Abstract
Five new taxa with affinities to extant lineages that diverged at an early stage from the main line of eudicot evolution are established from the Early Cretaceous (late Aptian or early Albian) Vale de Agua locality, Portugal. Staminate flowers of Lusistemon striatus and pistillate flowers of Lusicarpus planatus are unisexual without rudiments of the opposite gender. They are linked by the association of an unusual pollen type found in situ in the stamens and adhering to the stigmatic surface. The staminate flower, Lusistemon striatus, is composed of six stamens subtended by small perianth parts. The arrangement of the stamens is difficult to ascertain, but their variable sizes suggests a spiral arrangement. Pollen found in situ is tricolpate and striate with densely‐spaced, sparsely diverging and anastomosing muri that are aligned more or less parallel to the polar axis. The muri have a conspicuous supratectal ornamentation of fine transverse ridges. The granular infratectal layer forms an indistinct internal reticulum. The foot layer is thin. Pollen is closely similar to dispersed grains from the Aptian of Egypt described as STRIOTRI‐SEGMUR. It also resembles pollen of the dispersed pollen genus Rutihesperipites, as well as some dispersed pollen assigned to Striatopollis. Pistillate flowers of Lusicarpus planatus consist of a bicarpellate, syncarpous gynoecium borne on a short stalk. The styles are bent outwards and expose the double‐crested stigmatic regions on their ventral sides. The only organ preserved besides the gynoecium is a lateral scale‐like organ at the base of the stalk. Pollen of the same type found in Lusistemon striatus occurs on the stigmatic surface of the carpels. Comparisons with extant taxa demonstrate that Lusistemon and Lusicarpus share many characters with early diverging groups of eudicots, in particular Buxaceae. In addition to the Lusistemon‐Lusicarpus flowers, the Vale de Agua samples also contain three other pistillate reproductive structures that may be related to early diverging lineages of eudicots. Silucarpus camptostylus has a bicarpellate and syncarpous gynoecium with two styles; Valecarpus petiolatus and Aguacarpus hirsutus have tricarpellate gynoecia that are distinguished from each other in the shape and extension of the stigma as well as other details.
Early Cretaceous floral structures and in situ tricolpate‐striate pollen: New early eudicots from Portugal
The eudicot clade comprises about 75% of extant angiosperm species diversity. It is clearly defined by its distinctive triaperturate pollen (Doyle & Hotton, Citation1991; Drinnan et al., Citation1994; Magallón et al., Citation1999) and is also strongly supported by phylogenetic analyses based on molecular data (e.g., Soltis & Soltis, Citation2004). Triaperturate pollen grains are easy to recognize in the fossil record and the oldest palaeobotanical evidence of eudicots is provided by dispersed tricolpate pollen grains from the Barremian‐Aptian transition about 120 million years ago (Hughes & McDougall, Citation1990). Subsequently, from Aptian and Albian deposits there is a greater diversity of eudicot pollen types, indicating that the group was already well established at that time and that its initial diversification was well under way (Hughes & McDougall, Citation1990; Penny, Citation1991; Doyle, Citation1992). In addition, eudicots are represented by a diversity of leaf fossils in the later part of the Early Cretaceous and mid‐Cretaceous (e.g., Doyle & Hickey, Citation1976). There is also an expanding record of Early Cretaceous reproductive organs such as flowers, fruits and dispersed stamens that can be attributed to the group (e.g., Friis et al., Citation1988; Crane, Citation1989; Drinnan et al., Citation1991; Crane et al., Citation1993; Pedersen et al., Citation1994; Leng & Friis, Citation2003, Citation2006).
As in other areas of palaeobotanical research the fossil record of early eudicots comprises mostly fragmented and dispersed plant organs. One of the difficulties in interpreting this early fossil record is to determine, which of these dispersed plant organs were produced by the same fossil species. Discoveries of fossil flowers and detached reproductive structures with in situ pollen from the Early Cretaceous (e.g., Friis et al., Citation1988, 2000b; Drinnan et al., Citation1991; Crane et al., Citation1993; Pedersen et al., Citation1994) have provided valuable information on the parent plants of fossil eudicot pollen grains that were previously only known from the dispersed microfossil record. In this paper we provide a further example. We described five new fossil taxa from the Early Cretaceous (Aptian or Albian) of Portugal closely related to Buxales. Two of the taxa, Lusistemon striatus Pedersen, von Balthazar, Crane & Friis gen. et sp. nov. and Lusicarpus planatus Pedersen, von Balthazar, Crane & Friis gen. et sp. nov., comprise unisexual pistillate and staminate flowers as well as stamens with the same kind of tricolpate and striate pollen grains. Another pistillate structure from the Vale de Agua locality, Aguacarpus hirsutus Pedersen, von Balthazar, Crane & Friis gen. & sp. nov., also has tricolpate and striate pollen adhering to the stigmatic surface. This kind of pollen is diverse in Early Cretaceous (Aptian and early Albian) sediments (Penny, Citation1988) and the pollen described here corresponds to one of the earliest records of tricolpate‐striate pollen currently recognised as STRIOTRI‐SEGMUR from the Aptian of Egypt (Penny, Citation1988). The pollen grains also resemble dispersed Cretaceous pollen assigned to the pollen genus Rutihesperipites S.K. Srivastava (Srivastava, Citation1977) and Striatopollis Krutzsch (Norris, Citation1967; Singh, Citation1971; Ward, Citation1986). Striate tricolpate pollen has earlier been associated with the fossil flowers of Spanomera Drinnan, Crane, Pedersen & Friis (Drinnan et al., Citation1991). Several characters of Spanomera suggest a relationship to extant Buxaceae (Drinnan et al., Citation1991) and this is also the case for the fossils described here. There are also similarities with extant members of several other families of early diverging eudicots as well as with Saxifragales in the core eudicots. In addition to the fossil flowers with striate pollen, two other taxa of putative buxalean affinity are also described.
Material and methods
The material described here is from Early Cretaceous sediments exposed in the opencast clay pit complex near the small village of Vale de Agua in western Portugal (39°37′15″N, 8°51′30″W). The sediments at this locality consist predominantly of grey, reddish or greenish fine‐grained sediments of Early Cretaceous age that were previously assigned to the “Complexos gresosos de Nazaré e de Cós‐Juncal” (Carta Geologica de Portugal, 27‐A Vila Nova de Ourém, Zbyszewski et al., Citation1974). These sediments have subsequently been included in the Figueira da Foz Formation established by Dinis (Citation1999, Citation2001) and according to Dinis (Citation1999) the plant bearing sediments from the Vale de Agua locality probably belong to the basal part (Famalicão Member) of this formation. Based on in situ pollen and comparisons with other fossil floras from Portugal a pre‐Albian age (Barremian or Aptian) was suggested for the plant fossil assemblages from Vale de Agua (Friis et al., Citation2001). Dinis and collaborators (Dinis, Citation2001; Dinis et al., Citation2002), using mainly sedimentological and lithofacies correlations, suggested a late Aptian age for the base of the Figueira da Foz Formation. Recently, correlation of a marine sequence in the Algarve Basin (dated on dinoflagellates and carbon‐isotope records) with the sedimentary sequence in the Lusitanian (Western Portuguese) Basin has suggested that the base of the Figueira da Foz Formation could be slightly younger than previously thought (early Albian) (Heimhofer et al., Citation2007), but detailed correlation between the Figueira da Foz area (Beiro Litoral region) and the Vale de Agua area (Estremadura region) has not yet been established.
The fossil plant remains were extracted from the sediments by sieving in water followed by treatment with 40% HF, 10% HCl and rinsing in water. The fossils are typically small, charcoalified or lignitised, fragments of wood and twigs as well as detached reproductive organs. Most of the reproductive organs are fruits and seeds, but the flora also includes well‐preserved flowers that often have their three‐dimensional form intact. Angiosperms are diverse and associated pollen grains (in situ in stamens, adhering to gynoecia, or incorporated in coprolites) are mainly monoaperturate indicating that most of the diversity is with magnoliid angiosperms and monocots (Friis et al., Citation1997, Citation1999, Citation2000a , Citation b , Citation2001). The few triaperturate pollen types indicate the presence of early eudicots including the fossils described here.
The fossil specimens described in this paper were isolated from sediment samples Vale de Agua 19, 141, 328, 329 and 364 collected in 1989 (KRP, EMF, PRC), 1992 (KRP, EMF) and 2000 (KRP). The fossil material comprises bicarpellate and tricarpellate gynoecia, as well as staminate flowers and stamens with in situ striate pollen. For scanning electron microscopy (SEM) the specimens were mounted on SEM stubs, sputter coated with Au, and studied using a Hitachi S‐4300 field emission scanning electron microscope at 1–5 KV and a Philips SEM 515 scanning electron microscope at 15 KV. Pollen grains removed for ultrastructural examination of the pollen wall were dehydrated in an ethanol‐propylene oxide series, embedded in Epon, and sectioned with an ultramicrotome at 500–700 Å. Sections were stained with uranyl acetate and lead citrate and studied using a Jeol‐100S transmission electron microscope. One specimen of Lusicarpus was studied using synchrotron x‐ray tomographic microscope (SRXTNM) at the Swiss Light Source at the Paul Scherrer Institute, Villigen, Switzerland.
The specimens and preparations of the fossil material are deposited in the collections of the Swedish Museum of Natural History, Stockholm (S).
Results
Five different early eudicot reproductive structures are described here. The material includes: i) staminate structures with in situ tricolpate, striate pollen; ii) bicarpellate pistillate structures with similar tricolpate, striate pollen adhering to the stigmatic surface; iii) two different tricarpellate pistillate structures, one without and one with tricolpate and striate pollen adhering to the stigma; and iv) one bicarpellate pistillate structure with tricolpate and reticulate pollen. All of the fossils are isolated flowers or flower fragments and there is no information on inflorescence structure.
