Abstract
Many anemophilous, early‐flowering tree genera include allergy plants of world‐wide significance. We studied the synchronisation of high and low pollen years in the genera Betula, Alnus, Corylus, Salix and Populus and the cumulative effects that an increasing number of taxa has on the number of days of exposure to different levels of allergenic pollen in North Europe. The proximal causes of the inter‐annual variations of airborne pollen loads were analysed with a multiple regression analysis. The annual fluctuations of airborne pollen sums were compared between genera and found to be positively correlated among all combinations of genera at the three study sites. Most correlations were statistically significant (p<0.05). The comparison between Betula and Alnus is discussed first. Betula pollen was clearly the most abundant airborne pollen type. The presence of Alnus pollen, however, significantly increased the predisposal to allergenic pollen. At all sites, the number of days per year when the Betula and Alnus pollen counts together exceeded 10 and 100 grains m−3 of air, was found to be greater than the number of days when the Betula pollen counts alone exceeded 10 and 100 m−3 of air. The difference was statistically significant. In Kuopio, the difference was found to be statistically significant even for grains per 1 000 m−3 of air of Betula and Alnus together compared with the same count of Betula pollen alone. Betula, Alnus and Corylus belong to the order Fagales and have cross‐reacting main allergens. The flowering of Alder and Corylus culminate at the same time, two to four weeks earlier than that of Betula. Due to synchronization of high and low years and the mostly non‐overlapping flowering seasons, the time of exposure to pollen may be very long during the high years. Furthermore, Alnus and Corylus pollen may prime allergic people before the onset of the Betula season.
Synchronized inter‐annual fluctuation of flowering intensity affects the exposure to allergenic tree pollen in North Europe
The reproductive output of many forest tree species in the boreal and temperate zones varies intermittently and extensively from year to year (Koenig & Knops, Citation2000). Fluctuation in flowering is likely to have a major effect on the reproduction of plant populations. An exponential positive relationship exists between the amount of pollen production on the one hand, and pollination efficiency and seed viability on the other, of the tree genera belonging to the Betulaceae (birch family) (Sarvas, Citation1952; Shibata et al., Citation1998).
Many anemophilous, early‐flowering tree genera include allergy plants of world‐wide significance. In northern boreal and temperate zones, the species belonging to the order Fagales, birch (Betula), alder (Alnus), hazel (Corylus) and hornbeam (Carpinus) are among the most important agents of seasonal allergies. Especially in northern Europe, Betula is a very important allergy plant with 10–15% of the population sensitised to its allergens. It is also a significant cause of allergy in central Europe and North and Central Asia (D'Amato et al., Citation1991; Savitsky & Kobzar, Citation1996; WHO, Citation2003). In North America, Betula, Alnus and Corylus are regarded as allergy plants over wide geographic areas. Salix and Populus, the broad‐leaved genera belonging to the Salicaceae (willow family, order Malphigiales), have a circumpolar distribution and are also significant allergy plants (D'Amato et al., Citation1991; Hough, Citation1947; More & White, Citation2003; Savitsky & Kobzar, Citation1996).
Extensive fluctuation in flowering is characteristic for trees belonging to the Fagales. Sarvas Citation(1952) described large annual variations in the pollen catch and seed sets of two European Betula species, B. pendula Roth. (silver birch) and B. pubescens L. (downy birch). Likewise, Shibata et al. Citation(1998) observed the same phenomenon with seven Carpinus species in Japan. Many aerobiological studies have demonstrated the extensive year‐to‐year fluctuations of airborne birch pollen sums (Ranta et al., Citation2005; Spieksma et al., Citation1995). The annual fluctuations in the flowering of Alder, Corylus and the Salicaceae trees have been studied less (however, see Worrell et al., Citation1999). Nevertheless, the great annual variability in airborne pollen loads has been demonstrated in aerobiological and palynological studies (Autio & Hicks, Citation2004; Emberlin et al., Citation2007).
In boreal and temperate environments, the flowers of early‐flowering species, like those belonging to the Fagales and Salicaceae, develop during the growing season that precedes pollination (Edmonds, Citation1979). The inception of the flower primordial and the development of catkins are assumed to depend on resource storage, which in turn is affected, via photosynthesis, by weather parameters (Hampson et al., Citation1996; Koenig & Knops, Citation2000; Masaka & Maguchi, Citation2001; Schauber et al., Citation2002). It is obvious, however, that weather variables alone do not explain the year‐to‐year variation in flowering. For one thing, resource allocation within the tree varies from year to year. Allocation of resources to ripening seeds in particular, may strongly reduce leaf area, which results in less photosynthetic capacity and less catkin formation in the years following successful flowering (Dahl & Strandhede, 1993; Tuomi et al., Citation1982). Several independent studies have demonstrated that there is a positive correlation between flowering intensity and the summer temperature and flowering intensity of the previous year. Further, there is a negative autocorrelation in the reproductive output of successive years (Koenig & Knops, Citation2000; Koenig et al., Citation1994; Schauber et al., Citation2002; Turcotte & Houle, Citation2001).
