Abstract
Pollen is important for the nutrition of honeybees and it is necessary for their survival and reproduction. In this study, we collected daily the pollen pellets from four colonies and also recorded the plants in flower in the area around the apiary, over a two‐year period. Field records revealed the presence of 204 species with Asteraceae, Fabaceae and Rosaceae being the most specious families. Although honey bees collected more than 140 pollen types, the main pollen sources (>60% of the total weight) came from less than ten taxa. The most important pollen types with respect to total weight were Sisymbrium irio, Papaver rhoeas, Verbascum sp., Polygonum aviculare, Zea mays and Olea europaea. The use of pollen traps proved a more accurate method to record the type and the foraging period during which the honeybees collect pollen, compared to field observations.
Seasonal variation in vegetation and pollen collected by honeybees in Thessaloniki, Greece
The knowledge of the pollen flora of an area is a basic tool for the development of apiculture. Honeybees (Apis mellifera L.) depend both on nectar and pollen to sustain themselves (Winston, Citation1987), but pollen is necessary for raising the brood (Dietz, Citation1978). The absence of pollen can affect the strength of the colony and honey production (McLellan, Citation1974; Duff & Furgala, Citation1986; Nelson, Citation1987). Furthermore, the knowledge of the pollen flow periods could be used to determine when pollen substitutes or supplements should be supplied in an apiary resulting in the better growth of the colony.
Beekeeping in Greece plays a very important role. According to the Hellenic Ministry of Rural Development and Food Citation(2007) more than 23 000 families maintain colonies of honeybees. Greek beekeepers produce more than 14 000 t of honey annually; covering 98% of the domestic consumption. Although there are several studies about the nectar sources and honey types produced in Greece (Thrasyvoulou & Manikis, Citation1995; Tsigouri & Passaloglou‐Katrali, Citation2000; Tsigouri et al., Citation2004; Dimou et al., Citation2006b ), there are no records of pollen sources apart from few data that can be inferred from the honey analysis.
The objective of this study was to determine and evaluate the pollen sources of honeybees as well as the flowering periods and availability in the region of Thessaloniki. This study provides additional data about the pollen flora visited by the honeybees and the impact of the surrounding vegetation to the pollen foraged by the bees.
Material and methods
The study took place in the south‐east part of Thessaloniki, Greece at the farm of the Aristotle University, during 2003 and 2004. Plants in the study area are widely distributed Mediterranean therophytes and hemicryptophytes (Krigas, Citation2004). In addition, there are vineyards (Vitis vinifera L.) and several cultivated crops including: fruits (Pyrus spp. L., Prunus spp. L.), olive (Olea europaea L.), cotton (Gossypium hirsutum L.), wheat (Triticum durum L.), and corn (Zea mays L.) in the area. The neighbouring areas are of particular interest since the beekeeping activity is intense and more than 85 000 colonies are kept there.
Pollen traps are widely used to record the pollen flora of an area and to study the pollen diet of honey bees (Severson & Parry, Citation1981; Pearson & Braiden, Citation1990; Doull, Citation1966; Coffey & Breen, Citation1997; Nabors, Citation1997; Barth & da Luz, Citation1998; Cook et al., Citation2003; Webby, Citation2004; Andrada & Tellería, Citation2005). To record the pollen flora of the area we fitted pollen traps to the entrance of four honey bee hives. In each hive, the trap, containing a three‐day pollen sample, was removed every third day and all the collected pollen was cleaned of debris by hand, dried at room temperature and weighed. Then, 10% of the total amount of the pollen from each trap was sampled and the pollen loads were separated according to their colour (Dimou et al., Citation2006a ). At least two representative pellets of each colour fraction were mounded in glycerine jelly without acetolysis in order to identify the botanical origin of each colour. In each slide we counted at least 300 pollen grains to verify the homogeneity of each colour fraction (Percival, Citation1947; Pearson & Braiden, Citation1990). Pollen loads from the same flora source were weighed together in order to evaluate the contribution of each type. In each year the experiment lasted from March to November since during winter (December to February) the pollen collection is very low (less than 0.5 g per colony per day) and brood rearing is restricted.
