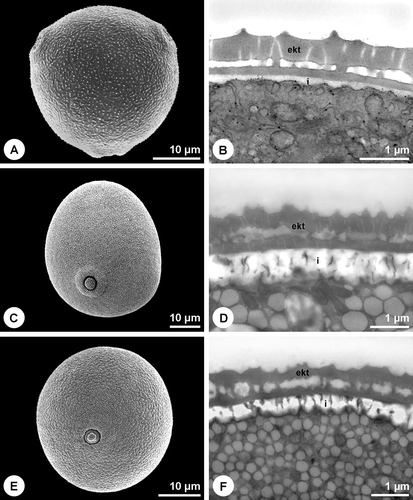
Figure 3 Pollen walls without compact or detectable endexine(continued). A. Chenopodium album. Overall view of the goosefoot pollen grain. B. Chenopodium album. Cross section of pollen grain wall. Endexine discontinuous. Primexine‐matrix in ektexine cavities (pm). PA+TCH+Sp. C. Brassica napus. Overall view of the rape pollen grain. D. Brassica napus. Cross section of pollen grain wall. Tryphine in ektexine cavities. PA+TCH+Sp (short). A, C. SEM. B, D. TEM (ekt = ektexine, (end) = discontinuous endexine, i = intine, tr = tryphine).
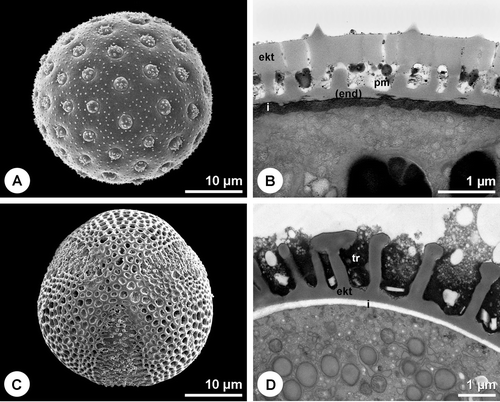
Figure 4 Pollen walls with compact endexine.A. Fraxinus excelsior. Overall view of the ash pollen grain. B. Fraxinus excelsior. Cross section of pollen grain wall. Compact endexine. TEM, PA+TCH+Sp (short). C. Platanus×hispanica. Overall view of the plane pollen grain. D. Platanus×hispanica. Cross section of pollen grain wall. Compact endexine. PA+TCH+Sp (short). E. Ambrosia artemisiifolia. Overall view of the ragweed pollen grain. F. Ambrosia artemisiifolia. Cross section of pollen grain wall. Laminated endexine. KMnO4. A, C & E. SEM. B, D & F. TEM (ekt = ektexine, end = endexine, i = intine).
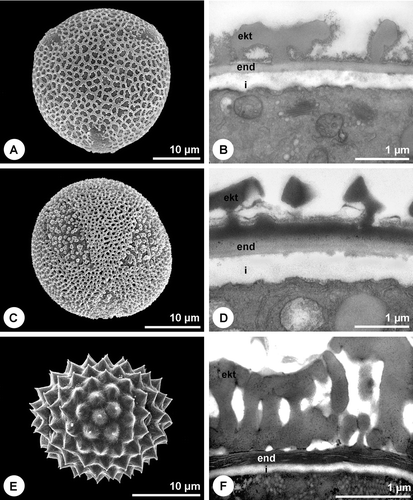
Table I. Most important hayfever inducing species, their allergenicity (according to the Austrian Pollen Forecast, http://www.pollenwarndienst.at ), pollen wall characteristics, types of pollen coating and reserve materials, respectively.
Discussion
The majority of allergenic plants are wind pollinated. Thus, the adaptation to wind pollination determines the morphological characters of a great proportion of the examined pollen grains. To ensure broad dispersal, pollen of anemophilous plants is small, the apertures are round (porus or ulcus), ornamentation is simple and pollenkitt is reduced (Nilsson & Praglowski, Citation1992; Culley et al., Citation2002). The observations of this study confirm the anemophilous pollen morphology of most of the major allergenic plants. This, however, does not necessarily mean that only wind pollinated species produce proteins that are able to cause a disproportionate reaction of the human immune system. It is mainly the availability of the pollen grains and consequently of the allergenic proteins in the ambient air that contributes to the allergenicity of these species. Pollen of insect pollinated plants very rarely occurs freely in the air. Furthermore entomophilous plants produce far less pollen than anemophilous ones (Nilsson & Praglowski, Citation1992). Hence the probability that pollen of wind pollinated plants reaches the human airways is higher.