Since the prevalence and severity of allergic symptoms are connected with the concentrations of airborne pollen (Viander & Koivikko, Citation1978), the annual fluctuations in airborne Betula pollen loads alone are likely to have substantial implications for public health and economy. Additionally, the exposure to extremely high levels of Betula pollen in infancy has been shown to increase the risk of sensitisation to birch pollen allergens, as well as increasing the risk of allergic asthma (Kihlström et al., Citation2002). The synchronous inter‐annual fluctuations in flowering among many allergy plants might have cumulative effects on allergy sufferers during high pollen years. Firstly, the number of days of exposure to pollen allergens may increase. Secondly, the species with cross‐reacting allergens may prime allergic people (Emberlin et al., Citation2007). This is especially the case if the pollination of allergy plants with homologous main allergens, like Betula, Alnus and Corylus (Valenta et al., Citation1991), should culminate in different times. And finally, the probability of becoming exposed to several types of allergenic pollen at the same time, such as the pollen of Fagales and Salicaceae trees, may increase.
We hypothesized that the same proximal causes, i.e. summer temperature and the negative autocorrelation of reproductive output between consecutive years, determine the amount of flowering in Betula, Alder, Corylus, Populus and Salix. Consequently, their flowering intensity should fluctuate synchronously, which in turn causes the synchrony of high‐ and low pollen‐years. Further, we assumed that the synchronous high pollen‐years of several species increase the time of exposure to allergenic pollen. We studied these assumptions in two ways. We compared the accumulated annual pollen sums of the genera in three sampling sites in Finland and North Europe and we studied the significance of the number of genera for the number of days per year with exposure to different levels of allergenic pollen. Finally, we analysed the effects of the previous year's summer temperature and reproductive output on annual airborne pollen loads by regression analyses.
Material and methods
Data
We used the time series of annual accumulated pollen sums in Finland, counted as a sum of the daily average pollen counts (pollen grains m−3 air), from the years 1974–2006 in Turku (60°32′N, 22°28E′), 1980–2006 in Kuopio (62°53′N, 27°38′E) and 1976–2006 in Oulu (65°04′N, 25°31′E) (Figure ). At all sites, pollen sampling was performed with the volumetric Burkard‐spore trap (Hirst, Citation1952) on an open rooftop. The technique of volumetric trapping is standard throughout most of Europe (British Aerobiology Federation, Citation1995). Pollen grains were counted and identified on randomised fields under microscopic observation (Mäkinen, Citation1981).
The Betula pollen data includes the pollen of two different birch species, B. pendula and B. pubescens. According to Hämet‐Ahti et al. Citation(1998), the natural distribution of B. pubescens covers the whole of Finland and B. pendula extends to about 65°N. Likewise, the Alnus pollen data also includes the pollen of two species, A. incana (L.) Moench (European alder) and A. glutinosa (L.) Gaertner (black alder). The distribution of A. incana covers the whole of Finland. A. glutinosa is found at all of the study sites, but its distribution does not cover the continental parts of Northern Finland. Corylus avellana L. (hazel) is common in Finland only in a narrow hemi‐boreal vegetation zone along the south‐western coast. Of all the study sites, it only belongs to the native flora in Turku. Within the order Fagales, Betula and Alnus belong to the Betulaceae family, while Corylus is a member of the Corylaceae. The distribution of Populus tremula L. (European aspen), the only native Populus species in Finland, covers the whole country. There are approximately 40 different Salix species in Finland, both trees and bushes, 10 to 15 of which grow at the study sites. Betula, Alnus, Populus, Corylus and Salix have a circumpolar distribution. Except the extreme north and south, the distributions of the species found in the study area, B. pendula, B. pubescens, A. glutinosa, A. incana, and P. tremula, cover most of Europe and continental Asia. A. glutinosa is also a native species in North America. C. avellana is native in Europe except the arctic and boreal zones, and in western Asia and North Africa (Hough, Citation1947; More & White, Citation2003).
The timing and length of the main pollination season was defined separately for each taxon at each study site. It was calculated as a mean of annual values, and the season was defined as starting when the accumulated pollen sum reaches 5% of the annual total and as ending when 95% of the annual total is reached (Dahl & Strandhede, Citation1996).
Meteorological data, measured at Turku, Kuopio and Oulu airports, was provided by the Finnish Meteorological Institute. The meteorological observation sites were located within a range of 20 km of the pollen monitoring sites. The variable used in the regression analyses was the daily mean temperatures between May and July.
Analyses
The annual pollen sums of all combinations of genera at each site separately were compared by using the Spearman's rank correlation analysis. Non‐parametric correlation was used because all data‐sets could not be normalized with the same transformation, and parametric correlation strictly assumes normally distributed data. For graphical comparisons, all data‐sets were standardized to mean = 0 and standard deviation = 1.
Regression analysis was performed for each genus separately for Turku, Kuopio and Oulu. The two variables used to explain the inter‐annual variation of the pollen sums were the annual pollen sum and the mean temperature (°C) between May and July in the year preceding pollination. The analysis was not carried out on Corylus in Turku or on Populus in Oulu due to very low annual amounts of airborne pollen.
