Abstract
The evolutionary and developmental origin of tricolpate pollen is of great interest because pollen of this kind defines a major clade of angiosperms (eudicots), a clade that is also well supported by molecular data. We examined evidence that tricolpate and monosulcate pollen types are produced alongside each other in the anthers of Nelumbo flowers, as has previously been reported. Observations of pollen in situ within individual anthers revealed mainly tricolpate pollen produced in tetrahedral tetrads, but also a small percentage of clearly aberrant pollen grains that have a great variety of aperture configurations. Previously published evidence for tetragonal tetrads is not supported, and previously reported monosulcate grains are part of a continuum of variation among the aberrant grains in aperture number, position and form. Other eudicots show similar variability in their pollen apertures. The variation in the pollen of Nelumbo is not exceptional, and may not be more significant than variation seen in the other taxa with regard to the origin of the tricolpate and tricolpate‐derived pollen characteristic of eudicots. Nevertheless further studies of aberrant pollen in Nelumbo and other eudicots, together with comparisons of pollen development in “normal” eudicots and closely related species that show radical, and developmentally fixed, reorganization of apertures and pollen polarity, may be helpful in understanding the processes that controlled the transition from the monosulcate to the tricolpate condition.
Aperture variation in the pollen of Nelumbo (Nelumbonaceae)
The evolutionary and developmental origin of tricolpate and tricolpate‐derived pollen apertures remains a topic of great interest because the production of pollen of this kind is a clear synapomorphy of the eudicot clade (Angiosperm Phylogeny Group II, Citation2003; Judd & Olmstead, Citation2004), and the evolution of tricolpate pollen has been suggested to be a potential key innovation underlying eudicot success (Furness & Rudall, Citation2004). The triaperturate grains that occur sporadically in monocotyledons and some magnoliid dicotyledons (Harley, Citation2004) do not appear to be homologous with the triaperturate condition in eudicots.
With regard to the evolution of tricolpate and tricolpate‐derived pollen, the Nelumbonaceae, a small family of aquatic angiosperms comprising only two extant species, have been of great interest to palynologists since Kuprianova (Citation1979) found a small percentage of monosulcate grains among the otherwise largely tricolpate pollen produced by Nelumbo. She suggested that this pollen variation provided evidence of the origin of tricolpate from monosulcate pollen. Kuprianova (Citation1979) also reported tetragonal (‘square’ or ‘cruciform’) tetrads among the otherwise tetrahedral tetrads. From this, and additional observations (Kreunen & Osborn, Citation1999) it has been inferred that successive microsporogenesis, that is otherwise only known in monocots and magnoliid angiosperms, also occurs in Nelumbo (e.g. Furness & Rudall, Citation1999, Citation2004).
In the past 15 years Kuprianova's observations have taken on additional significance because Nelumbo (together with Platanus and Proteaceae) is positioned by phylogenetic analyses based on molecular data as one of the earliest diverging groups of eudicots. Together with Nelumbo, Platanus and Proteaceae comprise a clade close to the base of the eudicots. In this study we review previous investigations of the apertures of Nelumbo pollen grains, and report our own observations.
Material and methods
Floral buds of Nelumbo ‘Sword Dance’ (Kew Accession Number, 20051137) and Nelumbo lutea (Kew Accession Number, 20051356NKSC) were taken on the first and second days of flowering respectively, from living collections at the Royal Botanic Gardens, Kew. The whole buds were immediately immersed in 70% ethanol, and stored in a refrigerator. Anthers were removed from different points in the androecium of each bud, and dehydrated in a graded ascending ethanol series up to 100%. They were then transferred into 100% acetone for a few minutes, drained on tissue, then placed on pre‐prepared SEM stubs with double‐sided adhesive tape. The anther locules were opened carefully with fine dissecting needles to reveal the pollen in situ. All preparations were coated with platinum using an Emitech K550X sputter coater and examined using a Hitachi S2400 or Hitachi S4700 FE‐SEM scanning electron microscope (SEM).