The reason why some pollen species are highly allergenic while others only exhibit minor or no allergenicity is still mostly unknown. It is, however, thought that high accessibility of the proteins determines the allergenicity to a significant degree (Vrtala et al., Citation1993; Aalberse et al., Citation2000; Castells et al., Citation2002; Hoidn et al., Citation2005). By comparing pollen of Artemisia vulgaris L. and Lilium longiflorum Thunb., Hoidn et al. (Citation2005) found that a fast release of total protein is not restricted to allergenic plants but is a general feature of hydrating pollen. Since the released proteins have a vital function in the pollen germination and fertilization process these results have to be expected, but they also observed that mugwort pollen released four times more proteins than Lilium pollen (Hoidn et al., Citation2005). Furthermore Vrtala et al. (Citation1993) detected major allergens earlier and in larger amounts in the supernatant of hydrated pollen than minor allergens like profilin. Reasons for this could be manifold including a potential initial difference in protein expression and different properties of the proteins. However, the question also arises if the morphology and ultrastructure of the pollen grain and the sporoderm in particular has an effect on the accessibility of pollen proteins.
Pollen of three quarters of the examined species contains starch (Table ) which is very high compared to a study conducted by Baker and Baker (Citation1979) who found starch in 24% of about 990 examined species. The occurrence of amyloplasts within the pollen grains might contribute to the allergenicity since they have been shown to contain major allergens like Bet v 1 (El‐Ghazaly et al., Citation1999) and grass group 5 allergens (Grote et al., Citation1994; Staff et al., Citation1999) in allergenic pollen. Furthermore they are small enough to reach the lungs and cause allergic asthma when they are set free (Casas et al., Citation1996; El‐Ghazaly et al., Citation1999; Schäppi et al., Citation1999). The disproportionate appearance of starch in the examined pollen grains is thereby due to their dispersal by wind. The production and storage of starch is metabolically more economic. However, pollinators prefer oils or lipids. Pollen of anemophilous plants does not need to be alimentary since it is not dependent on biotic pollinators. Thus, the production of starch is another adaptation to wind pollination (Baker & Baker, Citation1979). Being anemophilous, however, many minor or non‐allergenic plants like Populus sp., Ulmus sp. or the ambophilous Acer sp. and Tilia sp. have also been found to possess amyloplasts which does not seem to enhance their allergenicity and studies about allergen location in minor allergenic plants are mostly lacking.
The results of the study show that most of the major allergenic plants (Betulaceae, Poaceae, Chenopodiaceae, Ambrosia artemisiifolia) lack homogenous, electron‐dense pollenkitt (Table ) which is another feature of anemophilous plants (Hesse, Citation1980). Pollenkitt consists mainly of lipids and therefore, in addition to clumping pollen grains together, it also might help to keep sporophytic proteins within the exine cavities (Dickinson, Citation1973; Pacini & Franchi, Citation1996). The lipidic, hydrophobic nature of this pollen coating may function as a barrier that water soluble proteins are not able to pass easily which also includes allergenic proteins of hay fever inducing plants.
Furthermore the very striking lack of a clearly distinguishable, compact endexine in a great proportion of plants with high allergenic potential (Table ) might contribute to the allergenic potency. Even though several staining methods were applied, an endexine could not be detected in the examined Betulaceae or Poaceae. Chenopodiaceae either exhibited no endexine or a discontinuous, hardly detectable one (Figure ). The exact function of this sporoderm layer is yet to be clarified. Speculations tended towards a function in transport between the gametophyte and intine, respectively and the environment (Rowley, Citation1995). Staining electron dense with TCH+Sp provides evidence for lipidic compounds occurring in the endexine (Weber et al., Citation1998). Thus, it could well function as a barrier instead of playing a role in transport. Comparable to the pollenkitt, it might be hydrophobic and may prevent hydrophilic proteins from being eluted too easily or/and too soon from the pollen grain wall. This means that pollen grains with an endexine might have a lower leaking rate of proteins than those that lack this special layer. The lack of a compact endexine is thereby not restricted to anemophilous allergenic plants. In the examined insect pollinated species Brassica napus, Juglans regia L. and Rumex acetosa L. the inner layer of the exine is not detectable either. Those species, however, are referred to as being of only moderate allergenicity but their allergenic potency is highest within entomophilous plants. This would mean that species with an endexine could produce the same amount of gametophytic allergenic proteins as species without this wall layer but, due to the properties of the endexine as barrier, part of those proteins are not released. Additionally the existence of pollenkitt would reduce the availability of sporophytic allergenic proteins. Thus, those two factors, existence/absence of a compact endexine and pollenkitt, could have an influence on the allergenicity.