The effect of the increasing number of flowering genera on the number of days of exposure to different levels of allergenic pollen was analysed with the t‐test for paired (non‐independent) samples. Betula pollen is clearly the most abundant pollen type in the area. Therefore, the testing was performed by comparing 1) the number of days per year that have daily average pollen counts which exceed 10, 100 and 1 000 grains m−3 of air of Betula alone, with 2) the number of days that have daily average Betula and Alnus pollen counts that exceed 10, 100 and 1 000 grains m−3 of air. In Turku, the comparison was also made between the number of days per year that have daily average Betula and Alnus pollen counts exceeding 10 grains m−3 of air and 3) that have Betula and Alnus or Salix counts exceeding 10 grains m−3 of air.
The annual pollen sums and the principal pollination periods were counted using the statistical facilities of the European Aeroallergen Network. Statistical analyses were performed with the SAS Enterprise Guide (SAS Inst. Inc., Citation1999).
Results
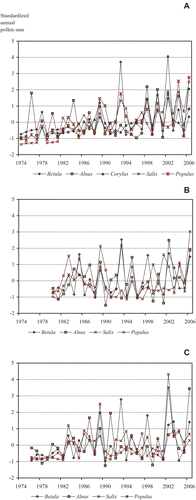
The inter‐annual fluctuations of airborne pollen sums were positively correlated among all combinations of genera at the three measuring sites. In Turku, the southernmost study site, all correlations except the suggestive (p = 0.096) correlation of Alnus and Corylus, were statistically significant (p<0.05, Table ). The correlations between Betula and Alnus were statistically significant at all three sites. In Kuopio, the correlations between Salix and Betula, Populus and Salix and Populus and Alnus were not statistically significant, as was the case in Oulu for the correlations between Salix and Betula, and Salix and Alnus (Table , Figure ). The graphical analysis (Figure ) of the inter‐annual fluctuations shows that at the southernmost study site (Turku), the synchronization among the different genera and the negative autocorrelation of pollen production between consecutive years has been strong during the last 15 years.
Table I. Comparison of annual pollen sums of Betula, Alnus, Salix, Populus and Corylus in Turku, Kuopio and Oulu using Spearmans' rank correlation analyses.
Figure 2 Standardized time series of the fluctuations of pollen sums in(A) Turku, (B) Kuopio and (C) Oulu.
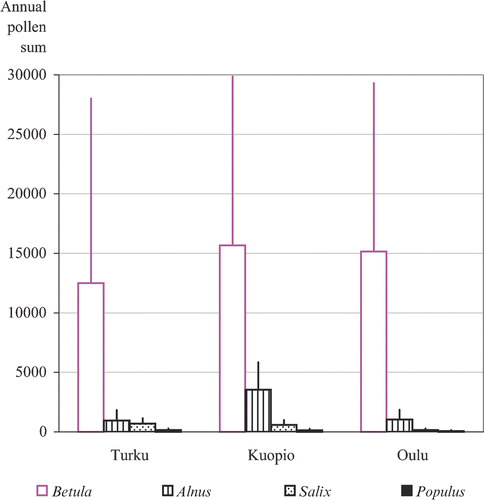
Betula pollen was clearly the most abundant airborne pollen type at the three measuring sites. The annual accumulated sums of airborne birch pollen were an order of magnitude, or more, greater than the values for Alnus, Salix and Populus (Figure ). In Kuopio, however, the average annual pollen sum of Alnus was as large as 3 537, which was approximately one fifth of the average annual sum of Betula at that site. The average annual pollen sums of Corylus ranged from only 6 to 268 pollen grains in Turku. In Kuopio and Oulu Corylus pollen was encountered only occasionally.
Figure 3 Annual average pollen sums ofBetula, Alnus, Salix and Populus at the three study sites. Number of year is 33, 27 and 31 for Turku, Kuopio and Oulu, respectively. Standard deviation is shown.
Despite the dominance of Betula, Alnus pollen had a significant effect on the number of days of predisposal to allergenic pollen. At all sites, the number of days per year when the sum of Betula and Alnus pollen exceeded 10 and 100 grains m−3 of air, and in Kuopio even that exceeding 1 000 grains m−3 of air, was statistically significantly greater than the number of days that had corresponding Betula pollen counts only (Table ).
Table II. Summary statistics of the number of days per year that had daily average pollen counts of Betula and Alnus exceeding 10, 100 and 1 000 pollen grains per m−3 of air.