Systematics
Order Buxales
Family insertae sedis
Lusistemon gen
nov.
Derivation of generic name
Composed of Lusitania (old Roman name for Portugal) and stemon (gr. thread) referring to the staminate nature of these Portuguese fossil flowers.
Generic diagnosis
Staminate flower with six small tepals and six stamens of different sizes and apparently placed opposite the tepals. Anthers basifixed, attached to filament without distinct joint; filaments free from each other. Anthers dithecal and tetrasporangiate; thecae placed laterally; anther dehiscence by longitudinal slits. Connective poorly developed between thecae, but extended apically into a short protrusion. Pollen tricolpate, tectate and striate with dichotomising and sometimes anastomosing muri that are ornamented with minute, transversely oriented ridges. Infratectal layer thin, granular and forming a fine internal reticulum; foot layer thin.
Type species
Lusistemon striatus sp. nov.
Comments on the genus
The genus is established based on staminate structures with distinctive in situ tricolpate and striate pollen. Spanomera Drinnan, Crane, Pedersen & Friis is another genus established from the Cretaceous to encompass similar staminate structures with in situ striate pollen (Drinnan et al., Citation1991), but staminate flowers of Spanomera are organised on a clear opposite and decussate plan and the in situ pollen grains are distinctively different (see also Discussion).
Lusistemon striatus sp
nov. (Figures – ).
Figure 1 Staminate flowers ofLusistemon striatus. SEM‐micrographs. A–E. Holotype; staminate flower with six stamens. S122091, sample Vale de Agua 141. A Flower showing unequal length of stamens and subtending organs. B. Same specimen remounted to show stamens from the other side. C. Close‐up of subtending organs. D,E. Apical part of holotype enlarged to show short connective protrusions and papillate epidermis along stomium. F–H. Fragmentary flower with one subtending organ partly preserved. S105028, sample Vale de Agua 19. F. Same specimen as in G remounted to show stamens from inside. G. Flower from outside showing tepals and stamens. H. Close‐up of G, showing extension of connective. Scale bars – 100 µm (A–H).
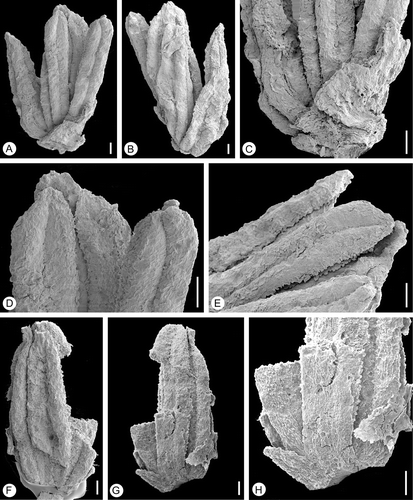
Figure 2 External morphology ofin situ Lusistemon striatus pollen. SEM‐micrographs. A. Pollen grains from specimen in Figure . S105028, sample Vale de Agua 19. B–E. Pollen grain from isolated stamen. S101284, sample Vale de Agua 141. B. Equatorial view showing full length of colpus and verrucae on colpus membrane. C,D. Polar view showing three colpi and parallel arrangement of muri across polar region. E. Detail of Figure showing striate ornamentation of verrucae on colpus membrane. F. Detail of pollen from holotype showing surface sculpture and muri that have distinct transverse ridges and muri that occasionally bifurcate. S122091, sample Vale de Agua 141. Scale bars – 10 µm (A–D); 1 µm (E, F).
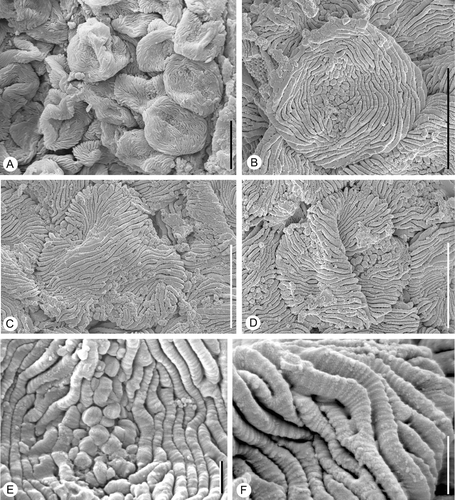
Figure 3 Internal structures ofin situ Lusistemon striatus pollen. SEM‐micrographs. A,B. Pollen grains from specimen in Figure . S105028, sample Vale de Agua 19. A. Pollen with muri partly missing exposing infratectal layer. B. Detail of infratectal layer showing reticulate clustering of granulae. C,D. Details of exine showing transverse ridges of muri and infratectal granular layer. Holotype, S122091, sample Vale de Agua 141. E. Detail of pollen wall showing infratectal reticulum. S101284, sample Vale de Agua 141. F. Fragmentary grain showing granular infratectal layer and foot layer (arrowheads). Holotype. Scale bars – 5 µm (A, F); 1 µm (B–E).
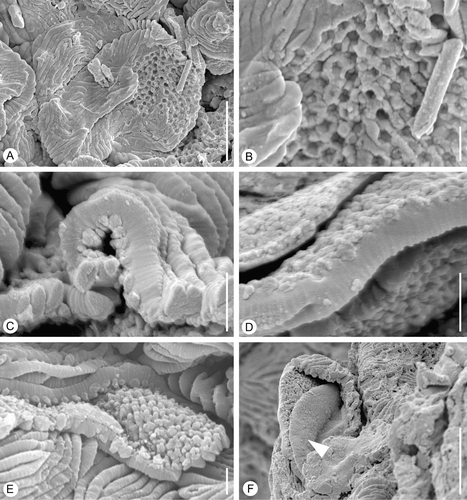
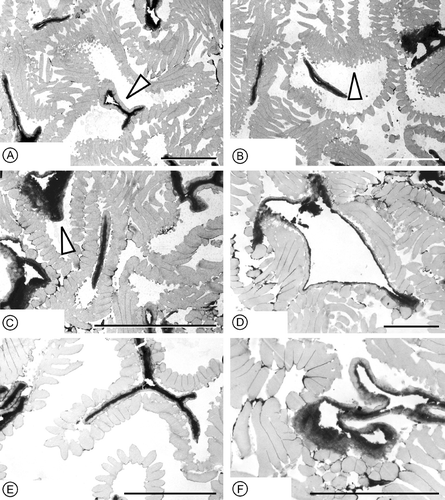
Figure 4 Pollen wall ultrastructure ofin situ Lusistemon striatus pollen. TEM‐micrographs. All sections from specimen S101284, sample Valede Agua 141. A–F. Sections show lighter staining ektexine (muri, infratectal layer and foot layer) and darker staining endexine. A. Cross‐section of grain in equatorial region showing detached foot layer and endexine in the center (arrow). B. Section through several pollen grains showing infratectal reticulum (arrow) and sharply triangular profile of muri. C. Section through several grains showing endexine in aperture regions (arrow) and detached foot layer of central grain. D. Cross‐section more or less parallel to equatorial plane showing thin foot layer still attached to granular infratectal layer. E. Section through grain outside aperture regions showing ektexine with distinct triangular profiles of muri, granular infratectal layer, partly detached foot layer, and endexine. F. Section of grain in apertural region showing expanded, finely granular endexine and verrucae of colpus membrane; note foot layer outside aperture region. Scale bars – 5 µm (A–F).
Derivation of species name
Referring to the striate pollen.
Specific diagnosis
As for the genus with the following additions: Tepals narrow. Epidermis of anthers along the stomium papillate. Pollen grains circular to slightly elliptical in equatorial view and subangular in polar view with the colpi positioned in the centre of the flattened sides. Tectum striate in non‐apertural regions. Muri of intercolpium aligned more or less parallel to the pollen grain polar axis forming a slightly undulating pattern, more strongly aligned over the poles. Granulae of infratectal layer form an internal reticulum. Colpus membrane verrucate with finely striate ornamentation on the verrucae.
Holotype
S122091 from sample Vale de Agua 141, illustrated Figures , , .
Paratypes
S105028 (sample Vale de Agua 19), S101284 (part on SEM stub; part sectioned for TEM) and S105042 (sample Vale de Agua 141).
Type locality
Clay pit complex near the village of Vale de Agua, western Portugal (39°37′15″N, 8°51′30″W).
Age and stratigraphy
Early Cretaceous (late Aptian or early Albian), “Complexos gresosos de Nazaré e de Cós‐Juncal” – basal part (Famalicão Member) of the Figueira da Foz Formation.
Description and remarks on the species
The staminate material comprises two flowers (S105028, S122091) as well as several isolated stamens and stamen fragments (S101284, S105042).
The best preserved staminate flower (S122091) is composed of six stamens and remains of several short bract‐like organs (Figure ). Most likely these structures are abraded and correspond to the narrow organs of specimen S105028 (Figure ) that are interpreted as tepals. No carpels or remains of carpels could be observed inside the stamens and the flower is most likely unisexual. The stamens are of variable sizes ranging from 0.9 mm to 1.3 mm in length; two are long, three are of intermediate size and one is relatively short (Figure ). It is difficult to ascertain the arrangement of the stamens, but their different sizes may indicate a spiral arrangement. The other flower (S105028) is more fragmentary. It consists of four stamens, three of which are more or less complete, all about 1.25 mm long (Figure ). In this flower only two organs are preserved outside the stamens, both are broken, one longer and one shorter, fused at the base (Figure ). They are interpreted as tepals and inferred from the position of these two organs opposite to the stamens the number of tepals were probably equal to the number of stamens.