There are, however, reports about inactivated but quite abundant pollenkitt in highly allergenic plants like certain grasses (Hesse, Citation1980) and Platanus acerifolia (Suarez‐Cervera et al., Citation1995). Furthermore lipids of the pollen exudates and pollen coatings themselves seem to play a crucial role in pollen hydration, cell‐cell recognition and pollen tube adhesion and navigation and are therefore also transferred upon hydration (Traidl‐Hoffmann et al., Citation2003). Non‐specific lipid transfer proteins have been shown to have the ability to transfer a broad range of lipids between membranes in vitro but have now been classified as pathogenesis‐related proteins (Colombo et al., Citation2003; Salcedo et al., Citation2004). Two major allergens of Parietaria (Par j 1 and Par j 2) as well as the allergens Art v 3 of Artemisia and Ole e 7 of Olea belong to this ubiquitous protein family which causes considerable cross‐reactivity between pollen and food like cabbage and Rosaceae fruits (Diaz‐Perales et al., Citation2000; Asturias et al., Citation2003; Vega‐Maray et al., Citation2004; Palacin et al., Citation2006; Tejera et al., Citation1999). Moreover, in an ontogenetic study El‐Ghazaly and Jensen (Citation1986) observed the formation of a rudimentary endexine in wheat pollen which was appressed until it was no longer detectable during the vacuolisation stage. This implies that highly allergenic pollen examined in this study could possess detectable endexine at least once during development. Furthermore pollen of some important allergenic plants examined in this study, like Oleaceae, Platanaceae and Ambrosia artemisiifolia does have a clearly distinguishable endexine. Also, the allergenic pollen of Zygophyllaceae and Cupressaceae species exhibits a compact endexine (Castells et al., Citation2002; Suarez‐Cervera et al., Citation2003). The massive endexine in the apertural region of Zygophyllum fabago L. is, however, crossed by irregular microchannels (Castells et al., Citation2002). Similar microchannels could be detected in this study in the endexine of the apertural region of Fraxinus excelsior (Figure ) and the ektexine of examined Betulaceae (Figures and ) and Poaceae (Figure ). Additionally the apertural endexine of Platanus is laminated (Figure ) as well as the interapertural endexine of Ambrosia artemisiifolia (Figure ). These microchannels and modifications could again enhance the allergenicity of these pollen species by allowing uninhibited diffusion.
The very special formation of the endexine of Ambrosia artemisiifolia, which is not compact but laminated, appears to be an unusual and therefore noticeable feature amongst Asteraceae and is also unique among the 61 allergenic plant species examined in this study. Laminated endexine was described by Van Campo and Lugardon (Citation1973) as being a typical feature of gymnosperm pollen. This was emphasized by many electron microscopic studies confirming the presence of a laminated endexine in many gymnosperm taxa such as Abies (Kurmann, Citation1989), Cunninghamia (Kurmann, Citation1990a ), Tsuga (Kurmann, Citation1990b ) and Cupressaceae (Kurmann, Citation1994). Since then, however, laminated endexine has been observed in several angiosperm species whereas in many cases they are only found underlying the apertures while an endexine is not detectable in the interapertural regions (Hesse, Citation2002; Doyle, Citation2005). There are, however, angiosperm species with continuously laminated endexine like Brasenia (Taylor & Osborn, Citation2006) and Ambrosia artemisiifolia as shown in this study. While the endexine lamellae are compressed and hardly distinguishable in the interapertural regions in Brasenia, they are distinct in Ambrosia.
Conclusion
The morphology as well as the ultrastructure of pollen of allergenic plants is influenced to a high degree by the anemophily of these species. Several features of the pollen might thereby have the potential to influence its allergenicity. Starch granules loaded with major allergens as well as microchannels in endexine and ektexine facilitating allergen transit across the pollen grain wall upon hydration could enhance allergenicity. The less abundant pollenkitt and less compact or hardly detectable endexine often encountered in allergenic pollen could also favour higher accessibility of those proteins. There are, however, various arguments against a role of the pollenkitt and the endexine in influencing allergenicity. Nevertheless a barrier function of these layers cannot completely be dismissed and further research will be required to investigate if pollen ultrastructure can alter the allergenicity of the pollen grain or if the allergenicity is mainly due to the expressed amount of allergenic protein and allergen properties like water solubility.