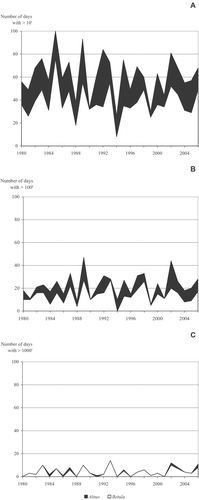
The average number of days per year that had daily average pollen counts of Betula and Alnus together exceeding 10, 100 and 1 000 pollen grains m−3 of air varied between 49 to 61, 17 to 22 and 3 to 4, at the three sites respectively. The range and standard deviations among the annual values were noted, indicating the great variability among years (Table , Figure ). The difference between the number of days that had Betula pollen only and the number of days that had Betula and Alnus together where the count exceeded 10 and 100 grains m−3 of air, ranged from 12 to 23 and 2 to 8 days respectively, at the three sites. The differences were the largest in Kuopio (Table , Figure ).
Table III. Comparison of the number of days per year that had daily average pollen counts of Betula exceeding 10, 100 and 1 000 grains m−3 of air, with the number of days with the daily average Betula and Alnus pollen counts exceeding 10, 100 and 1 000 grains m−3 of air.
Figure 4 The number of days per year that had daily average pollen counts ofBetula only or Betula and Alnus pollen counts together exceeding (A) 10, (B) 100 and (C) 1 000 grains m−3 of air in Kuopio, eastern Finland.
In Turku, where the proportion of Salix pollen is relatively high (Figure ), the number of days per year that had Betula and Alnus or Salix pollen counts exceeding 10 pollen grains m−3 of air was approximately two days greater than the number of days that had Betula and Alnus pollen counts only, that exceeded 10 pollen grains m−3 of air. The difference, however, was not statistically significant (Table ). The pollen of Populus had little effect. Both the daily average pollen counts as well as the annual pollen sums were constantly very low, the latter ranging from 148 (Turku) to 74 (Oulu).
The chronological order of the pollen seasons of the four genera was about the same at the three measuring sites. The allergenic season starts with Alnus, followed by Populus and Salix and finally Betula (Figure ). On average, the principal pollen season of Alnus begins between 44 (Turku) and 17 (Kuopio) days earlier than that of Betula, and between 40 (Turku) and 18 (Oulu) days earlier than Salix. The pollen seasons of Betula, Salix and Populus are mostly overlapping.
Figure 5 The timing of pollen seasons, defined as starting when the accumulated pollen sum reaches 5% of the annual total and as ending when 95% of the annual total is reached, in (A) Turku, (B) Kuopio and (C) Oulu.
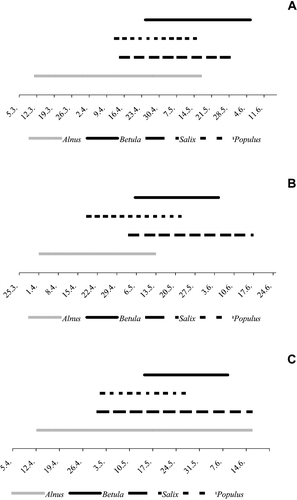
Table shows that the regression model was either statistically significant (p<0.05) or suggestive (p = 0.05–0.1) in all cases except for Populus in Turku and Salix in Kuopio. The statistically significant models explained 24–45% of the annual fluctuations of airborne pollen loads. A statistically significant or suggestive negative autocorrelation between the annual pollen sums of consecutive years was found in eight of 11 cases, and the positive association between the annual pollen sum and the average temperature in the May – July of the year before pollination was found in 7 of 11 cases.
Table IV. Multiple regression analysis of annual fluctuations of pollen sums for different locations and genera.
Discussion
The years with abundant and low airborne pollen sums of the genera belonging to the Fagales, Betula, Alnus and Corylus, tended to occur at the same time. In most cases, the situation was the same for the other combinations of the genera of the Fagales and Salicaceae. Such results have not been published before to our knowledge. Of the 22 comparisons between two genera, five were neither statistically significant nor suggestive. In four of these cases, the other object of comparison was Salix, the genus that includes species relying on both insects and wind, or on wind only as pollinating agents in the study area (Myklestad & Birks, Citation1993; Tollsten & Knudsen, Citation1992). This probably decreases the comparability of Salix to solely wind‐pollinated trees when airborne pollen data is used. However, most of the associations between the annual fluctuations in the pollination intensity of Salix and the other genera were also positive and statistically significant.
Many ecological studies exist that compare the inter‐annual variation of seed or flower production among different plant taxa. Shibata et al. Citation(1998) observed the strong synchronization of annual fluctuations in seed production among four Carpinus species in Japan. Likewise, Ranta et al. Citation(2006) observed the same for the number of male catkins of B. pendula and B. pubescens in Finland. Shibata et al. Citation(2002) found out that taxonomically distant tree species showed synchronized annual seed production with each other in a temperate deciduous forest in Japan. Likewise, Schauber et al. Citation(2002) showed indications of the same even between species of monocotyledons and dicotyledons in New Zealand. However, the synchronization of flowering is not a rule in nature; seed production patterns of species belonging to the same genus may be uncorrelated (Koenig et al., Citation1994; Shibata et al., Citation2002).
