Abstract
Male phenology, processes of microsporogenesis and pollen differentiation were studied for Cupressus sempervirens from the city of Tetouan (Morocco). Five phenological phases are described based on morphological and structural characters. Pollen mother cells stage, meiosis, microspore maturation and pollen development occurred in winter from December to February. Meiotic development shows typical coniferous pattern. Anthesis takes place two months after bud burst. The flowering phenophase takes place over one month and is associated with the main pollen emission period which started in the last week of February 2007. The pollen production of individual trees in C. sempervirens is also estimated. Tree size, cone density and number of pollen grains per cone are investigated. The results indicate that the number of pollen grains per cone and the total pollen production per tree were in the production range to those obtained in anemophilous trees. This study provides the first data on the onset of the pollen season and on the pollen production in C. sempervirens in Morocco and the contribution of this species to the total amount of airborne pollen content in Tetouan city.
Anemophilous plants are recognized to have small pollen that disperses very easily and in great quantities by wind. There is no information in Morocco about pollen production by common plants. The Cupressaceae are widely distributed over the Mediterranean area. They are also used as windbreaks to safeguard the qualitative and quantitative production of vegetable and fruit crops. Other uses such as ornamental urban and peri‐urban plantations shape the Mediterranean landscape. During winter when no other plants are flowering, they release pollen in very high quantities; accounting for some 70% of the pollen spectrum (Diaz de la Guardia et al., Citation2006) and causing pollinosis (Caiaffa et al., Citation1993; Charpin et al., Citation1993; D'Amato & Liccardi, Citation1994; Papa et al., Citation2001). In Morocco, up to 13.4% of patients suffering from pollinosis are sensitive to cypress pollen (Yazidi Alaoui & Bartal, Citation2000). The pollen grains of Cupressaceae family are stenopalynous (Bortenschlager, Citation1990; Kurmann, Citation1994). Therefore, it is impossible in the current state, to estimate the relative contribution of each species in the pollen spectrum of the aerobiological studies (Galán et al., Citation1998a ).
In the Mediterranean basin Cupressus is represented by three native and closely related species forming a circum‐Mediterranean group of cypresses (Ducrey et al., Citation1999). These are: C. sempervirens (common or evergreen cypress) native to the eastern Mediterranean region, C. atlantica Gaussen which is endemic to the Atlas Mountains in Morocco, and C. dupreziana A. Camus which is endemic to the Algerian Tassili N'Ajjer desert. Male cones of Cupressus conform to the Cupressaceae type showing microsporophylls in a cyclic disposition (Biswas & Johri, Citation1997). Cupressus have male reproductive cones with 8 to 20 opposite decussate scales. These microsporophylls bear 2 to 10 microsporangia abaxially attached near the cone axis.
The application of phenological knowledge to allergy studies has proved important, as the determination of starting date of flowering, and the ability to estimate the potential pollen emission by plants provides a predictive framework for the pollen content of the atmosphere. By combining sampling data with information about climatic factors that drive plant development an understanding of the variability observed in the pollen scores can be determined.
The present investigation reports a preliminary description of Cupressus sempervirens male phenology related to microsporogenesis events in Tetouan city (Morocco) where planted Cupressus sempervirens var. pyramidalis L. trees are responsible for allergic symptoms. The main objective of this study was to evaluate the period and amount of pollen production of an individual tree. This was done by estimating tree size, cone density and pollen grains per cone, thus allowing the knowledge of the contribution of this species to the total amount of airborne pollen.
Material and methods
The city of Tetouan is situated, in the eastern sector of the Tingitane Peninsula, in the northwest of Morocco (35°34′N; 5°22′W) and in the Thermomediterranean bioclimatic level (altitude: 65 m a.s.l.). The study area of M'hannech district, surrounding the University, is located 10–14 m above sea level. Climatic conditions are predominantly Mediterranean warm subhumid (Benabid, Citation1982). Annual rainfall averages 728 mm and temperature averages 18.2°C.
Phenological observations
Fifty‐two mature and developed individuals of the Cupressus sempervirens species were studied. Male cone phenology was carried out during winter 2006/2007. Twice a week observations were made during the main flowering period from November to March. This method is the best way to assess phenological evolution as indicated by previous studies (Hidalgo et al., Citation1999). Five phenological phases (Ph 1 to Ph 5) were defined according to the steps proposed by Hidalgo et al. (Citation2003): Ph 1 – male bud differentiation, Ph 2 – immature cone, Ph 3 – pre‐flowering, Ph 4 – blossoming cones and Ph 5 – senescent cones. They were recognized based on developmental stages of the cones and cytological observations. Forty‐five cones per tree were measured. The predominant phenophase (75%) in the pool of cones around the crown was recorded as the phenological stage of the tree.