Specimens investigated
Acer negundo L. Austria. Diethart & Sam, Herbarium WU Vienna
Acer platanoides L. Austria. Diethart & Sam, Herbarium WU Vienna
Acer pseudoplatanus L. Austria. Diethart & Sam, Herbarium WU Vienna
Acer saccharinum L. Austria. Diethart & Sam, Herbarium WU Vienna
Aesculus×carnea Hayne. Austria. Diethart & Sam, Herbarium WU Vienna
Aesculus hippocastanum L. Austria. Diethart & Sam, Herbarium WU Vienna
Ailanthus altissima (Mill.) Swingle. Austria. Diethart & Sam, Herbarium WU Vienna
Alnus glutinosa L. Austria. Sam, Herbarium WU Vienna
Alopecurus pratensis L. Austria. Diethart & Sam, Herbarium WU Vienna
Ambrosia artemisiifolia L. Austria. Diethart & Sam, Herbarium WU Vienna
Apera spica‐venti L. Austria. Diethart & Sam, Herbarium WU Vienna
Artemisia dracunculus L. Austria. Diethart & Sam, Herbarium WU Vienna
Artemisia vulgaris L. Austria. Diethart & Sam, Herbarium WU Vienna
Atriplex patula L. Austria. Diethart & Sam, Herbarium WU Vienna
Atriplex sagittata Borkh. Austria. Diethart & Sam, Herbarium WU Vienna
Atriplex tatarica L. Austria. Diethart & Sam, Herbarium WU Vienna
Avena sativa L. Austria. Diethart, Herbarium WU Vienna
Avenula pubescens Huds. Austria Diethart & Sam, Herbarium WU Vienna
Betula humilis Schrank. Austria. Diethart & Sam, Herbarium WU Vienna
Betula pendula Roth. Austria. Diethart, Herbarium WU Vienna
Brassica napus L. emend. Metzg. Austria. Diethart, Herbarium WU Vienna
Bromus inermis Leyss. Austria. Diethart, Herbarium WU Vienna
Carpinus betulus L. Austria. Sam, Herbarium WU Vienna
Castanea sativa Mill. Austria. Diethart & Sam, Herbarium WU Vienna
Chenopodium album L. Austria. Diethart & Sam, Herbarium WU Vienna
Chenopodium hybridum L. Austria. Diethart & Sam, Herbarium WU Vienna
Corylus avellana L. Austria. Diethart, Herbarium WU Vienna
Corylus colurna L. Austria. Diethart & Sam, Herbarium WU Vienna
Elymus repens L. Austria. Diethart & Sam, Herbarium WU Vienna
Festuca pratensis Huds. Austria. Diethart & Sam, Herbarium WU Vienna
Fraxinus excelsior L. Austria. Diethart & Sam, Herbarium WU Vienna
Fraxinus ornus L. Austria. Diethart, Herbarium WU Vienna
Juglans regia L. Austria. Sam, Herbarium WU Vienna
Kochia scoparia (L.) Schrad. Austria. Diethart & Sam, Herbarium WU Vienna
Parietaria officinalis L. Austria. Diethart & Sam, Herbarium WU Vienna
Phleum pratense L. Austria. Diethart & Sam, Herbarium WU Vienna
Platanus×hispanica Münchh. Austria. Diethart & Sam, Herbarium WU Vienna
Poa angustifolia L. Austria. Diethart & Sam, Herbarium WU Vienna
Populus alba L. Austria. Diethart & Sam, Herbarium WU Vienna
Populus nigra L. Austria. Diethart & Sam, Herbarium WU Vienna
Populus tremula L. Austria. Diethart & Sam, Herbarium WU Vienna
Quercus cerris L. Austria. Sam, Herbarium WU Vienna
Quercus robur L. Austria. Diethart & Sam, Herbarium WU Vienna
Robinia pseudacacia L. Austria. Diethart, Herbarium WU Vienna
Rumex acetosa L. Austria. Diethart & Sam, Herbarium WU Vienna
Rumex obtusifolius L. Austria. Diethart & Sam, Herbarium WU Vienna
Salix alba L. Austria. Diethart & Sam, Herbarium WU Vienna
Salix fragilis L. Austria. Diethart & Sam, Herbarium WU Vienna
Salix purpurea L. Austria. Diethart & Sam, Herbarium WU Vienna
Sambucus nigra L. Austria. Diethart & Sam, Herbarium WU Vienna
Secale cereale L. Austria. Diethart, Herbarium WU Vienna
Solidago canadensis L. Austria. Diethart, Herbarium WU Vienna
Syringa vulgaris L. Austria. Diethart & Sam, Herbarium WU Vienna
Tilia euchlora K. Koch. Austria. Diethart & Sam, Herbarium WU Vienna
Tilia tomentosa Moench. Austria. Diethart & Sam, Herbarium WU Vienna
Trisetum flavescens (L.) P. Beauv. Austria. Diethart & Sam, Herbarium WU Vienna
Triticum aestivum L. emend. Fiori et Paol. Austria. Diethart, Herbarium WU Vienna
Ulmus laevis Pall. Austria. Diethart & Sam, Herbarium WU Vienna
Ulmus minor Mill. Austria. Diethart & Sam, Herbarium WU Vienna
Urtica dioica L. Austria. Diethart, Herbarium WU Vienna
Zea mays L. Austria. Diethart, Herbarium WU Vienna
References
- Aalberse , R. C. 2000 . Structural biology of allergens. . J. Allergy Immunol. , 106 : 228 – 238 .