At the same time, we recorded the blooming period and the population size of the taxa around the apiary (approximately 1 km radius). Once a week, plants in flower around the apiary were collected, mounted, stored in the Laboratory and identified with the use of dichotomous keys and verified by comparison with identified specimens from the herbarium of the Laboratory of Systematic Botany (Department of Biology, Aristotle University of Thessaloniki). For the estimation of the population size of the taxa we used a semi‐quantitative scale with four classes adapted from Wittig Citation(1993). The located taxa were classified into four classes. Class I contained scarcely located taxa, while class IV contained the dominant taxa (taxa covering at least 30% of the collection site). Reference pollen slides were prepared from all collected taxa without acetolysis (Louveaux et al., Citation1978).
Results
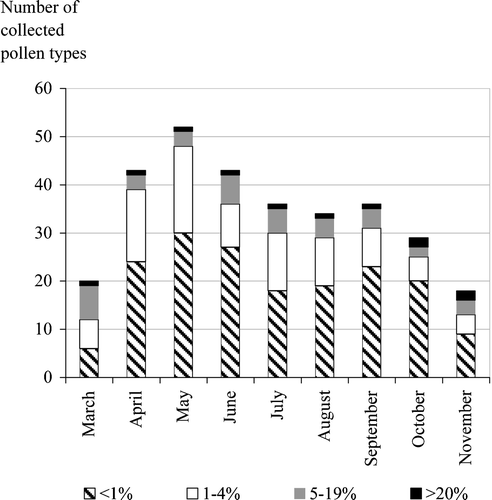
Over the two years we collected 674 samples from the colonies. Honey bees began collecting pollen during March, peaked during May and a decrease was recorded in the middle of November. On average, every colony collected about 6 kg of pollen each year. Most pollen (>50%) was collected during spring (mainly April–May) when the brood population was expanding. Over the two‐year period we recorded 142 different pollen types, and 101 of them during April, May and June–from which 55 were collected exclusively during these three months (Figure ). The mean number of collected pollen types per sample during spring was 9.7±0.5, during summer 11.6±2.6 and during autumn 8.0±3.2. Usually, in each trap sample two to three pollen sources were “dominant”, often exceeding 60% of the total trapped pollen. There were samples where the dominant pollen sources weighted more than 80% of the total collected pollen. These pollen loads came from cultivated crops such as Zea mays or plants with large populations and pollen flow such as Erica manipuliflora Salisb. Such phenomena occurred late in the summer or autumn where many pollen sources were scarce.
Figure 1 Average number of pollen types that the colonies collected in Thessaloniki during two apicultural periods(pollen abundance to the total weight).
Pollen was collected both from entomophilous and anemophilous plants. The main entomophilous pollen were from Sisymbrium irio L., Papaver rhoeas L., Verbascum sp., Polygonum aviculare L., Carduus ‐ Type, Reseda lutea L. and Rubus ulmifolius Schott (Figure ). The weight of these pollen sources reached 46% of the total trapped pollen during the study (Table ). In both years the main anemophilous plants were Pinus halepensis Miller, Acer negundo L., Zea mays, Olea europaea and Chenopodium sp. (Table ). The last three pollen sources contributed to more than 7% of the total weight each year. This is unusual, given that anemophilous pollen is characterized as having low nutrition value (Stanley & Linskens, Citation1974).
Figure 2 Weight(g) and foraging period of major pollen types collected by the honeybee colonies in Thessaloniki in 2003 and 2004. A. Sisymbrium irio; B. Papaver rhoeas; C. Carduus – Type; D. Polygonum aviculare.
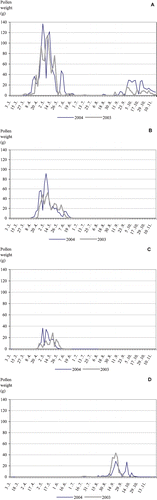
Table I. Overall total pollen type, foraging period and weight (%) of the collected pollen on average from the colonies in Thessaloniki during 2003 and 2004.