Results
Within anthers taken from the inner part of the androecium on the morning of the first day of flowering, we found many examples of tricolpate pollen with apertures fully formed, but with grains still adhering in tetrahedral tetrads (Figure , apertures arranged according to Fischer, Citation1890). We found a few tetrads in anthers from the middle part of the androecium, and none in anthers from the outer part of the androecium. From anthers collected on the second day of flowering, we found only two pollen tetrads in the uppermost part of one anther taken from the inner androecium. In all cases, the tetrads observed were tetrahedral; no other tetrad type was encountered.
The majority of Nelumbo pollen grains in all anthers observed were tricolpate (Figure ), with only a small percentage of the grains (2.38% in N. lutea and 9.96% in N. ‘Sword Dance’) having other types of apertures (Figure , ). Some of these ‘aberrant’ pollen grains had only one aperture (Figure , ), while others had two (Figure ) or four (Figure ). There is also variation in the position and shape of the apertures. Our observations do not indicate a clear distinction between tricolpate and monosulcate grains. Instead, most grains are tricolpate and the remainder (always small percentage) forms a continuum that is variable in aperture number, position and form. Pollen grains with aberrant apertures were found scattered singly in all parts of the anthers. In only one case did we observe four aberrant grains grouped together. This possible tetrad was irregularly tetragonal, rather than tetrahedral, although we cannot be certain that it was undisturbed. Percentages of grains with aberrant apertures in the samples that we examined are given in Table .
Figure 1 A–C. Pollen of Nelumbo lutea, from anthers of flowers on the first day of flowering, showing tricolpate pollen in tetrahedral tetrads with apertures fully developed and arranged according to Fischer's Law (Fischer, Citation1890). D–F. Various types of abnormal apertures seen in Nelumbo pollen. A. Nelumbo lutea, tetrahedral tetrad with one grain in the upper plane of view, and three in the lower plane. B. N. lutea, tetrahedral tetrad with two grains in the upper plane of view, and two below. C. N. lutea, tetrahedral tetrad with three grains in the upper plane of view, the fourth grain is lying below and is not visible. D. N. lutea, pollen grain with one curved aperture present. E. N. lutea, pollen grain showing one bifurcating aperture present. F. Nelumbo ‘Sword Dance’, tetracolpate grain in which the apertures almost join in two pairs. Scale bars – 20 µm.
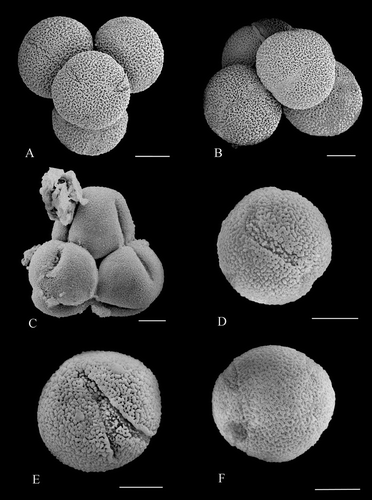
Figure 2 A–F. Pollen of Nelumbo with abnormal apertures. A. N. lutea, grain with one wide aperture, which is uneven in width, extending around the grain. B. N. lutea, grain with one curved aperture. C. N. lutea, grain with two apertures. D. N. lutea, grain with two apertures visible. E. N. lutea, grain with one bifurcating aperture visible. F. Nelumbo ‘Sword Dance’, tetracolpate grain with four apertures present. Scale bars – 20 µm.
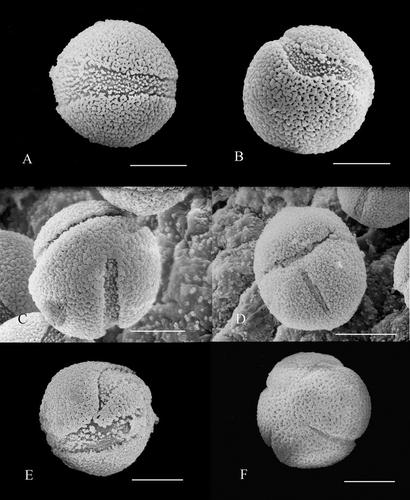
Table I. Number of ‘normal’ and ‘abnormal’ grains found in Nelumbo Adans. and Platanus L.