- Alche , J. , M'rani‐Alaoui , M. , Castro , A. J. and Rodriguez‐Garcia , M. I. 2004 . Ole e 1, the major allergen from olive (Olea europaea L.) pollen increases its expression and is released to the culture medium during in vitro germination. . Pl. Cell Physiol. , 45 : 1149 – 1157 .
- Andersson , K. and Lidholm , J. 2003 . Characteristics and immunobiology of grass pollen allergens. . Int. Arch. Allergy Immunol. , 130 : 87 – 107 .
- Asturias , J. A. , Gomez‐Bayon , N. , Eseverri , J. L. and Martinez , A. 2003 . Par j 1 and Par j 2, the major allergens from Parietaria judaica pollen, have similar immunoglobulin E epitopes. . Clin. Exp. Allergy , 33 : 518 – 524 .
- Bacsi , A. , Choudhury , B. K. , Dharajiya , N. , Sur , S. and Boldogh , I. 2006 . Subpollen particles: Carriers of allergenic proteins and oxidases. . J. Allergy Immunol. , 118 : 844 – 850 .
- Baker , H. G. and Baker , I. 1979 . Starch in angiosperm pollen grains and its evolutionary significance. . Am. J. Bot. , 66 : 591 – 600 .
- Behrendt , H. and Becker , W. M. 2001 . Localization, release and bioavailability of pollen allergens: The influence of environmental factors. . Curr. Opinion Immunol. , 13 : 709 – 715 .
- Casas , C. , Marquez , J. , Suarez‐Cervera , M. and Seoane‐Camba , J. 1996 . Immunocyto‐chemical localization of allergenic proteins in Parietaria judaica L. (Urticaceae) pollen grains. . Eur. J. Cell Biol. , 70 : 179 – 188 .
- Castells , T. , Arcalis , E. , Moreno‐Grau , S. , Bayo , J. , Elvira‐Rendueles , B. , Bechi , J. , Seoane‐Camba , J. and Suarez‐Cervera , M. 2002 . Immunocytochemical localization of allergenic proteins from mature to activated Zygophyllum fabago L. (Zygophyllaceae) pollen grains. . Eur. J. Cell Biol. , 81 : 107 – 115 .
- Chapman , M. D. , Pomes , A. , Breiteneder , H. and Ferreira , F. 2007 . Nomenclature and structural biology of allergens. . J. Allergy Immunol. , 119 : 414 – 420 .
- Colombo , P. , Bonura , A. , Assunta Costa , M. , Izzo , V. , Passantino , R. , Locorotondo , G. , Amoroso , S. and Geraci , D. 2003 . The allergens of Parietaria. . Int. Arch. Allergy Immunol. , 130 : 173 – 179 .
- Cosgrove , D. J. , Bedinger , P. and Durachko , D. M. 1997 . Group 1 allergens of grass pollen as cell wall‐loosening agents. . Proc. Natl. Acad. Sci. USA , 94 : 6559 – 6564 .
- Culley , T. M. , Weller , S. G. and Sakai , A. K. 2002 . The evolution of wind pollination in angiosperms. . Tr. Ecol. Evol. , 17 : 361 – 369 .
- Diaz‐Perales , A. , Lombardero , M. , Sanchez‐Monge , R. , Garcia‐Selles , F. J. , Pernas , M. , Fernandez‐Rivas , M. , Barber , D. and Salcedo , G. 2000 . Lipid‐transfer proteins as potential plant panallergens: Cross‐reactivity among proteins of Artemisia pollen, Castanea nut and Rosaceae fruits with different IgE‐binding capacities. . Clin. Exp. Allergy , 30 : 1403 – 1410 .
- Dickinson , H. G. 1973 . The role of plastids in the formation of pollen grain coatings. . Cytobios , 8 : 25 – 40 .
- Doyle , J. A. 2005 . Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. . Grana , 44 : 227 – 251 .