Anthers are basifixed and there is no distinct joint between anther and filament (Figure ). Filaments are short, broad and free from each other. Anthers are dithecal and tetrasporangiate, 0.8–1.2 mm long and 0.2–0.3 mm wide (Figure ). Thecae are lateral, and each appears to open by a longitudinal slit along its entire length (Figure ). The connective is poorly developed between thecae, but it extends apically into a short protrusion that is more or less triangular in shape (Figure ). The epidermis along the stomium is papillate (Figure ).
Pollen grains observed in situ in the anthers are tricolpate, tectate and striate (Figure ). They are circular to slightly elliptical in equatorial view (Figure ) and inter‐subangular in polar view (Figure ), about 14–15 µm in diameter, and 15–16 µm long. Colpi are short, about 7–8 µm long, resulting in a relatively extensive polar area (Figure ). In polar view they are positioned each in the centre of one of the flat sides of the grain. The tectum is striate in non‐apertural regions with narrow and densely‐spaced elongated sculpturing elements (muri) separated by deep, narrow furrows (striae). The muri are arranged more or less parallel to the polar axis forming a slightly undulating pattern in the intercolpium. They are more obvious parallel over the poles (Figure ). The muri sometimes dichotomise, sometimes anastomose or have free endings (Figure ). They are about 0.6 µm wide and about 4 µm high with flattened sides and narrow elliptical to narrow triangular profile, often with a sharp crest. Muri are ornamented with minute, transversely oriented ridges (Figure ). These transverse ridges are rounded and spaced in a dense and regular pattern. The ridges and interspacing furrows are of equal dimension, about 0.06–0.07 µm (Figure ). Over the colpus membrane the sculpture elements are verrucate (Figure ). Verrucae have finely striate ornamentation similar to that of the muri (Figure ). The muri are supported by a thin granular infratectal layer (Figure ) that forms an indistinct internal reticulum (Figure ). The foot layer is very thin and often separates from the infratectal layer (Figure ).
Ultra‐thin sections of the pollen wall show an outer lighter staining layer (ektexine) and an inner darker staining layer (endexine) (Figure ). The ektexine consists of tectum, about 0.6 µm thick, characterised by the densely spaced muri, a thin granular infratectal layer, about 0.2 µm thick. The infratectal layer consists of irregular granulae that cluster laterally to form a reticulate pattern. This reticulate arrangement of the granules is particularly clear in SEM‐micrographs of broken specimens (Figure ), but is also shown in TEM‐micrographs (Figure ). The foot layer is very thin, up to about 0.2 µm thick (Figure ). The endexine is granular, about 0.15 µm thick in non‐apertural regions, but thicker under the apertures (Figure ).
Lusicarpus gen
nov
Derivation of generic name
Composed of Lusitania (old Roman name for Portugal) and karpos (gr. fruit) referring to the pistillate nature of these Portuguese fossil flowers.
Generic diagnosis
Gynoecium bicarpellate, syncarpous, hypogynous and shortly stalked, supported by a narrow elongated bract. Gynoecium flattened dorsi‐ventrally with a rounded base. Carpels united for most of their length. Styles two, short and stout, bent outwards in anthetic flowers. Stigma double‐crested, not distinctly papillate and covered by secretion. Pollen grains adhering to stigma tricolpate and striate.
Type species
Lusicarpus planatus sp. nov.
Remarks on the genus
The genus Lusicarpus is established to encompass bicarpellate pistillate structures with tricolpate and striate pollen adhering to stigmatic surface. Lusicarpus is distinct from bicarpellate pistillate flowers of Spanomera in having carpels united for most of its length and a correspondingly much shorter stigmatic region (see also Discussion).
Lusicarpus planatus sp
nov. (Figures , ).
Figure 5 Bicarpellate gynoecium ofLusicarpus planatus. SEM‐micrographs. Holotype, S101305, sample Vale de Agua 141. A. Lateral view of gynoecium and scale‐like bract. B. Stylar region of gynoecium. C. Stigmatic area on ventral side of style. D. Scale‐like bract at base of gynoecium. E. Carpel surface showing isodiametric epidermal cells and ridges probably from vascular bundles. F. Detail of stigmatic surface showing tricolpate‐striate pollen grains (arrowheads) embedded in secretion. G. Stomata on carpel surface (arrowheads). Scale bars – 500 µm (A); 100 µm (B–E); 10 µm (F, G).
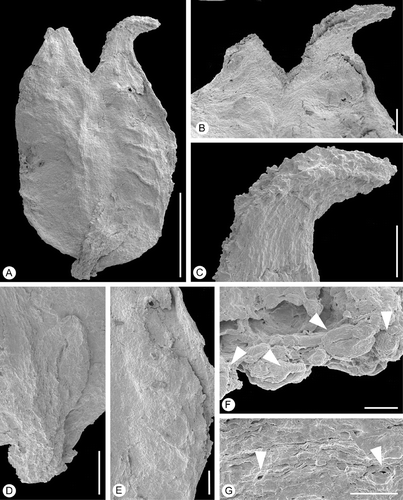
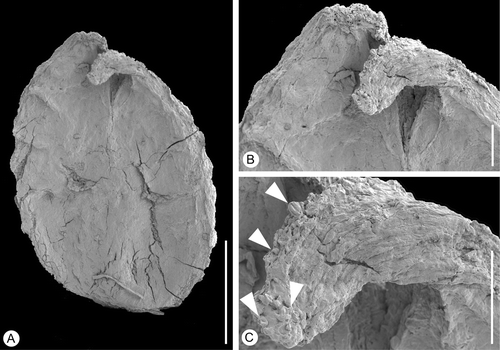
Figure 6 Bicarpellate gynoecium ofLusicarpus planatus. SEM‐micrographs. S153149, sample Vale de Agua 329. A. Lateral view of gynoecium. B. Stylar region of gynoecium. C. Detail of style showing ventral stigma and tricolpate‐striate pollen grains (arrowheads) embedded in secretion. Scale bars – 500 µm (A); 100 µm (B, C).
Derivation of species name
Referring to the flat shape of the gynoecium.
Specific diagnosis
As for the genus with the following additions: Carpels united up to about 4/5 of their length. Gynoecium wall glabrous. Stigmatic regions along ca 2/3 of the length of styles. Tricolpate striate pollen adhering to stigmatic surface similar to in situ pollen of Lusistemon striatus.
Holotype
S101305 from sample Vale de Agua 141, illustrated in Figure .
Paratypes
S153149 (sample Vale de Agua 329), S153156 (sample Vale Agua 328), S153518 (sample Vale de Aqua 19).
Type locality
Clay pit complex near the village of Vale de Agua, western Portugal (39°37′15″N, 8°51′30″W).
Age and stratigraphy
Early Cretaceous (late Aptian or early Albian), “Complexos gresosos de Nazaré e de Cós‐Juncal” – basal part (Famalicão Member) of the Figueira da Foz Formation.
Description and remarks on the species
The material comprises four pistillate structures, three with styles and stigmatic areas well preserved (S101305, S153149, S153156) and one in which most of the styles and stigmatic areas are broken off (S153518). Each pistillate structure is a bicarpellate, syncarpous, hypogynous gynoecium, borne on a short stalk (Figures , ), and supported by a small, narrow bract in a lateral position at the base of the stalk (Figure ). The gynoecia are about 1.5–1.7 mm long and 1 mm wide, strongly flattened dorsi‐ventrally and with a rounded base.
The carpels are united for about 4/5 of their length (Figures , ). The two styles are short and stout. In specimen S101305 the styles are bent outwards and the distal 2/3 of the ventral margin is composed of a double‐crested stigmatic region (Figure ). This specimen might be preserved in anthetic or post‐anthetic stage. In the three other specimens that have their styles preserved styles diverge much less prominently (Figure ). These specimens may be preserved in a younger, pre‐anthetic stage than specimen S101305. The stigma appears to be covered with the remains of secretion and was apparently wet. It is not distinctly papillate (Figure ). The dorsal surfaces of the styles exhibit numerous stomata (Figure ).
Synchrotron x‐ray tomographic microscopy showed that the ovary is clearly bilocular and the thin ovary wall is composed of small isodiametric cells. Unfortunately, all tissues were densely packed and the x‐ray studies did not reveal further information on ovule number, organisation, or placentation.
The gynoecium wall is thin and the fruit was probably either non‐fleshy, indehiscent or a capsule. The outer epidermis of the gynoecium is glabrous and composed of small isodiametric cells.
Tricolpate striate pollen identical to that in situ in the staminate flowers of Lusistemon striatus occurs abundantly on the stigmatic surface in specimen S101305 and S153518, partly or fully embedded in the secretion‐like substance (Figure ). The only grain encountered of another type was a single tricolpate and foveolate pollen grain.
Other putative buxalean gynoecia in the Vale de Agua assemblages
In addition to Lusicarpus planatus the Vale de Agua assemblages also include another bicarpellate gynoecium and two different types of tricarpellate gynoecia that also indicate the presence of early eudicots.
Silucarpus gen
nov.
Derivation of generic name
Anagram of Lusicarpus referring to the morphological similarity of the fruits.
Generic diagnosis
Gynoecium bicarpellate, syncarpous and hypogynous, flattened dorsi‐ventrally with a round base. Carpels united for most of their length. Styles two, short and stout, bent outwards at anthesis. Stigma double‐crested and papillate.
Type species
Silucarpus camptostylus sp. nov.
Comments on the genus
Silucarpus is based on a single fragmentary specimen. The specimen is closely similar to Lusicarpus, but differs mainly in having a distinct papillate stigmatic surface. Pollen grains observed on the stigmatic surface of Silucarpus are reticulate in contrast to the striate pollen that occurs on the Lusicarpus stigmas. In extant angiosperms the nature of the stigmatic surface is typically constant within a genus and the Lusicarpus and Silucarpus are therefore here assigned to separate genera.
Silucarpus camptostylus sp
nov. (Figure ).