Analytically the average results of the two apicultural periods are as follows:
Spring (March to May). During spring, 67 different pollen types were collected. Most important, with respect to their contribution to the total weight, were Sisymbrium irio (28.6%), Papaver rhoeas (15.6%), Reseda lutea (4.2%), Olea europaea (4.1%), Taraxacum officinale Weber (3.1%), Carduus ‐ Type (7.2%) and Malabaila graveolens (Sprengel) Hoffm. (2.7%).
Summer (June to August). During summer, 70 different pollen types were collected. In 20 of them the collection commenced during spring and continued for the first few weeks of summer. Most important, with respect to their contribution to the total weight, were Zea mays (12.6%), Verbascum sp. (12.3%), Papaver rhoeas (8.9%), Rubus ulmifolius L. (7.2%), Chenopodium sp. (3.7%) and Cichorium intybus L. (2.2%).
Autumn (September to November). During autumn, 41 different pollen types were collected. Thirty of them commenced during the summer and continued during autumn. Most important, with respect to their contribution to the total weight, were Polygonum aviculare (17.6%), Sisymbrium irio (15.7%), Liliaceae ‐ Type (10.1%), Erica manipuliflora (9.3%), Chenopodium sp. (6.7%), Cynanchum sp. (6.4%), Portulaca oleracea (7.7%) and Hedera helix L. (5.8%).
Overall, 204 taxa belonging to 68 families were recorded in the area around the apiary. Asteraceae, Fabaceae and Rosaceae contained the greatest number of taxa. More flowering taxa were recorded during April, May and June than during autumn. During spring and summer most flowering taxa had a small population size (class I or II), while the population size of less than eight taxa was characterized with scale class IV (Figure ).
Figure 3 Average number of taxa in bloom in the area around the apiary during two apicultural periods[population size according to Wittig's Citation(1993) scale].
![Figure 3 Average number of taxa in bloom in the area around the apiary during two apicultural periods[population size according to Wittig's Citation(1993) scale].](/cms/asset/cafb74a0-6842-4209-8ffe-684ec08ca9e0/sgra_a_276047_o_f0003g.gif)
Pollen from only 100 taxa in the surrounding area was found in the pollen pellets (Table ). The blooming period that was recorded using field observations was shorter than the period during which the pollen of those plants appeared in the traps. This difference between the two methods (pollen traps and field records) varied from one week (usually) to two weeks (occasionally). Some taxa with large population sizes such as Fallopia auberii (L. Herny) Holub (class III), Solanum eleagnifolium Cav. (class III) and Sambucus ebulus L. (class II) were not found in the pollen pellets. On the other hand, 42 taxa found in the trap were not recorded in the study area using field observations (Table ).
Table II. Number of taxa located in the area and pollen types collected by the honeybees during 2003 and 2004.
Although the number of pollen types collected by honey bees was smaller than the number of the taxa in flower in the area, the same pattern between the number of flowering taxa per month and the number of the pollen types in the pellets was noticed (Figures and ). Linear regression analysis showed positive correlation between the number of taxa in bloom and the number of the collected pollen types by the honeybees (p<0.001, Pearson Correlation 0.458). Similar correlation was noticed between the population size of the taxa and the weight contribution of the foraged pollen types.
Discussion
The honey bees collected a wide variety of pollen types throughout the year. The most pollen types and with greatest contribution to the total weight were collected during spring (March–May) because the brood population was expanding at that time and pollen is needed as a protein source for growth (Al‐Tikrity et al., Citation1972; Camazine, Citation1993; Hrassnigg & Crailsheim, Citation1998; Pankiw et al., Citation1998; Dreller et al., Citation1999; Dreller & Tappy, Citation2000). Although more than 130 pollen sources were collected each year, only a few of them were collected in quantities greater than 1%, and even fewer were responsible for the total collected amount of pollen. Trapped pollen samples usually contained eight to twelve pollen types with only two or three being dominant. Usually the weight of the dominant types exceeded 60% of the total trapped pollen. In these samples, the pollen loads came from cultivated plants such as Zea mays or plants with high population size and flow such as Sisymbrium irio, Papaver rhoeas, Erica manipuliflora and Rubus ulmifolius. Similar reports of the pollen foraged by honey bees for a limited number of plants are also found in other studies (Cortopassi‐Laurino & Ramalho, Citation1988; Parent et al., Citation1990; Villanueva, Citation2002). Several authors have reported that honeybees collect pollen mainly from plants with large population sizes near colonies (Severson & Parry, Citation1981; Coffey & Breen, Citation1997). In this study, the surrounding vegetation significantly affected the pollen collection of the honey bees both in terms of the collected amount and the number of pollen types collected.
