Abstract
The small aquatic family Hydatellaceae was recently assigned to the early‐divergent angiosperm order Nymphaeales. Pollen morphology is described using both SEM and LM for all 12 species of Hydatellaceae, and using TEM for one species (T. submersa). These observations are compared with pollen data from the other two families of Nymphaeales, Nymphaeaceae and Cabombaceae, including original observations for both genera of Cabombaceae. No significant interspecific variation in pollen morphology occurs in Hydatellaceae, though both of the perennial species (Trithuria inconspicua and T. filamentosa), which could be apomictic, possess at least partially sterile and collapsed pollen. Pollen of Trithuria is small, oblong or rounded (viewed from the distal pole) and monosulcate. The aperture has a distinct margin and extends the full length of the pollen grain. The exine is tectate‐columellate. The tectum is perforate: discontinuous with numerous small perforations randomly scattered over the entire non‐apertural surface. The exine surface is microechinate. In most species of Trithuria, a small percentage of pollen grains possess a trichotomosulcate aperture. In two species, rare atypical grains with striate or partially striate exine sculpturing were found. This striate pattern is very similar to exine sculpturing in Gymnotheca (Saururaceae, Piperales). The presence of anasulcate pollen does not contradict placement of Hydatellaceae within Nymphaeales, but this pollen type is also common among other early‐divergent angiosperms and monocots. Exine sculpturing differs between Cabombaceae, Hydatellaceae and Nymphaeaceae, but this character also differs between the two genera of Cabombaceae and among genera of Nymphaeaceae. Compared with other families of Nymphaeales, Hydatellaceae are relatively uniform in pollen morphology.
Hydatellaceae are a small aquatic or semi‐aquatic family of mostly annual herbs, distributed in Australia, New Zealand and India (Cooke, Citation1987; Yadav & Janarthanam, Citation1994; Hamann, Citation1998; Sokoloff et al., Citation2008a ). These plants (Trithuria and Hydatella) were formerly placed within the monocot family Centrolepidaceae (e.g., Hieronymus, Citation1888; Hutchinson, Citation1959), which is now assigned to the order Poales (APG II, Citation2003). However, several unique morphological characters led Hamann (Citation1976) to segregate them into a new family, Hydatellaceae, which was subsequently placed in a new monocot order Hydatellales (Takhtajan, Citation1980; Cronquist, Citation1981), though their highly unusual features made their precise phylogenetic placement uncertain (Dahlgren et al., Citation1985; Hamann, Citation1998; Stevenson et al., Citation2000). A recent molecular phylogenetic study (Saarela et al., Citation2007) discovered that Hydatellaceae are closely related to the aquatic early‐divergent angiosperm families Nymphaeaceae and Cabombaceae, and the family was subsequently placed in the order Nymphaeales (Stevens, Citation2007; Rudall et al., Citation2008a , Citation b ; Sokoloff et al., Citation2008a ).
This radical taxonomic readjustment for Hydatellaceae has provoked a series of comparative morphological studies to elucidate character evolution in this unusual family (Rudall et al., Citation2007, Citation2008a , Citation b ; Tillich et al., Citation2007; Sokoloff et al., Citation2008b ). Placement in Nymphaeales is well supported by data from gynoecium, ovule, embryo sac and seed morphology (Rudall et al., Citation2007, Citation2008a ; Saarela et al., Citation2007; Friedman, Citation2008), though evidence from seedling structure is equivocal (Tillich et al., Citation2007; Sokoloff et al., Citation2008b ), and the homologies of the unusual pseudanthial reproductive units (flowers or inflorescences) of Hydatellaceae remain uncertain (Rudall et al., Citation2007, Citation2008b ). Among early‐divergent angiosperms, members of Hydatellaceae are the most reduced, both in their morphology and duration of life cycle. Furthermore, no other Nymphaeales possess unisexual reproductive units (flowers). New morphological data on Hydatellaceae are required for a better understanding of structural evolution, both within Nymphaeales and in flowering plants as a whole. A comparative study of pollen morphology is especially important, because dispersed pollen grains are the first fossil evidence of angiosperms, pre‐dating angiosperm meso‐ and macrofossils (Hughes, Citation1976; Brenner, Citation1996; Friis et al., Citation2006).