- Durham , S. R. 1998 . The inflammatory nature of allergic disease. . Clin. Exp. Allergy , 28 : 20 – 24 .
- El‐Ghazaly , G. and Jensen , W. A. 1986 . Studies of the development of wheat (Triticum aestivum) pollen: I. Formation of the pollen wall and Ubisch bodies. . Grana , 25 : 1 – 29 .
- El‐Ghazaly , G. , Moate , R. , Cresti , M. , Walles , B. , Takahashi , Y. , Ferreira , F. and Obermeyer , G. 1999 . Localization and release of allergens from tapetum and pollen grains of Betula pendula. . Protoplasma , 208 : 37 – 46 .
- Grote , M. 1999 . In situ localization of pollen allergens by immunogold electron microscopy: Allergens at unexpected sites. . Int. Arch. Allergy Immunol. , 118 : 1 – 6 .
- Grote , M. , Vrtala , S. and Valenta , R. 1993 . Monitoring of two allergens, Bet v 1 and profilin, in dry and rehydrated birch pollen by immunogold electron microscopy and immunoblotting. . J. Histochem. Cytochem. , 41 : 745 – 750 .
- Grote , M. , Valenta , R. and Reichelt , R. 2003 . Abortive pollen germination: A mechanism of allergen release in birch, alder and hazel revealed by immunogold electron microscopy. . J. Allergy Immunol. , 111 : 1017 – 1023 .
- Grote , M. , Dolecek , C. , van Ree , R. and Valenta , R. 1994 . Immunogold electron microscopic localization of timothy grass (Phleum pretense) pollen major allergens Phl p I and Phl p V after anhydrous fixation in acrolein vapor. . J. Histochem. Cytochem. , 42 : 427 – 431 .
- Grote , M. , Swoboda , I. , Valenta , R. and Reichelt , P. 2005 . Group 13 allergens as environmental and immunological markers for grass pollen allergy: Studies by immunogold field emission scanning and transmission electron microscopy. . Int. Arch. Allergy Immunol. , 136 : 303 – 310 .
- Grote , M. , Vrtala , S. , Niederberger , V. , Valenta , R. and Reichelt , R. 2000 . Expulsion of allergen‐containing materials from hydrated rye grass (Lolium perenne) pollen revealed by using immunogold field emission scanning and transmission electron microscopy. . J. Allergy Immunol. , 105 : 1140 – 1145 .
- Grote , M. , Vrtala , S. , Niederberger , V. , Wiermann , R. , Valenta , R. and Reichelt , R. 2001 . Release of allergen‐bearing cytoplasm from hydrated pollen: A mechanism common to a variety of grass (Poaceae) species revealed by electron microscopy. . J. Allergy Immunol. , 108 : 109 – 115 .
- Halbritter , H. 1998 . Preparing living pollen material for Scanning Electron Microscopy using 2,2‐Dimethoxypropane (DMP) and Critical‐Point Drying. . Biotech. Histochem. , 73 : 137 – 143 .
- Hesse , M. 1980 . Entwicklungsgeschichte und Ultrastruktur von Pollenkitt und Exine bei nahe verwandten entomophilen und anemophilen Angiospermensippen der Alismataceae, Liliaceae, Juncaceae, Cyperaceae, Poaceae und Araceae. . Pl. Syst. Evol. , 134 : 229 – 267 .
- Hesse , M. 2002 . The uniquely designed pollen aperture in Lasioideae (Araceae). . Aroideana , 25 : 51 – 59 .
- Hoidn , C. , Puchner , E. , Pertl , H. , Holztrattner , E. and Obermeyer , G. 2005 . Nondiffusional release of allergens from pollen grains of Artemisia vulgaris and Lilium longiflorum depends mainly on the type of the allergen. . Int. Arch. Allergy Immunol. , 137 : 27 – 36 .
- Holgate , S. T. and Church , M. K. 1995 . Allergy , London : Mosby‐Wolfe .
- Knox , R. B. and Suphioglu , C. 1996 . Environmental and molecular biology of pollen allergens. . Tr. Pl. Sci. , 1 : 156 – 164 .
- Kurmann , M. H. 1989 . Pollen wall formation in Abies concolor and a discussion on wall layer homologies. . Can. J. Bot. , 67 : 2489 – 2504 .
- Kurmann , M. H. 1990a . Exine formation in Cunninghamia lanceolata (Taxodiaceae). . Rev. Palaeobot. Palynol. , 64 : 175 – 179 .
- Kurmann , M. H. 1990b . Development of the pollen wall in Tsuga canadensis (Pinaceae). . Nord. J. Bot. , 10 : 63 – 78 .