Cytological studies
Cones of Cupressus sempervirens were collected at the different phenophases. The sporogenous tissues and the different processes of microsporogenesis were observed after crushing the cones in diluted staining solutions. A solution of 1% acetocarmine was used to stain nucleus in sporogenous tissues and 1% aniline blue in acetic acid (1:4, v/v) to examine the nucleus of microspores. Samples were observed using light microscopy. The slides were photographed using a digital compact Olympus camera C‐5060 wide zoom.
Total surface area calculation
The surface of tree crown (S) was estimated, assuming that tree shape was a cone, using the following geometric formula: S = πr (h+r) where r and h are respectively the radius and the hypotenuse (Hidalgo et al., Citation1999).
Number of cones
The number of cones per branch (C/Br) was estimated in three short branches (20–30 cm long) per m2 in all individuals selected. The number of branches per m2 was measured using a flexible quadrate of 1 m2 randomly deposited. The total number of cones per tree (C/Tr) was obtained by multiplying the average number of cones per branch (C/Br) by the average number of branches per m2 (Br/m2) by the total surface area of the crown (S): C/Tr = C/m2×S.
Number of pollen grains per cone
The number of pollen grains per cone (PG/C) was estimated using the method of Cruden (Citation1977) also used by Hidalgo et al. (Citation1999). A total of 27 pre‐flowering cones were collected from nine well developed trees. Each cone was crushed in 10 ml distilled water stained with 0.1% fuchsine. The number of pollen was counted in three samples of 10 µl deposited in a chamber of a Mac‐Master slide. To fill up the slide cell, 140 µl of distilled water were added. Observations were realised using an optic microscope equipped with a 4× lens and a 10× ocular.
Total pollen production
The total pollen production (PG/Tr) was estimated by multiplying the number of cones per tree by the number of pollen grains per cone (PG/C): PG/Tr = C/Tr×PG/C.
This method has already been proven to be very efficient to estimate the pollen production in ten anemophilous trees (Tormo et al., Citation1996) and in three species of Cupressus (Hidalgo et al., Citation1999).
Correlation between traits
The correlation coefficients and their statistical significance (p<0.05) were computed between the following traits: the dimensions of the tree (height and surface crown and radius), the number of branches per square meter and the total productions of branches, cones and pollen grains. A factor analysis (correspondence analysis) was also carried out using the same above mentioned variables. All statistical analyses were performed using the software Statistica 6.0 (StatSoft Inc., 1984‐2003).
Results
Phenophases and microsporogenesis
Five different phenophases were delimited according to the size, colour, stage of microsporogenesis and blossoming event (Figure ).
Figure 1. Male phenological phases, microsporogenesis and pollen development ofC. sempervirens. A. Phenophase 1 (Starting date: 30/11/2006) – Bud cones. B, C. Phenophase 2 (Starting date: 10/12/2006) – Immature cones. D. Cluster of interconnected pollen mother cells (PMC) before meiosis. E. Isolated PMC with distinct nucleus. F. PMC at the dyad phase (D) showing equatorial division. G. Isobilateral tetrad (ITe) with four microspores. H. Tetrad (Te) enclosed in a common callose wall. I. Uninucleate free microspore. J. Early microspore maturation with conspicuous nucleus showing the intine formation (arrow). K. Phenophase 3 (Starting date: 12/02/2007) – Pre‐flowering cone showing pollen sacs between scales. L. Late microspore maturation with droplet inclusions as accumulated nutrients. M, N. Phenophase 4 (Starting date: 23/02/2007) – Blossoming cones showing basipetal (M) and acropetal (N) dehiscence. O. Phenophase 4 – Blossoming cone with completely opened scales and empty pollen sacs. P. Mature pollen grain showing a star‐like cytoplasm. Q. Phenophase 5 (Starting date: 25/03/2007) – Senescent brown cone with unfolded scales. Scale bars – 550 µm (A); 600 µm (B); 650 µm (C); 10 µm (D); 6 µm (E, H, P); 6.5 µm (F); 7 µm (G); 5 µm (I); 3 µm (J); 800 µm (K); 4 µm (L); 900 µm (M, N); 1 mm (O‐Q).