Two potential explanations that are not mutually exclusive exist for the synchronized fluctuations of airborne pollen loads among different tree genera. Firstly, the same proximal factors may regulate the pollen production of different taxa. Secondly, the weather conditions during the pollination period may increase the similarity of inter‐annual variation among genera with overlapping pollination periods. Pollination success depends heavily on unpredictable environmental features, such as wind, rain and temperature, during the flowering event (Edmunds, Citation1979; Peternel et al., Citation2005). Moreover, tree pollen originating in remote areas may significantly contribute to the pollen counts observed in Scandinavia. This has been shown with birch pollen (Hjelmroos, Citation1991; Ranta et al., Citation2006), but it may also apply to other tree pollen types with similar aerodynamic characters (Sofiev et al., Citation2006). In such cases, pollen grains of the genera with overlapping pollination seasons may be transported in the same air masses.
There are, however, several reasons to believe that the synchronized variations in annual pollen sums are genuine and largely due to the annual variation in pollen production. Firstly, in south‐eastern Scandinavia, the flowering of Alnus culminates two to four weeks before that of Betula, Salix or Populus (D'Amato et al., Citation1991; Ranta & Pessi, Citation2006). Thus the pollen of Alnus is not subject to the same weather conditions and/or transport events as the pollen of the other species. Moreover, the results from several plant ecological studies have demonstrated that the synchronous fluctuations in reproductive output do not involve the populations of only one plant species, but even different species and families in large geographic areas (Koenig et al., Citation1994; Schauber et al., Citation2002; Shibata et al., Citation2002). Hicks Citation(2001) also found, using a standardized deposition method, that in northern Finland major pollen years can be recognized in which pollen deposition tends to be high in several tree taxa, and frequently in dwarf shrub taxa, as well. Autio & Hicks Citation(2004) found an association between the amount of deposited pollen of several tree species and the previous years' summer temperature in the northern tree‐line area. On the other hand, Schauber et al. Citation(2002) and Tapper Citation(1996) suggest that the environmental cue that synchronizes the flowering of several distantly related plant species might be a deviation from the local annual mean temperature, not the absolute temperature.
Regression analyses
The regression model with the same explanatory variables, summer temperature and the pollen sum of the preceding year, was found to explain the annual fluctuations of the pollen sums of most of the taxa studied at the three sites. Similar results with data sets of airborne birch pollen have been presented earlier by Dahl & Strandhede Citation(1996) and Masaka & Magushi (Citation2001), for example. The statistically significant regression models explained 24–45% of the annual fluctuations in pollen sums, which is less than that detected in earlier studies with similar explanatory variables, but which were performed with shorter (10 to 12 year) time series of Betula pollen (Dahl & Strandhede, Citation1996; Masaka & Magushi, Citation2001; Ranta et al., Citation2005). An earlier comparison of results from regression analyses using the 12‐year and 20‐year sets of the same data on Betula pollen sums indicated that both the time‐span and the time‐interval of the data may greatly affect the power of such a model (Ranta et al., Citation2005). A probable reason for the differences is a long‐term change that affects the amount of airborne pollen. There may be extraneous factors underlying the development, such as changing vegetation and/or succession of broadleaved forests, or a trend towards a warmer climate with a non‐linear effect on flowering intensity. A constant increase in pollen loads of early‐flowering trees has been found, especially in Northern Europe during the last 30 years (WHO, Citation2003; Rasmussen, Citation2002). The strong synchronization among different genera and the negative autocorrelation of pollen production between consecutive years may be related to climatic warming; in some ranges it may favour plant growth and reproduction (reviewed by Norby & Luo, Citation2004). It can be speculated that under near‐optimal weather conditions, the resource allocation within trees is the most important factor regulating reproductive output.
In this study, the results obtained with the same regression model structure for the same genera were parallel but not identical at different sites. It must be noted that the annual airborne pollen sums of Salix and Populus were constantly low. In such cases especially, the annual pollen sums, calculated as a sum of daily average pollen counts m−3 air, may be strongly affected by stochastic environmental conditions, such as weather, during the pollination season (Edmonds, Citation1979; Peternel et al., Citation2005). In other words, the annual pollen sum incompletely describes the annual flowering intensity. In addition, morphological constraints and the readiness to form floral organs at a given time may contribute to the inter‐annual variation in flowering in some species (Hasegawa & Takeda, Citation2004; Yuceer et al., Citation2003). Although the whole model was not statistically significant for Alnus in Kuopio, it was found that the average temperature of the spring and summer preceding pollination had a statistically significant effect on the annual airborne pollen sum. In addition, the effect of the previous years' pollen sum was suggestive (p = 0.097).