Discussion
The aperture types found in pollen grains of Nelumbo in this study are similar to those noted and illustrated in previous work (Kuprianova, Citation1979; Kreunen & Osborn, Citation1999; Borsch & Wilde, Citation2000). The number, position and shape of apertures in mature pollen grains are the result of a series of developmental processes that include the type of microsporogenesis (linked to the resulting tetrad shape) as well as the processes that determine pollen polarity and govern pollen wall formation (Ressayre et al., Citation1998, Citation2002a , Citation b , Citation2003, Citation2005; Penet et al., Citation2005). No direct observations have been made on the type of microsporogenesis present in Nelumbo; the interpretations in all published studies (Kuprianova, Citation1979; Kreunen & Osborn, Citation1999) are based on observations of tetrad shape in mature or developing grains.
Among angiosperms there are two main kinds of microsporogenesis: successive (whereby callose cell walls are formed after meiosis I and then again after meiosis II) or simultaneous (whereby both nuclear divisions take place before callose cell walls are formed) (Ressayre et al., Citation1998, Citation2002a , Citation b , Citation2003, Citation2005; Furness & Rudall, Citation2004; Penet et al., Citation2005). Simultaneous microsporogenesis allows for all microspores to adopt positions within the tetrad as far away from their neighbours as possible, thus forming tetrahedral tetrads. In contrast, several different tetrad types can result from successive microsporogenesis because the first pair of daughter nuclei produced by meiosis I is separated by the formation of a callose wall. Their subsequent development occurs independently. Tetragonal tetrads can result from successive microsporogenesis. True tetrahedral tetrads have never been demonstrated to be the result of anything other than simultaneous microsporogenesis (Penet et al., Citation2005).
We interpret the tetrad of Nelumbo described as ‘crucifix’ or ‘square’ by Kuprianova (Citation1979) as a tetrahedral tetrad. The varied appearance of a tetrahedral tetrad when viewed from different orientations is well illustrated by Gray (Citation1991) using a model made of glass. Similarly, Ferguson and Banks (Citation1994) illustrate mature tetrahedral tetrads of legume pollen in various orientations. Some of these (Ferguson & Banks, Citation1994: Figures 10, 16, and 21) are similar to the tetrad illustrated by Kuprianova (Citation1979). It is also significant that in the illustration of Kuprianova (Citation1979), the grains of the tetrad overlap each other, and are therefore in different planes. A true tetragonal tetrad is uniplanar (Punt et al., Citation1994).
Using material of Nelumbo prepared for transmission electron microscopy, Kreunen and Osborn (Citation1999) inferred the presence of both tetragonal and tetrahedral tetrads based on a TEM section of a Nelumbo anther. However, Ferguson and Banks (Citation1994) illustrate sections through legume tetrahedral tetrads that cut through all four grains (Ferguson & Banks, Citation1994: Figures 4, 30) giving the same appearance as the tetrad described by Kreunen and Osborn (Citation1999) as tetragonal.
Our observations, as well as a review of previously published work, indicates that so far there is no strong evidence of anything other than tetrahedral tetrads in Nelumbo. We infer that, as in other eudicots, simultaneous microsporogenesis is the norm in the genus. It remains to be established whether aberrant grains are produced in aberrant tetrads. Our observations clearly show that the apertures in Nelumbo are positioned and formed before the free microspore stage. We observed pollen still in tetrads when the apertures were fully formed. This is contrary to Kreunen and Osborn (Citation1999) but their work is nevertheless broadly consistent with the conclusion that the apertures of Nelumbo form relatively late in pollen development (Flynn & Rowley, Citation1971 – see below).