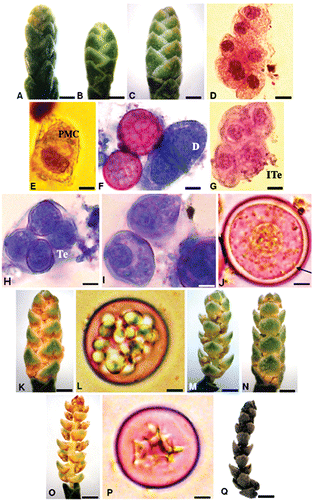
Phenophase 1. Starting date (SD): 30 November
Bud differentiation corresponds to bud burst in the first decade of December. The average size of the buds was 1.86 mm±0.2 (Figure ). The archesporial phase occurred during this stage.
Phenophase 2. SD: 10 December. Immature cones
The cones are developed but are not mature. The growing cones reach a mean size of 3.3 mm±0.6 (Figure ). Primary sporogenous tissue gave rise to interconnected mass of pollen mother cells (PMC, Figure ). At this developmental stage, the sporogenous tissue is composed of polyhedral PMCs with prominent nucleus (Figure ). As they mature, PMCs separate from the tapetal cells and from each other mother cell. This culminates at the onset of meiosis with the production of independent PMCs. After the completion of meiosis I, they enhance dyads (Figure ). Following the course of meiosis, the cytoplasm is separated with the callose wall into an isobilateral tetrad with four microspores (Figure ). At the end of meiosis II, the tetrads are enclosed in a common callose wall (Figure ). The microspores released from the tetrad were characterized by dense cytoplasm and conspicuous nucleus (Figure ). Early maturation of the microspore is achieved by the formation of the intine (Figure ).
Phenophase 3. SD: 12 February. Pre‐flowering
This is a brief period in which the cones change colour and the microsporangia appear between the scales (Figure ). The mean size was 4.9 mm±0.9. The increase in size of the microspores leads to the male cone swelling. This phase corresponds to the late pollen maturation characterized by cytoplasmic droplets accumulation as starch (Figure ).
Phenophase 4. SD: 23 February. Blossoming male cones
The majority of the microsporangia are opened and releasing mature pollen (Figure ). The microsporangia dehiscence occurs asynchronously. The cones (6.1 mm±0.9) dehisce both from the bottom or from the top (Figure ). The rachis grows during this phase and all the microsporophylls are opened (Figure ).
Phenophase 5. SD: 25 March Senescent male cones (6.4 mm±1)
The microsporangia are empty and the scales turning to a brown colour (Figure ). This is the end of the pollen emission period. Some cones drop down; some remain on the tree for one month or more.
Dendrological data
The mean, standard deviation and range of all dendrological data obtained are shown in Table . The average height of the trees was 11.27 m and the total crown surface area was estimated to 51.89 m2. The number of branches per 1 m2 was high (30 Br/m2, up to 49 per m2 in some specimens). The mean standard deviation was low indicating a regular shape of the trees. The number of cones per branch was very variable and reached as many as a thousand. The cones appeared on short branches and show a considerable floral density. In comparison to the mean value (384), a high mean standard deviation was observed. The number of 11 520 cones per m2 was calculated by multiplying the number of cones per branch by the number of branches per m2.
Table I. Dendrological data and total pollen production of Cupressus sempervirens in Tetouan (Morocco). (a)Cones/tree is calculated by multiplying the average number of cones/m2 by the total surface area average. (b)Total pollen/tree is obtained by multiplying the total cones/tree by the average number of pollen grains/cone.
Total pollen production
In the nine trees selected to estimate pollen production, the total production of cones per tree ranged from 2.5 thousand to 1.6 million (Table ). The number of 597 773 cones per tree (Table ) was obtained by multiplying the average number of cones per m2 by the total surface area average of all the trees. A mean of 460 778 pollen grains per cone was estimated. This production ranged between 386 000 and 504 667 showing a low standard deviation (Tables , ). Assuming that C. sempervirens contains 20 scales per cone, the number of pollen grains per microsporophyll is 23 039. Pollen production varied among trees between 1 000 million and a little over 800 000 million (Table ). The total pollen grains per tree increased when there was an increase in the number of cones per tree (Table ). Considering all the trees, the mean total pollen production was obtained by multiplying the average number of cones per tree by the average number of pollen grains per cone. The average production of pollen grains per tree is 275 440.65×106.