- Kurmann , M. H. 1994 . Pollen morphology and ultrastructure in the Cupressaceae. . Acta Bot. Gall. , 141 : 141 – 147 .
- Mothes , N. , Horak , F. and Valenta , R. 2004 . Transition from a botanical to a molecular classification in tree pollen allergy: Implications for diagnosis and therapy. . Int. Arch. Allergy Immunol. , 135 : 357 – 373 .
- Nilsson , S. and Praglowski , J. 1992 . Erdtman's Handbook of Palynology , Copenhagen : Munksgaard . (2nd ed.)
- Pacini , E. and Franchi , G. G. 1996 . Some cytological, ecological and evolutionary aspects of pollination. . Acta Soc. Bot. Pol. , 65 : 11 – 16 .
- Palacin , A. , Cumplido , J. , Figeroa , J. , Ahrazem , O. , Sanchez‐Monge , R. , Carillo , T. , Salcedo , G. and Blanco , C. 2006 . Cabbage lipid transfer protein Bra o 3 is a major allergen responsible for cross‐reactivity between plant foods and pollens. . J. Allergy Immunol. , 117 : 1423 – 1429 .
- Petersen , A. , Suck , R. , Lindner , B. , Georgieva , D. , Ernst , M. , Notbohm , H. , Wicklein , D. , Cromwell , O. and Becker , W.‐M. 2006 . Phl p 3: Structural and immunological characterization of a major allergen of timothy grass pollen. . Clin. Exp. Allergy , 36 : 840 – 849 .
- Radauer , C. and Breiteneder , H. 2006 . Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. . J. Allergy Immunol. , 117 : 141 – 147 .
- Rowley , J. R. 1995 . Are the endexines of pteridophytes, gymnosperms and angiosperms structurally equivalent? . Rev. Palaeobot. Palynol. , 85 : 13 – 34 .
- Rowley , J. R. and Dahl , A. O. 1977 . Pollen development in Artemisia vulgaris with special reference to glycocalyx material (1). . Pollen Spores , 19 : 169 – 184 .
- Salcedo , G. , Sanchez‐Monge , R. , Diaz‐Perales , A. , Garcia‐Casado , G. and Barber , D. 2004 . Plant non‐specific lipid transfer proteins as food and pollen allergens. . Clin. Exp. Allergy , 34 : 1336 – 1341 .
- Schäppi , G. F. , Taylor , P. E. , Pain , M. C. F. , Cameron , P. A. , Dent , A. W. , Staff , I. A. and Suphioglu , C. 1999 . Concentrations of major grass group 5 allergens in pollen grains and atmospheric particles: Implications for hay fever and allergic asthma sufferers sensitized to grass pollen allergens. . Clin. Exp. Allergy , 29 : 633 – 641 .
- Spurr , A. R. 1969 . A low‐viscosity epoxy resin embedding medium for electron microscopy. . J. Ultrastr. Res. , 26 : 31 – 43 .
- Staff , I. A. , Schäppi , G. and Taylor , P. E. 1999 . Localization of allergens in ryegrass pollen and in airborne micronic particles. . Protoplasma , 208 : 47 – 57 .
- Stumvoll , S. , Lidholm , J. , Thunberg , R. , DeWitt , A. M. , Eibensteiner , P. , Swoboda , I. , Bugajska‐Schretter , A. , Spitzauer , S. , Vangelista , L. , Kazemi‐Shirazi , L. , Sperr , W. R. , Valent , P. , Kraft , D. and Valenta , R. 2002 . Purification, structural and immunological characterization of a timothy grass (Phleum pratense) pollen allergen, Phl p 4, with cross‐reactive potential. . Biol. Chem. , 383 : 1383 – 1396 .
- Suarez‐Cervera , M. , Marquez , J. and Seaone‐Camba , J. 1995 . Pollen grain and Ubisch body development in Platanus acerifolia. . Rev. Palaeobot. Palynol. , 85 : 63 – 84 .
- Suarez‐Cervera , M. , Takahashi , Y. , Vega‐Maray , A. and Seoane‐Camba , J. A. 2003 . Immunocytochemical localization of Cry j 1, the mayor allergen of Cryptomeria japonica (Taxodiaceae) in Cupressus arizonica and C. sempervirens (Cupressaceae) pollen grains. . Sex. Pl. Repr. , 16 : 9 – 15 .
- Suarez‐Cervera , M. , Asturias , J. A. , Vega‐Maray , A. , Castells , T. , Lopez‐Iglesias , C. , Ibarrola , I. , Arilla , M. C. , Gabarayeva , N. and Seaone‐Camba , J. A. 2005 . The role of allergenic proteins Pla a 1 and Pla a 2 in the germination of Platanus acerifolia pollen grains. . Sex. Pl. Repr. , 18 : 101 – 112 .