Our study found a range of between about 2–10% of the grains to have aberrant apertures with the proportion higher in the cultivated form (Table ). This is consistent with Blackmore's unpublished observations of ca. 5 000 grains of Nelumbo nucifera that found about 5% of the grains to be non‐tricolpate (Blackmore & Crane, Citation1998). To investigate whether this proportion of aberrant grains is unusual in the context of other angiosperms, we examined pollen from two species of Platanus (P. racemosa and P. wrightii) and Platanus × hispanica. Platanus and Nelumbo are closely related and occupy a similar phylogenetic position in angiosperms as a whole. Significant variability in the apertures of Platanus pollen (including forms with 4‐colpate, twisted or otherwise distorted apertures) has recently been reported by Denk and Tekleva (Citation2006). In Platanus racemosa and Platanus wrightii the proportion of grains with aberrant apertures was small (0.19–0.34%). In Platanus × hispanica the proportion was much higher and more similar to what we observed in Nelumbo.
Beyond Nelumbo and Platanus, many species of eudicot are known to be variable in their pollen morphology, but in general such variation is under‐reported, because most studies focus on ‘normal’ rather than ‘abnormal’ pollen morphology. It is well known for example that while pollen of Alnus glutinosa typically has five equatorially arranged pores, triporate, tetraporate and hexaporate pollen also occurs in low percentages. In some eudicots the level of variation in pollen apertures is even more extreme. For example, in a study of Krameriaceae, Pozhidaev (Citation2003) found aberrant aperture arrangements in 28 of the 35 specimens of 16 species that he studied, based on examining 300 to 1 500 pollen grains per specimen. In five of the specimens aberrant pollen accounted for less than 1% of the sample, but in 23 specimens as much as 15% or more of the grains examined were aberrant. In this context, the percentage of aberrant aperture forms in Nelumbo may not be exceptional (Table ).
Pozhidaev (Citation1998) investigated aberrant aperture forms in 28 samples of angiosperm species taken from 20 (mainly eudicot) families. He placed the aberrant grains that he observed in a series that shows a continuous transition from forms with a single encircling aperture (‘sandwich’ form) to the three‐apertured ‘normal’ form occurring across all the studied families. He suggests that the patterns reflects spatial changes that occur in the microspores during development, and how the mistiming of developmental processes (for example, the exine developing relatively too early during ontogeny) leads to the aberrant aperture forms seen in mature pollen.
It is interesting that aberrant pollen of Nelumbo shows some of the same aberrant aperture configurations depicted by Pozhidaev (Citation1998, Figure ). For example, the illustration of the pollen grain with a single aperture from Kuprianova (Citation1979) shows a latitudinal orientation of the grain, which fits with the pattern of equatorial polarity suggested by Pozhidaev (Citation1998). The zonasulculus seen in the legume Duparquetia, which has simultaneous microsporogenesis, tetrahedral tetrads and equatorial aperture position (Banks et al., Citation2006), also recalls some of the aberrant forms illustrated by Pozhidaev (Citation1998). Further documentation of situations like this would be helpful in drawing attention to areas for future research.
It is also interesting that some of the aberrant aperture forms observed in Nelumbo (e.g. ‘tetracolpate’ form Figures , ) do not occur in the series observed by Pozhidaev (Citation1998), while other variants that might be expected do not appear to be produced. For example, Borsch and Wilde (Citation2000) noted that no trichotomosulcate grains were found, despite the screening of many populations, and that trichotomosulcate grains might be expected if a true monosulcate condition existed in Nelumbo. Similarly, pollen grains that lack apertures entirely have not been documented in Nelumbo.