Table II. Cone and pollen production in the nine studied trees: C/Tr – number of cones per tree; PG/C – number of pollen grains per cone; PG/Tr – number of pollen grains per tree. (a) Mean of three cones per tree.
Correlation between tree characteristics
From the data obtained, the correlation coefficients and their statistical significance (p<0.05) were computed (Table ). There is no correlation between the radius, the number of branches and of cones per tree and the height crown, but there is a positive and significant correlation between the pollen grains production and the height crown. The total production of branches and cones have a positive significant correlation with the radius and surface of the crown, the correlation with the surface being always superior to that of the radius. This means that branches bearing cones are abundant in the crown surface. This result is also related to the higher correlation of pollen grains production per tree with the surface. The correlation coefficients showed also that this production is very highly and positively related with the number of branches per tree and of cones per tree. The highest correlation one is with the total cones per tree. It seems that no correlation exists between the number of branches per square meter and all the others parameters of the tree.
Table III. Correlations between tree characteristics: Hc – height crown; Br/Tr – branches per tree; C/Tr – cones per tree; PG/Tr – pollen grains per tree; Br/m2 – branches per square meter. P<0.05
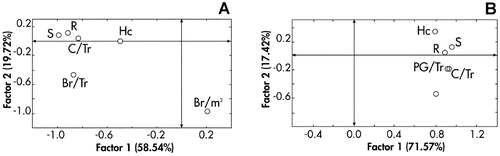
The results of factor analysis are shown in Figure . The accumulated proportion for the two axes in Figure is respectively 78.26 and 88.99. The factor 1 represents 58.54% of the variability when the 52 trees were analysed while it appears sufficient to explain the total production of pollen grains as it represents 71.57% of the variability when the same variables were used in addition to pollen grains production parameter (Figure ). The relation between the dimension parameters of the trees and the total productions of the reproductive structures was clearly evident in factor 1. Surface and radius are related to the number of cones per tree and to pollen production as they are together in Figure and maintain the same group when they were analysed with pollen grains per tree variable (Figure ). In the factor 2 axis, the branches density variable showed the highest contribution. The number of branches per square meter is strongly segregated from the other variables and it was inversely proportional to the height of the crown. However, the total number of short branches bearing cones increases more with the radius and the surface crown than with the height crown as is corroborated by the correlation coefficients.
Figure 2. Factor analysis diagrams obtained by using 6 or 7 variables and 52 and 9 trees(selected for pollen grains estimation) of C. sempervirens respectively in (A) and (B) diagrams. Variables used: height crown (Hc), surface (S), radius (R), branches per tree (Br/Tr), cones per tree (C/Tr), branches per square meter (Br/m2 ) and pollen grains per tree (PG/Tr).
Discussion
It is interesting to note that different patterns of development exist within gymnosperms (Andersson et al., Citation1969). Meiosis is started and completed during autumn in Cedrus, some Juniperus (J. chinensis, J. horizontalis, and J. virginiana) and Taxus. In Pinaceae, the male inflorescence went through winter dormancy in the PMC stage as small cone buds. Meiosis, free microspores and mature pollen occurred in spring. In Cupressus, anthesis takes place in winter two months later than bud cone differentiation. The microsporangia differentiate sporogenous tissue in the bud at the end of autumn. Meiotic development proved to be similar to the coniferous pattern. The PMCs separated from the archesporial tissue and underwent meiosis. It confirmed previous findings in Cupressus dupreziana A. Camus and C. macrocarpa (El Maâtaoui & Pichot, Citation2001; Hidalgo et al., Citation2003). In the majority of taxa in conifers, meiosis and early microspore maturation pollen develop over a long period in comparison to Picea (Lü et al., Citation2003), Pinus (Lü et al., Citation2001) and grass Leymus (Teng et al., Citation2005). The total duration is brief in Picea and Pinus and lasted about two weeks in Leymus chinensis. The long duration of immature cones phase in Cupressus is connected to the plant dormancy during this stage. Late microspore maturation during pre‐flowering phenophases, in addition to the increase in size of the microspore, is related to starch accumulation and exine‐, intine thickness.