Although the number of taxa that were recorded in the area was high, the honeybees only utilized around half of them. Similar results have been reported by Andrada and Tellería Citation(2005). The authors showed that the pollen that the bees collected in their study area did not exceed 25% of the taxa recorded by previous studies in the region. The use of pollen traps compared to hand collection of flowering plants in an area seems to have an obvious advantage: traps, not only are more accurate regarding the blooming periods, but also give information about the available pollen sources (not all plants supply pollen or are visited by the bees) and their contribution to the colony.
In summary, Thessaloniki is characterised by having a large variety of plants. Honeybees collected pollen from several sources. Uncultivated plants play a role in their nutritional needs. Field observations can give some information about the bee flora; however, the use of pollen traps gives more accurate results.
Acknowledgements
The authors would like to thank Dr. E. Chanlidou (Laboratory of Systematic Botany, Department of Biology, Aristotle University of Thessaloniki, Greece) for helping in the botanical classification of the collected taxa.
References
- Al‐Tikrity , W. S. , Bentin , A. , Hillman , R. C. and Clarke , W. W. 1972 . The relationship between the amount of unsealed brood in honey bee colonies and their pollen collection. . J. Apic. Res. , 11 : 9 – 12 .
- Andrada , A. C. and Tellería , M. C. 2005 . Pollen collected by honey bees (Apis mellifera L.) from south of Caldén district (Argentina): Botanical origin and protein content. . Grana , 44 : 115 – 122 .
- Barth , O. M. and Da Luz , C. F. P. 1998 . Melissopalynological data obtained from a mangrove area near to Rio de Janeiro, Brazil. . J. Apic. Res. , 37 : 155 – 163 .
- Camazine , S. 1993 . The regulation of pollen foraging by honey bees: How foragers access the colony's need for pollen. . Behav. Ecol. Sociobiol. , 32 : 265 – 272 .
- Coffey , M. F. and Breen , J. 1997 . Seasonal variation in pollen and nectar sources of honey bees in Ireland. . J. Apic. Res. , 36 : 63 – 76 .
- Cook , M. S. , Awmack , C. S. , Murray , D. A. and Williams , I. H. 2003 . Are honey bees' foraging preferences affected by pollen amino acid composition? . Ecol. Entomol. , 28 : 622 – 627 .
- Cortopassi‐Laurino , M. and Ramalho , M. 1988 . Pollen harvest by Africanized Apis mellifera and Trigona spinipes in Sao Paulo, botanical and ecological views. . Apidologie , 19 : 1 – 24 .
- Dietz , A. 1978 . “ Nutrition of the adult honey bee. ” . In The hive and the honey bee , Edited by: Graham , M . 125 – 156 . Carthage, IL : Dadant & Sons .
- Dimou , M. , Thrasyvoulou , A. and Tsirakoglou , V. 2006a . Efficient use of pollen traps to determine the pollen flora used by honey bees. . J. Apic. Res. Bee World , 45 : 42 – 46 .
- Dimou , M. , Katsaros , J. , Tzavella Klonari , K. and Thrasyvoulou , A. 2006b . A study on the botanical and geographical discrimination of pine and fir honeydew honeys by their microscopical characteristics. . J. Apic. Res. Bee World , 45 : 16 – 21 .
- Doull , K. M. 1966 . The relative attractiveness to pollen collecting honeybees of some different pollen. . J. Apic. Res. , 5 : 9 – 4 .
- Dreller , C. and Tappy , D. R. 2000 . Perception of the pollen need by foragers in a honey colony. . Anim. Behav. , 59 : 91 – 96 .