The developmental basis of the aberrant aperture forms seen in Nelumbo and other eudicots remains uncertain. In general, the formation of equatorial triaperturate pollen almost certainly reflects the duplication and repositioning of developmental processes that are usually restricted to the distal surface in monosulcate pollen (Blackmore & Crane, Citation1998). This repositioning may be linked to the development of a quadripolar spindle in tetrahedral tetrads (Blackmore & Crane, Citation1998), perhaps as a result of a shift to new kind of simultaneous microsporogenesis at the base of the eudicot clade. The very widespread occurrence of tricolpate or tricolpate‐derived pollen in eudicots (more than 175 000 species) indicates that the developmental mechanisms by which it is controlled have remained fixed and remarkably stable over a long period of time and through a massive process of diversification. In this context, while it is possible that the development of aberrant apertures in Nelumbo and other eudicots reflects instability in the formation of the quadripolar spindle, it seems more likely to reflect differences in the timing of pollen wall deposition and aperture formation in relation to relaxation of the control of aperture positioning.
Pozhidaev (Citation1998) suggests that mistiming of developmental processes, for example delayed or accelerated development of the exine, may lead to aberrant aperture forms in mature pollen. Similar shifts in developmental timing have been invoked as responsible for variation in other key features of pollen morphology (Blackmore & Crane, Citation1998). Similarly, Ressayre et al. (Citation1998, Citation2000a , Citation b , Citation2003, Citation2005) have related differences in the mature form of pollen apertures to changes in the deposition of the callose wall between daughter cells in a tetrad. In the case of Nelumbo, differentiation of the apertures appears delayed until relatively late in pollen development (Kreunen & Osborn, Citation1999). Flynn and Rowley (Citation1971) observed that apertures were not delimited at the primexine stage in their study of the development of Nelumbo pollen, in contrast to the pollen of Silene, Zea and Helleborus, which they also examined. If the determination of aperture position in Nelumbo is relatively late, it is possible that a slight acceleration in the timing of callose wall deposition may be sufficient to disrupt the positional control exerted by the quadripolar spindle and thereby result in aberrant grains. This hypothesis needs to be tested by future research. One approach would be to explore whether some of these processes (as reflected in the proportion of aberrant grains produced) may be partly influenced by environmental conditions and could therefore be manipulated experimentally. Another approach would be to undertake comparative developmental studies of situations like Duparquetia, where the stable production of “aberrant” grains could allow comparison of their development with that of normal tricolpate grains in their close relatives.
References
- Angiosperm Phylogeny Group (APG) . 2003 . An update of the Angiosperm Phylogeny Group classification for the orders and families of the flowering plants: APGII. . Bot. J. Linn. Soc. , 141 : 399 – 436 .
- Banks , H. , Feist‐Burkhart , S. and Klitgaard , B. 2006 . The unique pollen morphology of Duparquetia (Leguminosae: Caesalpinioideae): Developmental evidence of aperture orientation using confocal microscopy. . Ann. Bot. , 98 : 107 – 115 .
- Blackmore , S. and Crane , P. R. 1998 . “ The evolution of apertures in the spores and pollen grains of embryophytes. ” . In Reproductive biology , Edited by: Owens , S. J and Rudall , P. J . 159 – 182 . Kew : R. Bot. Gards .
- Borsch , T. and Wilde , V. 2000 . “ Pollen variability within species, populations, and individuals, with particular reference to Nelumbo. ” . In Pollen and spores: Morphology and biology , Edited by: Harley , M. M , Morton , C. M and Blackmore , S . 285 – 299 . Kew : R. Bot. Gards .
- Denk , T. and Tekleva , M. V. 2006 . Comparative pollen morphology and ultrastructure of Platanus: Implications for phylogeny and evaluation of the fossil record. . Grana , 45 : 195 – 221 .
- Ferguson , I. K. and Banks , H. 1994 . Tetrad pollen in the subfamily Caesalpinioideae (Leguminosae) and its significance. . Rev. Palaeobot. Palynol. , 83 : 31 – 42 .
- Fischer , H. 1890 . “ Beiträge zur vergleichenden Morphologie der Pollenkörner. Breslau: J. U. Kern. ” . In PhD. Thesis
- Flynn , J. J. and Rowley , J. R. 1971 . The primexine of Nelumbo nucifera. . Experientia , 27 : 227 – 228 .