A description of phenological stages is a helpful way to determine the starting date of pollen emission. The dehiscence mechanism of the cones corresponds to both basipetal and acropetal succession. In a basipetal succession, the cones dehisce from the bottom to the top. In an acropetal succession, they dehisce in the opposite way. These traits accompanied with the growth of the rachis and the swelling of the cones allow a long period emission. A different dehiscence mechanism was observed in Cupressus arizonica and Pinus roxburghii (Hidalgo et al., Citation2003; Khanduri & Sharma, Citation2000). The former dehisce only from the bottom to the top. The latter do it from the top to the basal cone insertion. This gradual opening is typical in anemophilous trees most of which have male flowers arranged in catkins with low maturation mechanism.
The pollen dispersal period in C. sempervirens is about one month. The conjunction of three species (C. arizonica Green, C. macrocarpa Hartweg and C. sempervirens L.) in Cordoba and Granada leads to continuous pollen emission between 45 to 147 days due to the overlapping of their main period of pollination (Galán et al., Citation1998b ; Diaz de la Guardia et al., Citation2006). Using C. sempervirens solely for ornamental trees in Tetouan city reduces the allergic exposure risk to one month, the pollen emission period in the winter season. The airborne pollen contribution of the native Cupressaceae such as Tetraclines articulata (Vahl) Masters and Juniperus phoenicea L. seems to be very scarce. Pollen release correlates with meteorological factors. Cone scales close with high humidity and open with low humidity to liberate pollen. The rainfall washes the particles in the air (Laaïdi et al., Citation1997).
The present study was carried out assuming Cupressus crown shape as a cone. The geometric formula πr (h+r) was then applied to estimate the surface area of the crown. In the evergreen species studied by Tormo et al. (Citation1996), all groups of inflorescences within a given area (1 m2) were counted and the results were extrapolated to the total estimated surface. Tormo et al. (Citation1996) considered the surface and the volume of the crown both as a sphere and a cylinder in the studied anemophilous trees. It confirmed previous findings (Hidalgo et al., Citation1999) suggesting that it is a helpful method to estimate pollen production. This method offers the possibility to compare efficiently the pollen production in different anemophilous trees. Such method should be used more often to monitor pollen production within a tree as well as among trees and to study the possible pollen production variation. The pollen production per scale in C. sempervirens was approximately 23 000 pollen grains. This is different to the 19 180 and 18 286 comparable to the mean value of approximately 20 000 pollen grains estimated respectively in C. macrocarpa and C. sempervirens studied by Hidalgo et al. (Citation1999). In C. sempervirens, a 5‐fold reduction of pollen in comparison to C. macrocarpa was obtained (Hidalgo et al., Citation1999). The main difference is due to cone density. The high mean standard deviation in C. sempervirens indicated an irregular distribution of the cones; some branches had many cones while others had none. This character can be influenced by both environmental and genetic factors. The results of factor analysis and since the production of cones per tree are strongly correlated with the surface and radius of the crown and not with height. This could indicate that the amount of available light is a limiting factor in the development of cones. However, the height does not have a negative effect. Similar findings where obtained in the anemophilous trees studied by Tormo et al. (Citation1996). This suggests that production is affected by environmental conditions. This result is also due to the formula used and confirmed the accuracy of the methodology undertaken.
The amount of pollen grains per cone observed in C. sempervirens is higher than previous estimations in same or different species of the same genera (Hidalgo et al., Citation1999). The value of 460 780 pollen grains per cone found in C. sempervirens of Tetouan is very different to the 383 600 and 365 722 pollen grains of C. macrocarpa and C. sempervirens of Cordoba respectively, although these species contain identical number of scales per cone. These variations could be attributed to localized climatic conditions between the Mediterranean Tetouan city and the continental Cordoba, which has more inland frequent temperature fluctuations. The amount in C. sempervirens is 3.5 times more than the number of pollen grains per cone found in Pinus pinaster, 2 times more than the value obtained in Olea europaea and is slightly higher (×1.35) than Platanus hispanica (Tormo et al., Citation1996) despite the difference between the spherical ball shaped inflorescences and the cones. Independently of the reproductive structures developed by these trees, the high pollen production tendency in anemophilous trees compensate for the reduced efficiency of the wind pollination (Faegri & van der Pijl, Citation1979). It could also be explained as a way to reach distant individuals within a dispersed population.