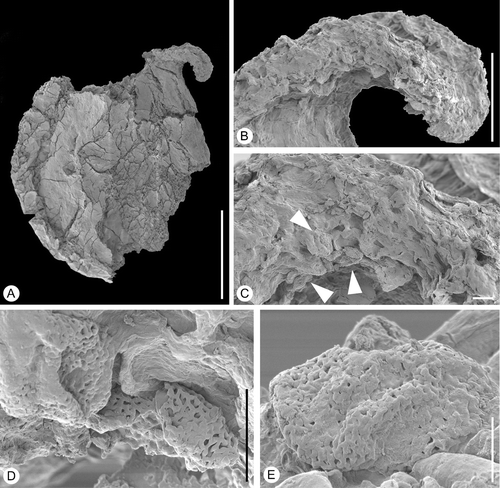
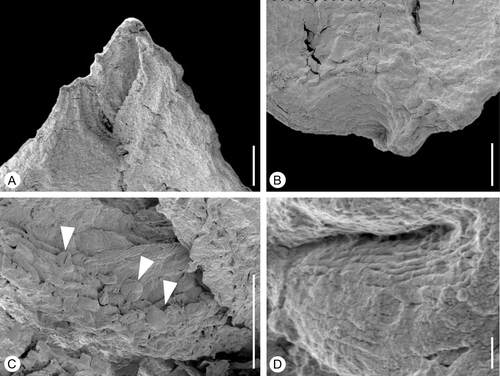
Figure 7 Bicarpellate gynoecium ofSilucarpus camptostylus. SEM‐micrographs. Holotype, S154521, sample Vale de Agua 364. A. Lateral view of gynoecium. B. Detail of style showing ventral stigma. C. Stigmatic region enlarged showing pollen grains (arrowheads) embedded in secretion. D. Reticulate pollen grains embedded in secretion. E. Pollen grain from stigmatic region enlarged. Scale bars – 500 µm (A); 100 µm (B); 10 µm (C, D); 5 µm (E).
Derivation of species name
From kamptos (gr. curved) and stylos (gr. pillar) referring to the curved styles of the fruit.
Specific diagnosis
As for the genus with the following additions: Carpels united up to about 5/6 of their length. Stigmatic regions along about 2/3 of the length of styles. Gynoecium wall glabrous.
Holotype
S154521 (from sample Vale de Agua 364, illustrated Figure ).
Type locality
Clay pit complex near the village of Vale de Agua, western Portugal (39°37′15″N, 8°51′30″W).
Age and stratigraphy
Early Cretaceous (late Aptian or early Albian), “Complexos gresosos de Nazaré e de Cós‐Juncal” – basal part (Famalicão Member) of the Figueira da Foz Formation.
Description and remarks on the species
The single specimen is slightly damaged and its epidermis is not well preserved. The gynoecium is flattened and composed of two carpels that are united to about 5/6 of their length (Figure ). It is about 1.1 mm broad at the level of the ovary and about 1.3 mm long.
Only one of the two styles is preserved. The style is bent outwards and has a double‐crested stigma on the ventral side (Figure ) composed of long papillae (Figure ). The stigma appears secretory.
The gynoecium wall is thin and the fruit was probably non‐fleshy, indehiscent or a capsule. The outer epidermis of the gynoecium is glabrous and composed of small isodiametric cells.
A few pollen grains are present along the rim of the stigma embedded in stigmatic secretion (Figure ). Pollen appears to be tricolpate, about 18 µm in polar diameter, tectate‐reticulate with smooth muri and narrow lumina (Figure ).
Valecarpus gen
nov.
Derivation of generic name
From Vale de Agua, and karpos (gr. fruit).
Generic diagnosis
Gynoecium tricarpellate, syncarpous and hypogynous, borne on a short stalk. Carpels united for most of their length. Ovary distinctly triangular in cross‐section. Stigma sessile, double‐crested, papillate, and secretory restricted to the distal parts of the three ventral slits; papillae short and rounded.
Type species
Valecarpus pedicellatus sp. nov.
Remarks on the genus
Valecarpus comprises small tricarpellate gynoecia/fruits with distinctly triangular cross‐section and short backward bending style. Valecarpus is distinct from Aguacarpus described below in having sessile stigmas and stigmatic area restricted to the distal potion of the ventral slit. It is further distinguished in having short stigmatic papillae.
Valecarpus pedicellatus sp
nov. (Figure ).
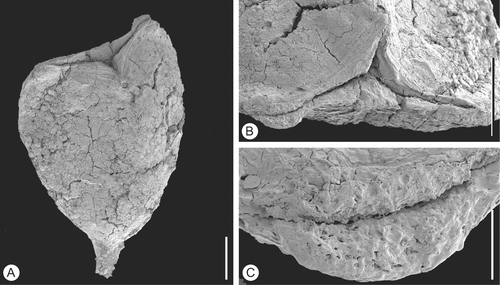
Figure 8 Tricarpellate gynoecium ofValecarpus pedicellatus. SEM‐micrograph. Holotype, S122094, sample Vale de Agua 19. A. Lateral view of gynoecium showing short stalk. B. Apical view of gynoecium showing the triangular shape. C. Double crested stigma along upper part of ventral slit. Scale bars – 500 µm (A, B); 100 µm (C).
Derivation of species name
Referring to the stalked gynoecium.
Specific diagnosis
As for the genus with the following additions: Stigmatic regions along the upper third of the apical suture. Gynoecium wall glabrous.
Holotype
S122094 (from sample Vale de Agua 19, illustrated Figure ).
Paratype
S122093 (sample Vale de Agua 19).
Type locality
Clay pit complex near the village of Vale de Agua, western Portugal (39°37′15″N, 8°51′30″W).
Age and stratigraphy
Early Cretaceous (late Aptian or early Albian), “Complexos gresosos de Nazaré e de Cós‐Juncal” – basal part (Famalicão Member) of the Figueira da Foz Formation.
Description and remarks on the species
Both specimens are gynoecia composed of three carpels that are united along almost their entire length (Figure ). They are about 2.5 mm long and about 1.8 mm broad at the level of the ovary. The gynoecium has a short stalk (Figure ). The region of the ovary is distinctly triangular in cross‐section.
The stigmas are short and bent backwards. The stigmatic region is formed by two broad crests in the upper part of the ventral slit (Figure ). The stigmatic surfaces are papillate with papillae that are short, rounded, and probably secretory (Figure ). There are no remains of associated stamens and no pollen grains were observed on the gynoecium.
The gynoecium wall is thin and the fruit was probably non‐fleshy, indehiscent or a capsule. The surface of the gynoecium is glabrous and epidermis cells small, isodiametric.
Aguacarpus gen
nov.
Derivation of generic name
From Vale de Agua, the locality where the fossils were found, and karpos (gr. fruit).
Generic diagnosis
Gynoecium tricarpellate, syncarpous, and hypogynous, borne on a short stalk. Carpels united for almost their entire length. Ovary triangular in cross‐section. Styles three, short, and stout. Stigma double‐crested, papillate, and secretory, extending for the full length of the ventral suture; stigmatic papillae long, multicellular, covered with remains of secretion.
Type species
Aguacarpus hirsutus sp. nov.
Remarks on the genus
The genus Aguacarpus is established for small tricarpellate gynoecia with distinctly triangular cross‐section and short stout styles. It is distinguished from Valecarpus in having more differentiated stylar area with stigmas that is extended for the full length of the ventral slit. Further the stigmatic papillae are longer than in Valecarpus.
Aguacarpus hirsutus sp
nov. (Figures , ).
Figure 9 Tricarpellate gynoecium ofAguacarpus hirsutus. SEM‐micrographs. Holotype, S153517, sample Vale de Agua 141. A. Lateral view of gynoecium. B. Stylar regions enlarged showing ventral stigmas. C. Detail of stigmatic surface showing distinct papillae. D. Close‐up of carpel epidermis showing densely‐space trichomes and stomata. Scale bars – 500 µm (A); 100 µm (B, D); 50 µm (C).
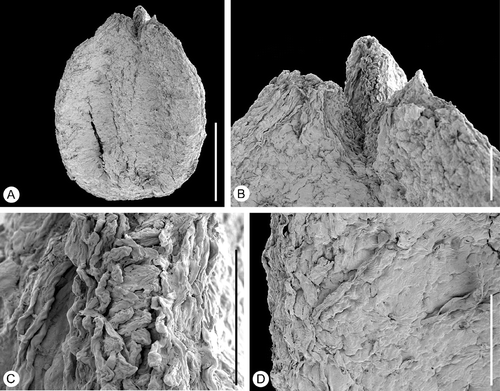
Figure 10 Tricarpellate gynoecium ofAguacarpus hirsutus. SEM‐micrographs. S101426, sample Vale de Agua 141. A. Apical part of gynoecium showing stylar region with stigmas. B. Basal part of fruit showing short stalk. C. Stigmatic surface with pollen grains (arrowheads) embedded in secretion showing two of three colpi. D. Detail of pollen showing striate sculpture and transverse ridges of muri. Scale bars – 100 µm (A, B); 50 µm (C); 1 µm (D).
Derivation of species name
Referring to the hirsute surface of the gynoecium.
Specific diagnosis
As for the genus with the following additions: Gynoecium with long trichomes and conspicuous stomata.
Holotype
S153517 from sample Vale de Agua 141, illustrated Figure .
Paratype
S101426 (sample Vale de Agua 141).
Type locality:
Clay pit complex near the village of Vale de Agua, western Portugal (39°37′15″N, 8°51′30″W).
Age and stratigraphy
Early Cretaceous (late Aptian or early Albian), “Complexos gresosos de Nazaré e de Cós‐Juncal” – basal part (Famalicão Member) of the Figueira da Foz Formation.