- Dreller , C. , Page , R. E. and Fondrk , M. K. 1999 . Regulation of pollen foraging in honeybee colonies: Effect of young brood, stored pollen, and empty space. . Behav. Ecol. Sociobiol. , 45 : 227 – 233 .
- Duff , S. R. and Furgala , B. 1986 . Pollen trapping in honey bee colonies in Minnesota, Part II: Effect on foraging activity, honey production, honey moisture content, and nitrogen content of adult workers. . Am. Bee J. , 126 : 755 – 758 .
- Hellenic Ministry of Rural Development and Food. 2007. HMRD&F Website: www.minagric.gr
- Hrassnigg , N. and Crailsheim , K. 1998 . The influence of brood on the pollen consumption of worker bees (Apis mellifera L.). . J. Insect Physiol. , 44 : 393 – 404 .
- Krigas , N. 2004 . Flora and human activities in the area of Thessaloniki: Biological approach and historical considerations , Thessaloniki : Aristotle University . Ph. D. Thes. (in Greek)
- Louveaux , I. , Maurizio , A. and Vorwohl , G. 1978 . Methods of melissopalynology. . Bee World , 59 : 139 – 157 .
- McLellan , A. R. 1974 . Some effects of pollen traps on colonies of honeybees. . J. Apic. Res. , 13 : 143 – 148 .
- Nabors , R. A. 1997 . Trapping pollen collection of the honeybee Apis mellifera L. to determine pollen flow periods. . Am. Bee J. , 137 : 215 – 216 .
- Nelson , D. L. 1987 . The effect of continuous pollen trapping on sealed brood, honey production and cross income in Northern Alberta. . Am. Bee J. , 127 : 648 – 650 .
- Pankiw , T. , Page , R. E. Jr. and Fondrk , W. K. 1998 . Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). . Behav. Ecol. Sociobiol. , 44 : 139 – 198 .
- Parent , J. , Feller‐Demalsy , M. J. and Richard , P. J. H. 1990 . Les sources de pollen et nectar dans la region de Rimouski, Quebec, Canada. . Apidologie , 21 : 431 – 445 .
- Pearson , W. D. and Braiden , V. 1990 . Seasonal pollen collection by honey bees from grass/shrub highlands in Canterbury, New Zealand. . J. Apic. Res. , 29 : 206 – 213 .
- Percival , M. 1947 . Pollen collection by Apis mellifera. . New Phytol. , 46 : 142 – 173 .
- Severson , D. W. and Parry , J. E. 1981 . A chronology of pollen collection by honey bees. . J. Apic. Res. , 20 : 97 – 103 .
- Stanley , R. G. and Linskens , H. F. 1974 . Pollen. Biology, biochemistry and management , Berlin/New York/Heidelberg : Springer .
- Thrasyvoulou , A. and Manikis , J. 1995 . Some physicochemical and microscopic characteristics of Greek unifloral honeys. . Apidologie , 23 : 441 – 452 .
- Tsigouri , A. and Passaloglou‐Katrali , M. 2000 . A scientific note on the characteristics of thyme honey from the Greek islands of Kithira. . Apidologie , 31 : 457 – 458 .
- Tsigouri , A. , Passaloglou‐Katrali , M. and Sabatakou , O. 2004 . Palynological characteristics of different unifloral honeys from Greece. . Grana , 43 : 122 – 128 .
- Villanueva , G. R. 2002 . Polliniferous plants and foraging strategies of Apis mellifera (Hymenoptera: Apidae) in the Yucatan Peninsula, Mexico. . Rev. Biol. Trop. , 50 : 1035 – 1043 .
- Webby , R. 2004 . Floral origin and seasonal variation of bee‐collected pollens from individual colonies in New Zealand. . J. Apic. Res. , 43 : 83 – 92 .
- Winston , M. L. 1987 . The biology of the honeybee , London : Harvard Univ. Press .
- Wittig , R. 1993 . “ Flora and vegetation. ” . In Stadtökologie , Edited by: Sukopp , H and Wittig , R . 198 – 238 . Stuttgart/Jena/New York : G. Fisher .