Effects of the increasing number of flowering genera
Hjelmroos et al. (Citation2006) suggest that instead of using individual pollen taxa, pollen categories should be used to describe the daily exposure estimates in epidemiologic studies. Betula, Alnus, Carpinus, Corylus and Ostrya, all belonging to Fagales, form one category because of their similarity as allergenic agents. They are closely related, early flowering anemophilous trees with cross‐reactive main allergens (Valenta et al., Citation1991), and their pollen is known to release submicronic, allergen‐bearing particles after precipitation (Grote et al., Citation2003). The results from this study indicated, that even in areas where birch pollen is dominant, the pollen of Alnus significantly increases the time of predisposal to moderate and even abundant levels of Betulaceae pollen. The result was the same at all three study sites, although the effect was most pronounced in Kuopio, where the proportion of airborne Alder pollen was the greatest. Due to the synchronization of high and low years and the largely non‐overlapping flowering seasons of Alnus and Betula, the time of exposure to Betulaceae pollen may be very long during the high years. In our study, the records were 100 (Kuopio; 1985), 47 (Kuopio; 1989) and 15 (Oulu; 1993) days per year of exposure to Betulaceae‐pollen counts that exceeded 10, 100 and 1 000 grains m−3 of air, respectively. Moreover, since Alnus and Corylus flower simultaneously but earlier than Betula, their pollen may prime allergic people before the start of the Betula season (Emberlin et al., Citation2007).
An extensive analysis of pollen records in Europe (data from 1974 to 2002, 17 pollen types, over 450 stations) showed that the pollen seasons of many early‐flowering taxa, including the tree genera studied here, have not only come to start earlier, but also lengthened (WHO, 2002). This may increase the risk of long and continuous pollen seasons. The possible changes over decades in the timing and length of the pollen seasons of Betula and Alnus, separately and/or in relation to each other, should be addressed in future studies because of their potential significance to allergic subjects.
Synchronous fluctuations in flowering intensity occur over large geographic areas. For instance, populations of Betula, Quercus, Pinus and Abies, that lie over 500 km from each other, have been found to flower synchronously (Koenig & Knops, Citation2000). Therefore, years of abundant pollen of allergy trees may occur over very large areas at the same time. Depending on the area in question, the additive effects similar to those of Betula and Alnus found in this study are likely to exist among the other genera of the Fagales. The inter‐annual fluctuations in the pollen sums of Corylus, Betula and Alnus were also found to be correlated. The significance of Corylus as an allergy plant is greater in central and southern Europe than in the north (D'Amato et al., 1993). Similar possibilities exist with the many Carpinus species occurring across the northern temperate regions (Hough, Citation1947; Shibata et al., Citation2002).
Salix and Populus
A tendency towards simultaneous high and low pollen years was also observed between Salix and Populus, and between these and the Fagales trees. In practice this could mean, that a multi‐sensitized person may become predisposed to high levels of many allergenic pollen types during the same year. Salix can be regarded as a potential allergy plant in the study area since the daily average pollen counts of Salix may exceed 100 grains per m−3 air in south and central Finland. The pollen season has two peaks, the first being generated by the species flowering before leaf bud break, and the second by the species flowering at the same time with it. The first peak overlaps with the latter half of the Alnus and Corylus seasons, and the latter with the Betula season (Ranta & Pessi, Citation2006). Very little is known about the intergeneric variation of pollen allergen content of different Salix species. The observed amounts of airborne Populus pollen were low, and potential effects may be masked by the other genera. Further, the allergenicity of pollen from P. tremula is poorly known. Some other Populus species, like P. alba L. in south and central Europe and P. deltoides Bartr. ex Marsh. in North America are known to cause sensitization (Lin et al. Citation2002; Yazicioglu et al., Citation2004).
Conclusions
The high and low annual pollen years of early‐flowering broadleaved arboreal taxa with allergenic pollen tends to occur at the same time. A probable reason for the phenomenon is that to some extent, the same proximal factors regulate the reproductive output of different tree species. Moreover, the same weather conditions during the pollination period are likely to increase the similarity in the inter‐annual variation of airborne pollen loads among plants that share the same flowering time.
The time of exposure to allergenic pollen, in this study, those of Betula and Alnus, which have cross‐reacting main allergens, may be very long in high pollen years in northern Europe. The results from this study indicated that, even in areas where birch pollen is clearly dominant, the pollen of Alnus significantly increases the time of predisposal to moderate and even abundant levels of Betulaceae pollen. The clinical consequences of long‐lasting predisposal to allergenic pollen or simultaneous exposure to high levels of different allergenic pollen types are not clear.
The weather conditions during the growing season and resource allocation within the tree have been identified as being important proximal factors affecting the intensity of flowering. However, the development of a universally applicable, powerful model for predicting the inter‐annual fluctuations of the flowering intensity of tree genera with these variables has not been successful. A probable reason for this is that the measured amounts of airborne pollen do not adequately reflect the amount of pollen production. Also, extraneous factors affecting airborne pollen loads, such as the succession of broadleaved forests, or a trend towards a warmer climate with anon‐linear effect on flowering intensity, would bias the predictions.
Acknowledgements
We thank Auli Rantio‐Lehtimäki for constructive comments. The pollen data for Turku, Kangasala and Oulu was produced by the Finnish Aerobiology Unit. The study was supported by the Finnish Academy.
References
- Autio , J. and Hicks , S. 2004 . Annual variations in pollen deposition and meteorological conditions on the fell Aakenustunturi in northern Finland: Potential for using fossil pollen as a climate proxy. . Grana , 43 : 31 – 47 .