- Furness , C. A. and Rudall , P. J. 1999 . Microsporogenesis in monocotyledons. . Ann. Bot. , 84 : 475 – 499 .
- Furness , C. A. , Rudall , P. J. and Sampson , F. 2002 . Evolution of microsporogenesis in angiosperms. . Int. J. Pl. Sci. , 163 : 235 – 260 .
- Furness , C. A. and Rudall , P. J. 2004 . Pollen aperture evolution – a crucial factor for eudicot success? . Trends Pl. Sci. , 9 : 154 – 158 .
- Gray , J. 1991 . “ Tetrahedraletes, Nodospora and the ‘cross’ tetrad: An accretion of myth. ” . In Pollen and spores: Patterns of diversification , Edited by: Blackmore , S and Barnes , S . 49 – 87 . Oxford : Clarendon Press . Syst. Assoc. Sp. Vol 44
- Harley , M. M. 2004 . Triaperturate pollen in the monocotyledons: Configurations and conjectures. . Pl. Syst. Evol. , 247 : 75 – 122 .
- Judd , W. S. and Olmstead , R. G. 2004 . A survery of tricolpate (eudicot) pylogenetic relationships. . Am. J. Bot. , 91 : 1627 – 1644 .
- Kreunen , S. S. and Osborn , J. M. 1999 . Pollen and anther development in Nelumbo (Nelumbonaceae). . Am. J. Bot. , 86 : 1662 – 1676 .
- Kuprianova , L. A. 1979 . On the possibility of the development of tricolpate pollen from monosulcate. . Grana , 18 : 1 – 4 .
- Penet , L. , Nadot , S. , Ressayre , A. , Forchioni , A. , Dreyer , L. and Gouyon , P. H. 2005 . Multiple developmental pathways leading to a single morph: Monosulcate pollen (examples from the Asparagales). . Ann. Bot. , 95 : 331 – 343 .
- Pozhidaev , A. E. 1998 . Hypothetical way of pollen aperture patterning. 1. Formation of 3‐colpate patterns and endoaperture geometry. . Rev. Palaeobot. Palynol. , 104 : 67 – 83 .
- Pozhidaev , A. E. 2003 . Hypothetical way of pollen aperture patterning. 3. A family‐based study of Krameriaceae. . Rev. Palaeobot. Palynol. , 127 : 1 – 23 .
- Punt , W. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 1994 . Glossary of pollen and spore terminology Utrecht: LPP Found., LPP Contrib. Ser. 1
- Ressayre , A. , Godelle , B. , Mignot , A. and Gouyon , P.‐H. 1998 . A morphogenetic model accounting for pollen aperture pattern in flowering plants. . J. Theor. Biol. , 193 : 321 – 334 .
- Ressayre , A. , Raquin , C. , Mignot , A. , Godelle , B. and Gouyon , P.‐H. 2002a . Correlated variation in microtubule distribution, callose deposition during male post‐meiotic cytokinesis, and pollen aperture number across Nicotiana species (Solanaceae). . Am. J. Bot. , 89 : 393 – 400 .
- Ressayre , A. , Godelle , B. , Raquin , C. and Gouyon , P.‐H. 2002b . Aperture ontogeny in angiosperms. . J. Exp. Zool. , 294 : 122 – 135 .
- Ressayre , A. , Mignot , A. , Siljak‐Yakovlev , S. and Raquin , C. 2003 . Postmeiotic cytokinesis and pollen aperture number determination in eudicots: Effect of the cleavage wall number. . Protoplasma , 221 : 257 – 268 .
- Ressayre , A. , Dreyer , L. , Triki‐Teurtroy , S. , Forchioni , A. and Nadot , S. 2005 . Post‐meiotic cytokinesis and pollen aperture pattern ontogeny: Comparison of development in four species differing in aperture pattern. . Am. J. Bot. , 92 : 576 – 583 .