Description and remarks on the species
The gynoecium is tricarpellate, borne on a short stalk (Figure ), and with the carpels united along almost their entire length (Figures and ). The gynoecium is about 1.4 mm long and 0.9 mm broad at the level of the ovary.
The styles are short and the stigmatic regions run along the entire ventral side (Figures , ). The stigmatic surfaces are distinctly papillate with long, multicellular papillae (Figure ). They are covered with remains of secretion and were probably wet.
The gynoecium wall is thin and the fruit was probably non‐fleshy, indehiscent or a capsule. The epidermis cells of the gynoecium are small and isodiametric. Numerous long trichomes or trichome bases, as well as stomata are scattered over the entire gynoecial surface (Figure ).
Pollen grains occur abundantly on the stigmatic surface of specimen S101426. Grains are tricolpate, tectate and striate with transverse ridges on the muri, about 15 μm long (Figure ).
Discussion
Comparison with dispersed striate pollen from the Cretaceous
A variety of tricolpate, striate pollen grains have been reported from Cretaceous and Tertiary palynological assemblages. Angiosperm pollen grains of this type have their first occurrences in the early Aptian of Egypt and Gabon (Penny, Citation1988; Doyle, Citation1992, Citation1999). Among dispersed pollen, the grains in situ within Lusistemon show the closest similarity to those described from the Early Cretaceous of Egypt (Mersa Matruh borehole 1) by Penny (Citation1988) who documented four different kinds of striate tricolpate pollen using SEM. All four taxa occur only in the Aptian part of the sequence, and extend from the latest Early Aptian to the end of the Middle Aptian. Three of the Egyptian striate pollen types have muri that are smooth or almost smooth and that either lack supratectal ornamentation or that have only faint ornamentation. The fourth type, referred to by Penny (Citation1988) as STRIOTRI‐SEGMUR, is closely similar to the pollen of Lusistemon. Muri in the intercolpium regions are narrow, densely‐spaced, arranged parallel to the polar axis, and have a supratectal ornamentation of fine transverse ridges. The sculpturing elements over the aperture membrane are verrucate. There is also agreement in pollen size, STRIOTRI‐SEGMUR being about 14.5–19.5 µm long compared to 14–15 µm for the pollen of Lusistemon. Penny (Citation1988) also mentions that STRIOTRI‐SEGMUR has a secondary layer in the sexine, which sometimes forms a pseudoreticulum. This is not illustrated but it probably corresponds to the unusual reticulate meshwork of the infratectal granular layer that occurs in the Lusistemon pollen. There are only minor differences between the two kinds of grains: muri in the Lusistemon pollen have a sharper profile than in STRIOTRI‐SEGMUR and STRIOTRI‐SEGMUR has granulae and shorter muri interspaced between the elongated muri, which does not occur in the Lusistemon pollen. The verrucae of the colpus membrane also lack supratectal ornamentation in STRIOTRI‐SEGMUR.
The dispersed pollen grains from Egypt described by Penny (Citation1988) were not given classic binominals, but similar dispersed striate grains from other Cretaceous palynological assemblages are typically referred to various species of the dispersed pollen genus Striatopollis. Striopollenites Rouse and Rutihesperipites, are sometimes regarded as junior synonyms of Striatopollis (for a more comprehensive list of other tricolpate and striate dispersed pollen genera see Srivastava, Citation1977). However, the type species for Striatopollis and Striopollenites, as well as many other dispersed pollen grains from the Cretaceous and Tertiary, have only been studied and illustrated by light microscopy (LM). Details of supratectal ornamentations or wall stratification are usually lacking, which precludes detailed comparison with the in situ pollen considered here and other pollen grains studied using scanning electron microscopy (SEM).
Closer examination of the many different kinds of dispersed tricolpate, striate pollen grains shows considerable variation in exine ornamentation. Grains of this kind were evidently produced by several systematically distinct groups of eudicots. The type species of Striatopollis, Striatopollis sarstedtensis Krutzsch, was described from the earliest Paleocene of Wehmingen, Germany (Krutzsch, Citation1959). It is characterised by non‐anastomosing and generally non‐diverging muri that run parallel to the polar axis in a slightly twisted arrangement. It is thus distinct from the fossil pollen of the Lusistemon flower, which has anastomosing and divergent muri and is also distinct from many other dispersed Cretaceous pollen assigned to Striatopollis. The circumscription of Striopollenites Rouse (type species Striopollenites terasmaei Rouse from the Eocene of British Columbia, Canada; Rouse, Citation1962) is closely similar to that of Striatopollis and the name is therefore regarded as a junior synonym of Striatopollis (Potonié, Citation1966; Ward, Citation1986).
The genus Rutihesperipites is well documented by both SEM and LM. The type species, Rutihesperipites trochuensis (S. K. Srivastava) S. K. Srivastava, from the Maastrichian of Alberta was first described as Salixipollenites trochuensis S.K. Srivastava (Srivastava, Citation1966). It shares many features with the pollen of Lusistemon described here. Grains are about 17–30 µm in equatorial diameter and about 21–24 µm in polar length with a densely striate exine. Muri are mostly parallel to the polar axis, but anastomose regularly, and are ornamented by fine, closely spaced transverse supratectal ridges. However, Rutihesperipites trochuensis differs from the pollen found in Lusistemon in having a much denser pattern of striations and muri with rounded profiles. Ward (Citation1986) transferred Rutihesperipites trochuensis to Striatopollis, but the striation pattern of Rutihesperipites trochuensis is distinctly different from that of the type species of Striatopollis. In addition, the pollen wall of Rutihesperipites trochensis is much thinner. Because of these differences, and because Rutihesperipites is well documented by SEM, we suggest that Rutihesperipites is maintained for dispersed tricolpate and striate pollen grains with anastomosing muri bearing transverse supratectal ornamentation.
Dispersed pollen reported from the Albian of Portugal as Striatopollis trochuensis and Striatopollis cf. trochuensis (Heimhofer et al., Citation2007) have a different striation pattern than the type material of Rutihesperipites trochuensis and clearly do not belong to this species. These dispersed Portuguese grains are illustrated by LM‐images only and a detailed comparison with the in situ pollen from Lusistemon is therefore not possible. However, the dispersed grains are larger (20–25 µm) and are probably not conspecific with the in situ grains.
Grains described as Striatopollis cf. paraneus (Norris) Singh from the Cenomanian of Bathurt and Mornington Islands, eastern Australia (Dettmann, Citation1973) were documented by SEM. They resemble Rutihesperipites and the pollen of Lusistemon in size, general morphology and sculpturing, but differ in the much more distinct and sharper supratectal ridges and the rounded profile of the muri.
Comparison with other fossil reproductive structures from the Cretaceous
A variety of eudicot reproductive structures have been described from the Cretaceous. Most of those from the Late Cretaceous belong to core eudicots (for a review see Friis et al., Citation2006a ) and none of them resemble Lusistemon or Lusicarpus. From the Early Cretaceous the fossil record of eudicot floral structures is much less extensive. Most of these Early Cretaceous taxa are related to the Platanaceae (Crane et al., Citation1993; Pedersen et al., Citation1994) and while the flowers are also unisexual they differ from Lusistemon‐Lusicarpus in their pentamerous organisation, apocarpous gynoecia, and the foveolate to reticulate sculpture of the pollen wall. However, flowers of Spanomera mauldinensis Drinnan, Crane, Friis & Pedersen and Spanomera marylandensis Drinnan, Crane, Friis & Pedersen from the Potomac Group sediments of eastern North America (Drinnan et al., Citation1991) are much more similar to the fossil flowers described here.
Spanomera includes staminate and pistillate floral structures that are sometimes preserved in fragments of inflorescences. In situ pollen of Spanomera is tricolpate with a coarse tectum sculpture formed from high vermiform muri with sharply triangular profiles. Grains of Spanomera and Lusistemon are similar in several respects but those of Spanomera are distinguished by having a distinctive columellate infratectal layer and a very thick foot layer. Grains of Spanomera mauldinensis are striate and the muri have fine transverse striations as in the pollen of Lusistemon. They were compared to dispersed grains assigned to Striatopollis paraneus. Grains of Spanomera marylandensis are striate‐rugulate to reticulate‐rugulate with smooth muri. They were compared to dispersed pollen assigned to Striatopollis vermimuris.
The reproductive structures of Spanomera mauldinensis are the most completely preserved and include inflorescences with both staminate and pistillate flowers. The staminate flowers are composed of one whorl of five bract‐like tepals, one whorl of five stamens opposite the tepals and a central pistillode (Drinnan et al., Citation1991). Both the whorl of tepals and the whorl of stamens have an opposite and decussate arrangement. The staminate flowers of Lusistemon differ in having a much less prominent perianth and in lacking a pistillode. They also seem to have a spiral arrangement of the stamens in contrast to the whorled arrangement in Spanomera. The basifixed anthers of Lusistemon also contrast with the dorsifixed anthers in Spanomera, but in both the thecae are long, laterally placed and open by longitudinal slits along their entire length. In both taxa the anthers also have short and pointed apical connective protrusions.
The pistillate flowers of Spanomera are bicarpellate with two bract‐like tepals, one opposite each carpel, and two additional bract‐like tepals in lateral position (Drinnan et al., Citation1991). Lusicarpus is also bicarpellate but in Spanomera the carpels are fused only near the base, the stigmatic area extents along the full length of the ventral suture, and the stigmatic papillae are long and prominent. In Lusicarpus there are no remains or scars of stamens and tepals outside the carpels. The scale‐like bract has a lateral position similar to the inner perianth whorl of Spanomera.