- British Aerobiology Federation . 1995 . Airborne pollen and spores, a guide to trapping and counting , Harpenden : BAF . (1st ed.)
- Dahl , A. and Strandhede , S.‐O. 1996 . Predicting the intensity of the birch pollen season. . Aerobiologia , 12 : 97 – 170 .
- D'Amato , G , Spieksma , F. T. M and Bonini , S , eds. 1991 . Allergenic pollen and pollinosis in Europe , London : Blackwell Sci. Publ .
- Edmonds , R. L. 1979 . Aerobiology. The ecological systems approach , Stroudsburg, PA : Dowden, Hutchinson & Ross, Inc .
- Emberlin , J. , Smith , M. , Close , R. and Adams‐Groom , B. 2007 . Changes in the pollen season of the early flowering trees Alnus spp. and Corylus spp. in Worcester, United Kingdom, 1996–2005. . Int. J. Biometeorol. , 51 : 181 – 191 .
- Grote , M. , Valenta , R. and Reichelt , R. 2003 . Abortive pollen germination, mechanisms of allergen release in birch, alder, and hazel revealed by immunogold electron microscopy. . J. Allergy Clin. Immunol. , 111 : 1017 – 1023 .
- Hampson , C. R. , Azarenko , A. N. and Potter , J. R. 1996 . Photosynthetic rate, flowering, and yield component alteration in hazelnut in response to different light environments. . J. Am. Soc. Hortic. Sci. , 121 : 1103 – 1111 .
- Hasegawa , S. F. and Takeda , H. 2004 . Current‐year shoot based approach for annual variation in the reproductive output in Siberian alder (Alnus hirsuta var. sibirica) . Trees , 18 : 436 – 441 .
- Hicks , S. 2001 . The use of annual arboreal pollen deposition values for delimiting tree‐lines in the landscape and exploring models of pollen dispersal. . Rev. Palaeobot. Palynol. , 117 : 1 – 29 .
- Hirst , J. M. 1952 . An automatic volumetric spore trap. . Ann. Appl. Biol. , 39 : 29 – 33 .
- Hjelmroos , M. 1991 . Evidence of long‐distance transport of Betula pollen. . Grana , 30 : 215 – 228 .
- Hjelmroos‐Koski , M. , Macher , J. M. , Hammond , K. and Tager , I. 2006 . Considerations in the grouping of plant and fungal taxa for an epidemiologic study. . Grana , 45 : 261 – 287 .
- Hough , R. M. 1947 . Handbook of the trees of the Northern States and Canada East of the Rocky Mountains , New York : MacMillan Co .
- 1998 . Retkeilykasvio , Hämet‐Ahti L, Suominen J, Ulvinen T, Uotila P, eds. 4th ed. Helsinki. Bot. Mus. Finn. Mus. Nat. Hist, (In Finnish)
- Kihlström , A. , Lilja , G. and Pershagen , G. 2002 . Exposure to birch pollen in infancy and development of atopic disease in childhood. . J. Allergy Clin. Immunol. , 110 : 78 – 84 .
- Koenig , W. D. and Knops , J. M. H. 2000 . Patterns of annual seed production by northern hemisphere trees: A global perspective. . Am. Nat. , 155 : 59 – 69 .
- Koenig , E. D. , Mumme , R. L. , William , C. J. and Tanback , M. T. 1994 . Acorn production by oaks in central coastal California: Variation within and among years. . Ecology , 75 : 99 – 109 .
- Lin , R. Y. , Clauss , A. E. and Bennett , E. S. 2002 . Hypersensitivity to common tree pollens in New York City patients. . Allergy Asthma Proc. , 23 : 253 – 258 .
- Masaka , K. and Maguchi , S. 2001 . Modelling the masting behaviour of Betula platyphylla var. japonica using the resource budget model . Ann. Bot. , 88 : 1049 – 1055 .
- More , D. and White , J. 2003 . Cassell's Trees of Britain and Northern Europe , London : Orion Publ. Group .
- Myklestad , Å. and Birks , H. J. B. 1993 . A numerical analysis of the distribution patterns of Salix L. species in Europe. . J. Biogeogr. , 20 : 1 – 32 .
- Mäkinen , Y. 1981 . Random sampling in the study of microscopic slides. . Aerobiol. Lab. Univ. Turku Rep. , 5 : 27 – 43 .
- Norby , R. J. and Luo , Y. 2004 . Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi‐factor world: A review. . N. Phytol. , 162 : 281 – 293 .
- Peternel , R. , Srnec , L. , Hrga , I. , Hercog , P. and Culig , J. 2005 . Airborne pollen of Betula, Corylus and Alnus in Zagreb, Croatia. A three‐year record. . Grana , 44 : 187 – 191 .
- Ranta , H. , Oksanen , A. , Hokkanen , T. , Bondestam , K. and Heino , S. 2005 . Masting by Betula‐ species; Applying the resource budget model to north European data sets. . Int. J. Biometeorol. , 49 : 146 – 151 .