The total pollen grains production per tree oscillated between 100 000 million and 500 000 million in Platanus, Salix, some specimens of Olea and Fraxinus and Quercus (Tormo et al., Citation1996). In this study we found 276 000 million pollen grains per tree corresponding to a mean value within the above mentioned species. A high correlation of the total pollen grains production per tree was observed with the crown surface, the number of branches per tree and of cones per tree. The number of pollen grains per cone found here showed little variation (9%) implying that the amount of pollen is constant. This production seems to be a genetically fixed constant value. Because cones production depends on light availability, these results lead us to believe that pollen production is affected by both genetic factors and environmental conditions. It is well established that the capacity of pollen production is genetically constant (Subba Reddi & Reddi, Citation1986) and also varies from one year to another influenced by environmental factors (Stanley & Linskens,Citation1974; Rogers, Citation1993).
This study allowed a partial estimate of an individual pollen emission and provides new knowledge on the contribution to the airborne pollen content from a particular species during a period of the year. Once the density of the plants in a city is known, we can evaluate approximately the total level reached. However, the exact quantity depends on the duration of flowering period and on the meteorological factors that affect yearly or seasonally pollen fluctuations. Temperature, humidity and rainfall prior flowering were the main parameters that directly influence this variability (Diaz de la Guardia et al., Citation2006). The higher minimum temperatures noticed in October and the scarce rainfall registered during autumn are the main variables affecting the two weeks delayed pre‐flowering and blossoming phenophases compared with C. sempervirens in Cordoba (Hidalgo et al., Citation2003).
The total individual production of pollen and the temporal pattern of pollen emission are of great importance for aerobiological studies, pollen shedding in the air is able to be surveyed and models predicting the beginning of the season as well as the pollen content in the air to warn the atopic population developed.
Conclusions
Cupressus sempervirens, a widespread cultivated species with allergenic pollen, has a different dehiscence mechanism of the male cones, corresponding to both basipetal and acropetal succession, compared to C. arizonica and P. roxburghii. The number of pollen grains per cone and the total pollen production per tree were in the production range of the anemophilous trees. C. sempervirens showed a higher pollen production per cone in the Mediterranean area than Cupressus species in continental zones. By only using Cupressus sempervirens as ornamental tree the allergenic exposure risk is reduced to one month, the pollen emission period in the winter season.
Acknowledgements
We thank Pr. Nard Bennas for her technical support and Ibrahim Aboulaich for his field assistance.
References
- Andersson , E. , Ekberg , I. and Eriksson , G. 1969 . A summary of meiotic investigations in conifers. . Stud. For. Suec. , 70 : 1 – 20 .
- Biswas , C. and Johri , B. M. 1997 . The gymnosperms , Berlin : Springer .
- Bortenschlager , S. 1990 . Aspects of pollen morphology in the Cupressaceae. . Grana , 29 : 129 – 137 .
- Benabid , A. 1982 . Etude phytoécologique, biogéographique et dynamique des associations et séries sylvatiques du Rif occidental (Maroc) , Aix‐en‐Provence : Fac. Sc. Tech. St. Jérome. Univ. D'Aix‐Marseille . Ph. D. Thes
- Bhatia , D. S. and Malik , C. P. 1996 . “ Significance of callose in reproduction of higher plants with special reference to male gametophyte. ” . In Pollen‐spore research emerging strategies , Edited by: Malik , C. P . 221 – 240 . New Delhi : Today & Tomorrow's Prints & Publs . Adv. Pollen‐Spore Res. 21
- Caiaffa , M. F. , Macchia , L. , Strada , S. , Bariletto , G. , Scarpelli , F. and Tursi , A. 1993 . Airborne Cupressaceae pollen in Southern Italy. . Ann. Allergy , 71 : 45 – 50 .
- Charpin , D. , Hughes , B. and Mellea , M. 1993 . Seasonal allergenic symptoms and their relation to pollen exposure in south‐east France. . Clin. Exp. Allergy , 23 : 435 – 439 .
- Cruden , R. W. 1977 . Pollen‐ovule ratios: A conservative indicator of breeding systems in flowering plants. . Evolution , 31 : 32 – 46 .
- D'Amato , G. P. and Liccardi , G. 1994 . Pollen‐related allergy in the European Mediterranean area. . Clin. Exp. Allergy , 24 : 210 – 219 .
- Díaz de la Guardia , C. , Alba , F. , De Linares , C. , Nieto‐Lugilde , D. and López Caballero , J. 2006 . Aerobiological and allergenic analysis of Cupressaceae pollen in Granada (Southern Spain). . J. Investig. Allergol. Clin. Immunol. , 16 : 24 – 33 .