Although the Portuguese fossils differ from Spanomera in details of flowers and pollen grains, and cannot be included in the same genus, the combination of characters is nevertheless similar and suggests that Lusistemon‐Lusicarpus and Spanomera may belong to the same evolutionary lineage of early eudicots (see also systematic discussion below). The other pistillate structures described from Portugal (Silucarpus, Valecarpus, Aguacarpus) are not similar to other pistillate structures described from the Cretaceous, but may also be part of the same systematic complex as Spanomera and Lusistemon‐Lusicarpus (see Discussion below).
Comparison with extant angiosperms: Systematic position of Lusistemon and Lusicarpus
The occurrence of distinctive striate pollen in situ in the stamens, and adhering to the stigmatic region, indicates that the staminate flowers of Lusistemon and the pistillate flowers of Lusicarpus were produced by the same kind of plant. In the following character analysis they are therefore discussed together. The tricolpate pollen clearly places the floral structures among eudicot angiosperms and characters of the fossil further indicate a probable relationship to lineages that diversified early in the eudicot radiation.
Floral organization
Unisexual flowers are common in many early diverging groups of eudicots (e.g., Buxaceae, Didymelaceae, Platanaceae, some Ranunculales, some Sabiaceae) as well as many early diverging groups of core eudicots (e.g., Myrothamnaceae, Gunneraceae) (Drinnan et al., Citation1994; von Balthazar & Endress, Citation2002a , Citation b ; von Balthazar et al., Citation2003). However, unisexual flowers also occur widely among other angiosperms especially in generally wind‐pollinated taxa of Fagales or Saxifragales (e.g., Daphniphyllaceae, Cercidiphyllaceae, Altingiaceae of Saxifragales; Endress, Citation1993b ).
Staminate structure
In the extant taxa considered above, staminate flowers have a simple perianth as inferred for Lusistemon, or a perianth is lacking as in Eupteleaceae, Achlys DC. (Berberidaceae), Trochodendron Sieb & Zucc. (Trochodendraceae), Styloceras Juss. (Buxaceae) and perhaps Didymelaceae of early diverging eudicots, as well as in Altingiaceae, Cercidiphyllaceae, Daphniphyllaceae p.p. of Saxifragales (Endress, Citation1986, Citation1989, Citation1993a , Citation b ; Sutton, Citation1989; von Balthazar & Endress, Citation2002a ).
Six stamens are the rule in various families of early diverging eudicots (Papaveraceae, Eupteleaceae, Lardizabalaceae, Menispermaceae, Berberidaceae, Notobuxus Oliv. in Buxaceae) and may be present in Saxifragales (Hamamelidaceae p.p., Daphniphyllaceae) (for an overview see von Balthazar et al., Citation2005). However, the arrangement of stamens is mostly whorled in these taxa and the flowers often organised in a clear dimerous plan. The stamens of the fossil are of different sizes and this may indicate an initiation in succession, but whether in a spiral or a whorl is unclear.
Filaments are short in the fossil flower, but extension of the filaments at anthesis cannot be excluded. Short filaments in anthetic extant flowers are known from various taxa in early diverging eudicots (Lardizabalaceae, Berberidaceae, Buxaceae, Didymelaceae, Proteales) as well as Saxifragales (Daphniphyllaceae, Altingiaceae). Anthers of the fossil are basifixed, which is also common among these extant groups. Anther dehiscence in the fossil is lateral, most probably by longitudinal slits along the entire length of the thecae. Anther dehiscence by longitudinal slits is found among many angiosperms including early diverging eudicots and early diverging core eudicots, for example in some wind‐pollinated taxa of the Saxifragales such as Daphniphyllaceae (Endress, Citation1993b ). Connective protrusions as in the fossil are common among extant taxa at this level of angiosperm evolution (Hufford & Endress, Citation1989; Endress & Stumpf, Citation1991; von Balthazar & Endress, Citation2002b ). A particular feature of the fossil is its papillate epidermal cells along the stomium; similar epidermal cells have been observed in Styloceras (Buxaceae; von Balthazar & Endress, Citation2002b ).
Pollen
Many early diverging eudicots have tricolpate pollen, in contrast to the mostly tricolporate pollen of core eudicots (e.g., Drinnan et al., Citation1994; Magallón et al., Citation1999). However, tricolpate pollen occurs also scattered among core eudicots, for example among certain Saxifragales such as Daphniphyllaceae and Hamamelidaceae (Zavada & Dilcher, Citation1986).
Pollen with striate surface ornamentation is present in a wide range of angiosperm families, both among early diverging eudicots and various orders of core eudicots (e.g., Santalales, Saxifragales, Geraniales, Myrtales, Fabales, Rosales, Sapindales, Ericales, Gentianales, Lamiales, Solanales, Asterales, sensu APG II, Walker & Doyle, Citation1975; Ward, Citation1986). However, pollen comparable to the fossil grains at a more detailed level is rare and occurs in Berberidaceae (Achlys, Vancouveria C. Morr. & Decne., Jeffersonia Bart., Nowicke & Skvarla, Citation1981; Nowicke & Skvarla, Citation1982), Ranunculaceae (Hydrastis Ellis ex L., Trollius L., Kumazawa, Citation1936; Nowicke & Skvarla, Citation1981; Lee & Blackmore, Citation1992), Trochodendraceae (Endress, Citation1986), Circaeasteraceae (Circaeaster Maxim., Kingdonia Balf. f. & W.W. Smith, Nowicke & Skvarla, Citation1982), Sapindaceae (Acer L., Halbritter & Hesse, 2000 Citationonwards), and Fabaceae (Crudia Schreb., Anthonotha Beauv., Isoberlinia Craib & Stapf., Germeraad et al., Citation1968). Among these the fossil grains are particularly similar to those of Circaeaster, which are about the same size and that have an irregularly striate tectum. In Circaeaster as in the fossil the foot layer and endexine is much thinner than tectum and infratectal layer, but muri are apparently smooth without supratectal ornamentation (Nowicke & Skvarla, Citation1982). Unfortunately there is no detail on the infratectal layer. The other member of the Ranunculales mentioned above as well as Acer also have an extremely thin foot layer (Nowicke & Skvarla, Citation1982; Halbritter & Hesse, 2000 Citationonwards), a character that is otherwise very rare among eudicot angiosperms. However, all of these taxa lack the distinct transverse ribbing of the muri. Pollen grains of Buxaceae differ from those of Lusistemon‐Lusicarpus in being tricolporate to polyporate with reticulate to rugulate or crotonoid exine sculpture (Drinnan et al., Citation1991; Köhler, Citation2007).
Pistillate structure
The presence of a bicarpellate superior ovary, as is clearly present in the fossil, is characteristic for several families among early diverging eudicots core eudicots (basal Papaveraceae, and Gunneraceae) as well as Saxifragales (Hamamelidaceae). Similar pistillate structures also occur in some Menispermaceae, Circaeasteraceae, Ranunculaceae, Buxaceae, Sabiaceae, Myrothamnaceae and Daphniphyllaceae.
Syncarpous pistillate structures, as observed in Lusicarpus, occur in Papaveraceae, Buxaceae, Sabiaceae, Trochodendraceae, Daphniphyllaceae, some Hamamelidaceae, and Altingiaceae. The stigma is double‐crested and decurrent to a greater or lesser extent in several extant taxa (Buxaceae, Didymelaceae, Platanaceae, Myrothamnaceae, Trochodendraceae, Saxifragales: Altingiaceae, Cercidiphyllaceae, Daphniphyllaceae, some wind‐pollinated Hamamelidaceae; Endress & Igersheim, Citation1999). In Lusicarpus the stigmatic area is double‐crested and decurrent only in the upper half of the free styles. The stigma of the fossil is not clearly papillate compared to the generally distinctly unicellular‐papillate, often pear‐shaped papillate stigmas of early diverging eudicots and early diverging core eudicots (Endress & Igersheim, Citation1999). Also, in some of the extant eudicots mentioned above rather than being papillate the stigmatic surface has multicellular protuberances [e.g. Podophyllum L. (Berberidaceae), Hydrastis (Ranunculaceae), Daphniphyllaceae, and Hamamelidaceae; Endress & Igersheim, Citation1999)]. The fossil stigma appears to be secretory as is also the case in most of the extant taxa considered here.
The only other organ that occurs in Lusicarpus in addition to the gynoecium is a small bract‐like organ. Since no abscission scars have been observed between the bract‐like organ and the ovary base it is likely that no staminate organs were present. Whether additional perianth organs were present outside the bract‐like organ cannot be determined with the material available. The lateral position of the bract‐like organ in respect to the gynoecium axis might indicate that it is a subtending bract. Flowers of eudicots with only bract‐like organs outside the gynoecium are found in extant Circaeasteraceae, Buxaceae, Didymelaceae, Trochodendraceae and Myrothamnaceae p.p. among early diverging eudicots and core eudicots, as well as in some Saxifragales, such as Daphniphyllaceae (Drinnan et al., Citation1991, Citation1994; Hoot et al., Citation1999; von Balthazar & Endress, Citation2002a ). A perianth is also lacking in the bisexual or pistillate flowers of Eupteleaceae, Trochodendraceae, Myrothamnaceae, Altingiaceae, Cercidiphyllaceae, and Daphniphyllaceae (Drinnan et al., Citation1991, Citation1994; Hoot et al., Citation1999; von Balthazar & Endress, Citation2002a ).
Non‐fleshy fruits, either indehiscent or capsular as inferred for Lusicarpus occur in several of the families considered above including the Buxaceae. Fruits of Daphniphyllaceae are fleshy drupes with a thick endocarp. They are further distinguished in being unilocular in contrast to the bilocular fruits of Lusicarpus.
Systematic conclusion
Lusistemon and Lusicarpus display a mosaic of characters that are found in various extant eudicot families, predominantly among early diverging eudicots and core eudicots, as well as in some Saxifragales. Floral characters are particularly similar to those of Buxaceae, but are also similar to those of the distantly related Daphniphyllaceae. Flowers of Buxaceae, like those of several other early diverging eudicots, are characterized by a low level of synorganisation between floral organs. In contrast, in the context of other Saxifragales, the flowers of Daphniphyllaceae are adapted to a specific pollination syndrome (i.e. wind‐pollination).