- Ranta , H. , Kubin , E. , Siljamo , P. , Sofiev , M. , Linkosalo , T. , Oksanen , A. and Bondestam , K. 2006 . Long‐ distance pollen transport cause problems for determining the timing of birch pollen season in Fennoscandia by using phenological observations. . Grana , 45 : 297 – 304 .
- Ranta , H and Pessi , A.‐M , eds. 2006 . The Finnish Pollen Bulletin. Supplement , Turku : Digipaino .
- Rasmussen , A. 2002 . The effects of climate change on the birch pollen season in Denmark. . Aerobiologia , 18 : 253 – 265 .
- Sarvas , R. 1952 . On the flowering of birch and the quantity of seed crop. . Comm. Inst. Forest. Fennica , 40 : 1 – 38 .
- SAS Institute Inc . 1999 . SAS/STAT user's guide , Cary, NC : SAS Inst. Inc . vers. 8, vol. 2 [Computer manual]
- Savitsky , V. D. and Kobzar , V. N. 1996 . Aerobiology in Russia and neighbouring countries, 1980–1993 ‐ A bibliographic review. . Grana , 35 : 314 – 318 .
- Schauber , E. M. , Kelly , D. , Turchin , P. , Simon , C. , Lee , W. G. , Allen , R. B. , Payton , I. J. , Wilson , P. R. , Cowan , P. E. and Brockie , R. E. 2002 . Masting by eighteen New Zealand plant species: The role of temperature as a synchronizing cue. . Ecology , 83 : 1214 – 1225 .
- Shibata , M. , Tanaka , H. , Iida , S. , Abe , S. , Masaki , T. , Niiyama , K. and Nakashiuzuka , T. 2002 . Synchronized annual seed production by 16 principal tree species in a temperate deciduous forest, Japan. . Ecology , 83 : 1727 – 1742 .
- Shibata , M. , Tanaka , H. and Nakashizuka , T. 1998 . Causes and consequences of mast seed production of four, co‐occurring Carpinus species in Japan. . Ecology , 79 : 54 – 64 .
- Sofiev , M. , Siljamo , P. , Ranta , H. and Rantio‐Lehtimäki , A. 2006 . Towards numerical forecasting of long‐range air transport of birch pollen: Theoretical considerations and a feasibility study. . Int. J. Biometeorol. , 50 : 392 – 402 .
- Solomon , W. R. 2002 . Airborne pollen: A brief life. . J. Allergy Clin. Immunol. , 109 : 895 – 900 .
- Spieksma , F. T. M. , Emberlin , J. C. , Hjelmroos , M. , Jaeger , S. and Leuschner , R. M. 1995 . Atmospheric birch (Betula) pollen in Europe – trends and fluctuations in annual quantities and the starting dates of the season. . Grana , 34 : 51 – 57 .
- Tapper , P. G. 1996 . Long‐term patterns of mast fruiting in Fraxinus excelsior. . Ecology , 77 : 2567 – 2572 .
- Tollsten , L. and Knudsen , J. T. 1992 . Floral scent in dioecious Salix (Salicaceae) – a cue determining the pollination system? . Pl. Syst. Evol. , 182 : 229 – 237 .
- Tuomi , J. , Niemelä , P. and Mannila , R. 1982 . Resource allocation on dwarf shoots of birch (Betula pendula): Reproduction and leaf growth. . N. Phytol. , 91 : 483 – 487 .
- Turcotte , J. and Houle , G. 2001 . Reproductive costs in Salix planifolia spp. planifolia in Subarctic Quebec, Canada . Ecoscience , 8 : 506 – 512 .
- Valenta , R. , Breiteneder , H. , Pettenburger , K. , Breitenbach , H. , Rumpold , H. , Kraft , D. and Scheiner , O. 1991 . Homology of the major birch‐pollen allergen, Bet VI, with the major pollen allergens of alder, hazel, and hornbeam at the nucleic‐acid level as determined by cross‐hybridization. . J. Allergy Clin. Immunol. , 87 : 677 – 682 .
- Viander , M. and Koivikko , A. 1978 . The seasonal symptoms of hyposensitized and untreated hay fever patients in relation to birch pollen counts: Correlation with nasal sensitivity, prick tests and RAST. . Clin. Allergy , 8 : 387 – 396 .
- WHO . 2003 . Phenology and human health: Allergic disorders , Copenhagen : WHO Eur. Reg. Off .
- Worrell , R. , Gordon , A. G. , Lee , R. S. and McInroy , A. 1999 . Flowering and seed production of aspen in Scotland during heavy seed year. . Forestry , 72 : 27 – 34 .
- Yazicioglu , M. , Oner , N. , Celtik , C. , Okutan , O. and Pala , O. 2004 . Sensitization to common allergens, especially pollens, among children with respiratory allergy in the Trakya region in Turkey. . Asian Pac. J. Allergy Immunol. , 22 : 183 – 190 .
- Yuceer , C. , Land , S. B. , Kubiske , M. E. and Harkess , R. L. 2003 . Shoot morphogenesis associated with flowering in Populus deltoides (Salicaceae). . Am. J. Bot. , 90 : 196 – 206 .