- Suck , R. , Hagen , S. , Cromwell , O. and Fiebig , H. 2000 . The high molecular mass allergen fraction of timothy grass pollen (Phleum pratense) between 50–60 kDa is comprised of two major allergens: Phl p 4 and Phl p 13. . Clin. Exp. Allergy , 30 : 1395 – 1402 .
- Swoboda , I. , Grote , M. , Verdino , P. , Keller , W. , Singh , M. B. , De Weerd , N. , Sperr , W. R. , Valent , P. , Balic , N. , Reichelt , R. , Suck , R. , Fiebig , H. , Valenta , R. and Spitzauer , S. 2004 . Molecular characterization of polygalacturonases as grass pollen‐specific marker allergens: Expulsion from pollen via submicronic respirable particles. . J. Allergy Immunol. , 104 : 797 – 802 .
- Taylor , M. L. and Osborn , J. M. 2006 . Pollen ontogeny in Brasenia (Cabombaceae, Nympheales). . Am. J. Bot. , 93 : 344 – 356 .
- Taylor , P. E. , Flagan , R. C. , Miguel , A. G. , Valenta , R. and Glovsky , M. M. 2004 . Birch pollen rupture and the release of aerosol of respirable allergens. . Clin. Exp. Allergy , 34 : 1591 – 1596 .
- Tejero , M. L. , Villalba , M. , Batanero , E. and Rodriguez , R. 1999 . Identification, isolation and characterisation of Ole e 7, a new allergen if olive tree pollen. . J. Immunol. , 172 : 6490 – 6500 .
- Thiéry , J. P. 1967 . Mise en évidence des polysaccharides sur coupes fines en microscope électronique. . J. Microsc. , 6 : 987 – 1018 .
- Traidl‐Hoffmann , C. , Kasche , A. , Menzel , A. , Jakob , T. , Thiel , M. , Ring , J. and Behrendt , H. 2003 . Impact of pollen on human health: More than allergen carriers? . Int. Arch. Allergy Immunol. , 131 : 1 – 13 .
- Valenta , R. , Steinberger , P. , Duchêne , M. and Kraft , D. 1996 . Immunological and structural similarities among allergens: Prerequisite for a specific and component‐based therapy of allergy. . Immunol. Cell Biol. , 74 : 187 – 194 .
- Van Campo , M. and Lugardon , B. 1973 . Structure grenue infratectale de l'ectexine des pollens de quelques Gymnospermes et Angiospermes. . Pollen Spores , 15 : 171 – 187 .
- Vega‐Maray , A. M. , Fernandez‐Gonzalez , D. , Valencia‐Barrera , R. , Polo , F. , Seoane‐Camba , J. and Suarez‐Cervera , M. 2004 . Lipid transfer proteins in Parietaria judaica L. Pollen grains: Immunocytochemical localization and function. . Eur. J. Cell Biol. , 83 : 493 – 497 .
- Venarske , D. and deShazo , R. D. 2003 . Molecular mechanisms of allergic disease. . S. Med. J. , 96 : 1049 – 1054 .
- Vinckier , S. , Cadot , P. , Grote , M. , Ceuppens , J. L. and Smets , E. 2006 . Orbicules do not significantly contribute to the allergenic micro‐aerosol emitted from birch trees. . Allergy , 61 : 1243 – 1244 .
- Vrtala , S. , Grote , M. , Duchêne , M. , van Ree , R. , Kraft , D. , Scheiner , O. and Valenta , R. 1993 . Properties of tree and grass pollen allergens: Reinvestigation of the linkage between solubility and allergenicity. . Int. Arch. Allergy Immunol. , 102 : 160 – 169 .
- Weber , M. and Frosch , A. 1995 . The development of the transmitting tract in the pistil of Haquetia epipactis (Apiaceae). . Int. J. Pl. Sci. , 156 : 615 – 621 .
- Weber , M. , Halbritter , H. and Hesse , M. 1998 . The spiny pollen wall in Sauromatum (Araceae) – with special reference to the endexine. . Int. J. Pl. Sci. , 159 : 744 – 749 .
- Wopfner , N. , Gadermaier , G. , Egger , M. , Asero , R. , Ebner , C. , Jahn‐Schmid , B. and Ferreira , F. 2005 . The spectrum of allergens in ragweed and mugwort pollen. . Int. Arch. Allergy Immunol. , 138 : 337 – 346 .