Pollen in the fossil material is distinct and shows a greater similarity to pollen of other members of early diverging eudicots. The combined evidence from reproductive structures and pollen indicates that the fossil is probably best placed among the early diverging lineages of eudicots and probably close to extant Buxaceae.
Inferences of habit and pollination biology
A characteristic that is particularly noticeable among the flowers of early diverging eudicots with striate pollen [e.g., Achlys, Vancouveria, Jeffersonia (Berberidaceae), Hydrastis (Ranunculaceae), and Kingdonia and Circaeaster (Circaeasteraceae)] is the preponderance of white or greenish flowers. Such flowers are mostly open and have non‐specific pollinators. In addition, many of these are herbaceous plants of the forest understorey that flower early in the season in temperate regions. Such flowers are often pollinated by a variety of smaller insects, in particular by flies and solitary bees (Motten, Citation1986).
Systematic position of Silucarpus, Valecarpus and Aguacarpus
The additional three pistillate reproductive structures from Vale de Agua also show similarities to Buxaceae. The tricarpellate gynoecium of Valecarpus with its decurrent, double‐crested stigmatic areas along the upper part of the styles or ventral suture is particularly similar to extant Buxus L. and Notobuxus species (Buxaceae, von Balthazar & Endress, Citation2002b ). The interstylar areas of the fossil gynoecium appear undifferentiated. However, in many extant Buxus species interstylar nectariferous bulges are present, although they are less differentiated or lacking in many African Buxus species and Notobuxus (von Balthazar & Endress, Citation2002b). The fossil stigma also appears to consist of unicellular‐papillae, as also occurs in Buxaceae and many other early diverging eudicots.
The tricarpellate gynoecium of Aguacarpus is comparable to Lusicarpus in the degree of union of the carpels, the decurrent stigmas, and the presence of very similar tricolpate, striate pollen adhering to the stigmatic surface. It also shows similarity to some Buxaceae, particularly Notobuxus. It is unfortunate that more detailed comparisons are not possible because internal features are unknown.
The bicarpellate gynoecium of Silucarpus with its reticulate pollen offers a limited number of characters for comparison with extant taxa. Two carpels comprising a superior ovary and with decurrent, double‐crested stigmatic areas occur in a similar variety of extant taxa to the pistillate structures mentioned above (basal Papaveraceae, Sabiaceae, Gunneraceae, and some Menispermaceae, Circaeasteraceae, Ranunculaceae, Buxaceae, Myrothamnaceae of early diverging eudicots and early diverging core eudicots as well as Hamamelidaceae and Daphniphyllaceae of Saxifragales, Endress & Igersheim, Citation1999). However, the stigma appears papillate and is more similar to that of early diverging eudicots and early diverging core eudicots. Reticulate pollen is present in various families of these groups, but the reticulum of the fossil pollen is similar to that of some Buxaceae (Buxus, although these pollen are usually zono‐colporate, pantocolporate or pantoporate, Köhler & Brückner, Citation1982; Köhler, Citation2007), Sabiaceae (Erdtman, Citation1952), Platanaceae (Denk & Tekleva, Citation2006), Menispermaceae (e.g., Ferguson, Citation1975; Thanikaimoni, Citation1984), as well as Hamamelidaceae (e.g., Erdtman, Citation1952; Zavada & Dilcher, Citation1986) and Cercidiphyllaceae in Saxifragales (Zavada & Dilcher, Citation1986).
Conclusions
The fossil reproductive organs from Vale de Agua add to the expanding record of Early Cretaceous eudicot angiosperms. Comparisons with extant plants indicate that the flowers of Lusistemon and Lusicarpus are related to early diverging groups of eudicots and are particularly close to extant Buxales. There is therefore good correspondence between the early stratigraphic position of these fossils and their likely position as an early diverging group in eudicot phylogeny.
The in situ pollen grains are very similar to dispersed grains reported from the early and mid‐Aptian of Egypt. Although the fossil grains from Egypt are probably not conspecific with the pollen found in Lusistemon, unusual details of the pollen wall (supratectal striation and infratectal reticulum) strongly suggest that the two taxa belong to the same evolutionary lineage and probably the same genus. This implies that the buxalean lineage, which was certainly present (based on Spanomera) by the late Aptian, may already have been established by the early Aptian. The presence of three other, probably closely related, taxa in the Vale de Agua flora further indicate a considerable diversity of early diverging eudicots supporting the palynological data of diverse and widespread occurrences of Eudicots in the Aptian‐Albian.
Acknowledgments
We thank B. Kunderup for help in preparing TEM sections and G. Dancher for permission to use the TEM facilities at the Institute of Anatomy, University of Aarhus. We also thank Pat Herendeen for helpful comments on the text. We thank Marco Stampanoni for help with SRXTM performed at the Swiss Light Source, Paul Scherrer Institut, Villigen, Switzerland. Financial support from the Swedish Research Council (EMF), the Carlsberg Foundation (KRP) is gratefully acknowledged. SRXTM was funded by the Swiss Light Source, European Union FP6 (to P. C. J. Donoghue and S. Bengtson).
References
- Crane , P. R. 1989 . Palaeobotanical evidence on the early radiation of nonmagnoliid dicotyledons. . Pl. Syst. Evol. , 162 : 165 – 191 .
- Crane , P. R. , Pedersen , K. R. , Friis , E. M. and Drinnan , A. N. 1993 . Early Cretaceous (Early to Middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of Eastern North America. . Syst. Bot. , 18 : 328 – 344 .
- Denk , T. and Tekleva , M. V. 2006 . Comparative pollen morphology and ultrastructure of Platanus: Implications for phylogeny and evaluation of the fossil record. . Grana , 45 : 195 – 221 .
- Dettmann , M. E. 1973 . Angiospermous pollen from Albian to Turonian sediments of eastern Australia. . Spec. Publ. Geol. Soc. Aust. , 4 : 3 – 34 .
- Dinis , J. L. 1999 . Estratigraphia sedimentologia da formação de Figueira da Foz. Aptiano a Cenomaniano do sector norte da Bacia Lusitânica , Coimbra : University of Coimbra .
- Dinis , J. L. 2001 . Definicão da Formacão da Figueira da Foz – Aptiano a Cenomaniano do sector central da margem oeste ibérica /Definition of the Figueira da Foz Formation – Aptian to Cenomanian of the central sector of the western Iberian margin). . Comun. Inst. Geol. Min. , 88 : 127 – 160 .
- Dinis , J. L. , Rey , J. and Graciansky , P.‐C. de. 2002 . Le bassin lusitanien (Portugal) à l'Aptien supérieur‐Albien: organisation séquentielle, proposition de corrélations, évolution. . C.R. Geosci. , 334 : 757 – 764 .
- Doyle , J. A. 1992 . Revised palynological correlations of the lower Potomac Group (USA) and the Cocobeach sequence of Gabon (Barremian‐Aptian). . Cret. Res. , 13 : 337 – 349 .
- Doyle , J. A. 1999 . “ The rise of angiosperms as seen in the African Cretaceous pollen record. ” . In Palaeoecology of Africa & Surr. Is. Vol. 26 , Edited by: Heine , K . 3 – 29 . Rotterdam : A. A. Balkema 3–29 .
- Doyle , J. A. and Hickey , L. J. 1976 . “ Pollen and leaves from the mid‐Cretaceous Potomac Group and their bearing on early angiosperm evolution. In C. B. Beck (Ed.), ” . In Origin and early evolution of Angiosperms , 139 – 206 . New York : Columbia Univ. Press .
- Doyle , J. A. and Hotton , C. L. 1991 . “ Diversification of early angiosperm pollen in a cladistic context. In S. Blackmore & S. H. Barnes (Eds), ” . In Pollen and spores: Patterns of diversity , 169 – 195 . Oxford : Clarendon Press .
- Drinnan , A. N. , Crane , P. R. and Hoot , S. B. 1994 . Patterns of floral evolution in the early diversification of non‐magnoliid dicotyledons (eudicots). . Pl. Syst. Evol. [Suppl.] , 8 : 93 – 122 .
- Drinnan , A. N. , Crane , P. R. , Pedersen , K. R. and Friis , E. M. 1991 . Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac Group (mid‐Cretaceous) of eastern North America. . Am. J. Bot. , 78 : 153 – 176 .
- Endress , P. K. 1986 . Floral structure, systematics, and phylogeny in Trochodendrales. . Ann. Mo. Bot. Gard. , 73 : 297 – 324 .
- Endress , P. K. 1989 . Chaotic floral phyllotaxis and reduced perianth in Achlys (Berberidaceae). . Bot. Acta , 102 : 159 – 163 .
- Endress , P. K. 1993a . “ Eupteleaceae. ” . In The families and genera of vascular plants. II Flowering plants – dicotyledons. Magnoliid, hamamelid and caryophyllid families , Edited by: Kubitzki , K , Rohwer , J. G and Bittrich , V . 299 – 301 . Berlin, Heidelberg : Springer .
- Endress , P. K. 1993b . “ Hamamelidaceae. In K. Kubitzki, J. G. Rohwer & V. Bittrich (Eds), ” . In The families and genera of vascular plants. II Flowering plants – dicotyledons. Magnoliid, hamamelid and caryophyllid families , 322 – 331 . Berlin, Heidelberg : Springer .
- Endress , P. K. and Igersheim , A. 1999 . Gynoecium diversity and systematics of the basal eudicots. . Bot. J. Linn. Soc. , 130 : 305 – 393 .