- Ducrey , M. , Brofas , J. , Andreoli , C. and Raddi , P. 1999 . “ The genus Cupressus. ” . In Cypress: A practical handbook , Edited by: Teissier du Cros , E , Ducrey , M , Barthelemy , D , Pichot , C , Giannini , A , Raddi , P , Roques , A , Sales‐Louis , J and Thibaud , B . 9 – 25 . Florence : Studio Leonardo .
- El Maâtaoui , M. and Pichot , C. 2001 . Microsporogenesis in the endangered species Cupressus dupreziana A. Camus: Evidence for meiotic defects yielding unreduced end abortive pollen. . Planta , 213 : 543 – 549 .
- Faegri , K. and van der Pijl , L. 1979 . The principle of pollination ecology , Oxford/New York/Toronto/Sydney/Paris/Frankfurt : Pergamon Press . 3rd ed. by K. Faegri & L. van der Pijl
- Galán , C. , Fuillerat , M. J. , Comtois , P. and Domínguez‐Vilches , E. 1998a . Predictive study of Cupressaceae pollen season onset, severity, maximum value and maximum value data. . Aerobiologia , 14 : 195 – 199 .
- Galán , C. , Fuillerat , M. J. , Comtois , P. and Domínguez‐Vilches , E. 1998b . Bioclimatic factors affecting daily Cupressaceae flowering in southwest Spain. . Int. J. Biometeor. , 41 : 95 – 100 .
- Hidalgo , P. J. , Galán , C. and Domínguez , E. 1999 . Pollen production of the genus Cupressus. . Grana , 38 : 296 – 300 .
- Hidalgo , P. J. , Galán , C. and Domínguez , E. 2003 . Male phenology of three species of Cupressus: Correlation with airborne pollen. . Trees , 17 : 336 – 344 .
- Khanduri , V. P. and Sharma , C. M. 2000 . Development of groups of male strobili, anthesis and microsporangium dehiscence in Pinus rouxburghii. . Grana , 39 : 169 – 174 .
- Kurmann , M. H. 1994 . Pollen morphology and ultrastructure in the Cupressaceae. . Acta Bot. Gallica , 141 : 141 – 148 .
- Laaidi , K. , Laaidi , M. and Besancenot , J. P. 1997 . Pollens, pollinoses et météorologie. . Météorologie, 8 Ser. N. , 20 : 41 – 56 .
- Lü , S. Y. , Li , Y. F. , Chen , Z. K. and Lin , J. X. 2001 . On pollen development of conifers. . Chin. Bull. Bot. , 18 : 340 – 346 .
- Lü , S. Y. , Li , Y. F. , Chen , Z. K. and Lin , J. X. 2003 . Pollen development in Picea asperata. . Flora , 198 : 112 – 117 .
- Papa , G. , Romano , A. , Difonso , M. , Viola , M. , Artesani , M. , Di Gioacchino , M. and Venuti , A. 2001 . Prevalence of sensitization to Cupressus sempervirens: A 4‐years retrospective study. . Sci. Tot. Environ. , 270 : 83 – 87 .
- Rogers , C. A. 1993 . Application of aeropalynological principles in Palaeoecology. . Rev. Palaeobot. Palynol. , 79 : 1133 – 1140 .
- Stanley , R. G. and Linskens , H. F. 1974 . Pollen: Biology, biochemistry, management , Berlin : Springer .
- Subba Reddi , C. and Reddi , N. S. 1986 . Pollen production in some anemophilous angiosperms. . Grana , 25 : 55 – 61 .
- Teng , N. , Huang , Z. , Mu , X. , Jin , B. , Hu , Y. and Lin , J. X. 2005 . Microsporogenesis and pollen development in Leymus chinensis with emphasis on dynamic changes in callose deposition. . Flora , 200 : 256 – 263 .
- Tormo Molina , R. , Muñoz Rodríguez , A. , Silva Palacios , I. and Gallardo López , F. 1996 . Pollen production in anemophilous trees. . Grana , 35 : 38 – 46 .
- Yazidi Alaoui , A. and Bartal , M. 2000 . Sensibilisation cutanée au pollen de Cyprès au Maroc: Étude multicentrique. . Allergol. Immunol. , 32 : 113 – 116 .