- Endress , P. K. and Stumpf , S. 1991 . The diversity of stamen structures in ‘Lower’ Rosidae (Rosales, Fabales, Proteales, Sapindales). . Bot. J. Linn. Soc. , 107 : 217 – 293 .
- Erdtman , G. 1952 . Pollen morphology and plant taxonomy. Angiosperms , Stockholm : Almquist & Wiksell .
- Ferguson , I. K. 1975 . Pollen morphology of the tribe Triclisieae of the Menispermaceae in relation to its taxonomy. . Kew Bull. , 30 : 49 – 75 .
- Friis , E. M. , Crane , P. R. and Pedersen , K. R. 1988 . Reproductive structure of Cretaceous Platanaceae. . Biol. Skr. Dan. Vid. Selsk. , 31 : 1 – 55 .
- Friis , E. M. , Crane , P. R. and Pedersen , K. R. 1997 . Anacostia, a new basal angiosperm from the Early Cretaceous of North America and Portugal with monocolpate/trichotomocolpate pollen. . Grana , 36 : 225 – 244 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 1999 . Early angiosperm diversification: The diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. . Ann. Mo. Bot. Gard. , 86 : 259 – 296 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2000a . Fossil floral structures of a basal angiosperm with monocolpate, reticulate‐acolumellate pollen from the Early Cretaceous of Portugal. . Grana , 39 : 226 – 245 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2000b . Reproductive structure and organization of basal angiosperms from the Early Cretaceous (Barremian or Aptian) of Western Portugal. . Int. J. Pl. Sci. , 161 (6 Suppl.) : S169 – S182 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2001 . Fossil evidence of Water Lilies (Nymphaeales) in the Early Cretaceous. . Nature , 410 : 357 – 360 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2006a . Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction. . Palaeogeogr. Palaeoclimatol. Palaeoecol. , 232 : 251 – 293 .
- Friis , E. M. , Pedersen , K. R. and Schönenberger , J. 2006b . Normapolles plants: A complex of extinct fagalean lineages. . Pl. Syst. Evol. , 260 : 107 – 140 .
- Germeraad , J. H. , Hopping , C. A. and Muller , J. 1968 . Palynology of Tertiary sediments from tropical areas. . Rev. Palaeobot. Palynol. , 6 : 189 – 348 .
- Halbritter , H. and Hesse , M. 2000 onwards . Acer campestre. PalDat – a palynological database: Descriptions, illustrations, identification, and information retrieval. http://www.paldat.org/ . R. Buchner & M. Weber (Eds)
- Heimhofer , U. , Hochuli , P. A. , Burla , S. and Weissert , H. 2007 . New records of Early Cretaceous angiosperm pollen from Portuguese costal deposits: Implications for the timing of the early angiosperm radiation. . Rev. Palaeobot. Palynol. , 144 : 39 – 76 .
- Hoot , S. B. , Magallon , S. and Crane , P. R. 1999 . Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. . Ann. Mo. Bot. Gard. , 86 : 1 – 32 .
- Hufford , L. D. and Endress , P. K. 1989 . The diversity of stamen structures and dehiscence patterns among Hamamelididae. . Bot. J. Linn. Soc. , 99 : 301 – 46 .
- Hughes , N. F. and McDougall , A. B. 1990 . Barremian‐Aptian angiospermid pollen records from southern England. . Rev. Palaeobot. Palynol. , 65 : 145 – 151 .
- Krutzsch , W. 1959 . Einige neue Formgattungen und ‐Arten von Sporen und Pollen aus der mitteleuropäischen Oberkreide und dem Tertiär. . Palaeontogr. B , 105 : 125 – 157 .
- Kumazawa , M. 1936 . Pollen grain morphology in Ranunculaceae, Lardizabalaceae and Berberidaceae. . Jpn. J. Bot. , 8 : 19 – 46 .
- Köhler , E. 2007 . “ Buxaceae. In K. Kubitzki (Ed.), ” . In The Families and Genera of Vascular Plants, Flowering Plants. 9. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae , 1 – 509 . Berlin : Springer .
- Köhler , E. and Brückner , P. 1982 . Die Pollenmorphologie der afrikanischen Buxus‐ und Notobuxus‐arten (Buxaceae) und ihre systematische Bedeutung. . Grana , 21 : 71 – 82 .
- Lee , S. and Blackmore , S. 1992 . A palynotaxonomic study of the genus Trollius (Ranunculaceae). . Grana , 31 : 81 – 100 .
- Leng , Q. and Friis , E. M. 2003 . Sinocarpus decussatus gen. et sp. nov., a new angiosperm with syncarpous fruits from the Yixian Formation of Northeast China. . Pl. Syst. Evol. , 241 : 77 – 88 .
- Leng , Q. and Friis , E. M. 2006 . Angiosperm leaves associated with Sinocarpus Leng et Friis infructescences from the Yixian Formation (mid‐Early Cretaceous) of NE China. . Pl. Syst. Evol. , 262 : 173 – 187 .
- Magallón , S. , Crane , P. R. and Herendeen , P. S. 1999 . Phylogenetic pattern, diversity and diversification of eudicots. . Ann. Mo. Bot. Gard. , 86 : 297 – 372 .
- Motten , A. F. 1986 . Pollination ecology of the spring wildflower community of atemperate deciduous forest. . Ecol. Monogr. , 56 : 21 – 42 .
- Norris , G. 1967 . Spores and pollen from the Lower Colorado Group (Albian‐?Cenomanian) of central Alberta. . Palaeontogr. B , 120 : 72 – 115 .
- Nowicke , J. W. and Skvarla , J. J. 1981 . Pollen morphology and phylogenetic relationships of the Berberidaceae. . Smiths. Contrib. Bot. , 50 : 1 – 83 .
- Nowicke , J. W. and Skvarla , J. J. 1982 . Pollen morphology and the relationships of Circaeaster, of Kingdonia, and of Sargentodoxa to the Ranunculaceae. . Am. J. Bot. , 69 : 990 – 998 .
- Pedersen , K. R. , Friis , E. M. , Crane , P. R. and Drinnan , A. N. 1994 . Reproductive structures of an extinct platanoid from the Early Cretaceous (latest Albian) of eastern North America. . Rev. Palaeobot. Palynol. , 80 : 291 – 303 .
- Penny , J. H. 1988 . Early Cretaceous striate pollen from the Borehole Mersa Matruh 1, North West Desert, Egypt. . J. Micropalaeontol. , 7 : 201 – 215 .
- Penny , J. H. J. 1991 . Early Cretaceous angiosperm pollen from the borehole Mersa Matruh 1, North West desert, Egypt. . Palaeontogr. B , 222 : 31 – 88 .
- Potonié , R. 1966 . Synopsis der Gattungen der Sporae dispersae. IV Teil: Nachträge zu allen Gruppen (Turmae). . Beih. Geol. Jb. , 72 : 1 – 244 .
- Rouse , G. E. 1962 . Plant microfossils from the Burrard Formation of Western British Columbia. . Micropaleontol. , 8 : 187 – 218 .
- Singh , C. 1971 . Lower Cretaceous microfloras of the Peace river area, northwestern Alberta. . Res. Counc. Alberta Bull. , 28 : 1 – 542 .
- Soltis , P. S. and Soltis , D. E. 2004 . The origin and diversification of angiosperms. . Am. J. Bot. , 91 : 1614 – 1626 .
- Srivastava , S. K. 1966 . Upper Cretaceous microflora (Maestrichtian) from Scollard, Alberta, Canada. . Pollen Spores , 8 : 497 – 552 .
- Srivastava , S. K. 1977 . A new fossil pollen genus Rutihesperipites. . Pollen Spores , 19 : 531 – 543 .
- Sutton , D. A. 1989 . “ The Didymelales: A systematic review. ” . In Evolution, Systematics, and Fossil History of the Hamamelidae. Vol. 1. Introduction and “Lower” Hamamelidae , Edited by: Crane , P. R and Blackmore , S . 279 – 284 . Oxford : Clarendon Press .
- Thanikaimoni , G. 1984 . Menispermacées: palynologie et systematique. Pondichery: Inst. Fr. . Trav. Sect. Sci. Tech. , 5
- Walker , J. W. and Doyle , J. A. 1975 . The bases of angiosperm phylogeny. . Ann. Mo. Bot. Gard. , 62 : 664 – 723 .
- Ward , J. V. 1986 . Early Cretaceous angiosperm pollen from the Cheyenne and Kiowa Formations (Albian) of Kansas, USA. . Palaeontogr. B , 202 : 1 – 81 .
- von Balthazar , M. and Endress , P. K. 2002a . Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. . Int. J. Pl. Sci. , 163 : 847 – 876 .
- von Balthazar , M. and Endress , P. K. 2002b . Reproductive structures and systematics of Buxaceae. . Bot. J. Linn. Soc. , 140 : 193 – 228 .
- von Balthazar , M. , Pedersen , K. R. and Friis , E. M. 2005 . Teixeiraea lusitanica gen. et sp. nov., a Ranunculalean flower from the Early Cretaceous of Portugal. . Pl. Syst. Evol. , 255 : 55 – 75 .
- von Balthazar , M. , Schatz , G. E. and Endress , P. K. 2003 . Female flowers and inflorescences of Didymelaceae. . Pl. Syst. Evol. , 237 : 199 – 208 .
- Zavada , M. S. and Dilcher , D. L. 1986 . Comparative pollen morphology and its relationship to phylogeny of pollen in the Hamamelidae. . Ann. Mo. Bot. Gard. , 73 : 348 – 381 .
- Zbyszewski , G. , Manupella , G. and Da Veiga Ferreira , O. 1974 . Carta geológica de Portugal na escala de 1/50 000. Notícia explicativa da folha 27‐A Vila Nova de Ourém , Lisboa : Serviços Geol. Port .