In this paper we present new data on pollen morphology of all twelve species of Hydatellaceae described so far, together with comparative data on other Nymphaeales. Taxonomy is after Sokoloff et al. (Citation2008a ); note that Hydatella is now placed in synonymy of Trithuria. Previous studies of pollen morphology in Hydatellaceae are relatively scarce and mostly based on light microscopy. Bortenschlager et al. (Citation1966) documented monocolpate pollen in Trithuria macranthera ( = T. occidentalis); this observation was subsequently confirmed using Scanning Electron Microscopy (SEM) by Linder & Ferguson (Citation1985, see also Hamann, Citation1998). Hamann (Citation1975) described pollen of Trithuria submersa as monocolpate, and pollen of Hydatella australis ( = Trithuria australis) as ‘somewhat modified’ monocolpate. Ladd (Citation1977) used SEM to confirm the monocolpate condition in T. submersa. Bortenschlager et al. (Citation1966) and Hamann (Citation1975) described pollen grains of the perennial species Hydatella inconspicua ( = Trithuria inconspicua) as frequently adherent in tetrads, irregular in shape and more or less shrunken because of degeneration. Hamann (Citation1975) suggested that H. inconspicua is apomictic. According to Yadav & Janarthanam (Citation1995) and Gaikwad & Yadav (Citation2003), the sole Indian species, Trithuria konkanensis, has monosulcate and psilate pollen grains.
To our knowledge, the only previous Transmission Electron Microscopy (TEM) study of Hydatellaceae pollen was that of Linder & Ferguson (Citation1985) for T. macranthera ( = T. occidentalis). They described exine as tectate‐columellate and concluded that details of exine stratification are different from those found in Restionales/Poales (i.e., graminoid Poales s.l. in the current classification). They noted wide inter‐columellar spaces and columellae that are much higher than the thickness of the foot layer. According to Linder & Ferguson (Citation1985: 73), “there is not yet an adequate understanding of the variation in exine stratification within the monocots, and it is not possible to find any possible allies for this taxon on the basis of this character”.
Material and methods
A list of species investigated is given at the end of the paper. Dried herbarium material was used for most species. For Trithuria inconspicua, in which stamen formation is extremely rare (Edgar, Citation1966), we used microtome sections of stamens made for U. Hamann (University of Bochum). For T. konkanensis, FAA‐fixed material was used, and for Trithuria submersa, T. australis, T. occidentalis, T. lanterna and T. austinensis, both FAA‐fixed and herbarium material were used. Species of Cabombaceae examined <1?show=[to]?>for comparison (Cabomba aquatica and Brasenia schreberi) were obtained from the Kew Herbarium spirit collection.
For the herbarium material, anthers were directly mounted onto SEM stubs. For the fixed material, individual stamens or entire reproductive units were removed from scapes and dehydrated in an ethanol series up to 100% ethanol, then critical‐point dried using a Autosamdri‐815B CPD (Tousimis Research Corporation, USA) at the Royal Botanic Gardens, Kew (RBGK). Anthers were mounted onto SEM stubs using double‐sided sticky tape and opened using fine dissecting needles to reveal the pollen. All preparations were coated with platinum using an Emitech K550X sputter coater and examined using a Hitachi S4700 scanning electron microscope at RBGK.
Measurements were taken using light microscopy with a Leica DMLB light microscope. Temporary slides were made of pollen grains submerged in a drop of glycerol solution. At least thirty measurements were taken from each sample. In T. polybracteata, T. cowieana and T. cookeana we were unable to measure pollen using Light Microscopy (LM), because these newly‐described species are rare in collections (two of the three species are known from one specimen each) and all available pollen material was used for SEM.
For TEM we used plants of T. submersa cultivated at RBGK from seeds collected by R. Tuckett at Mersea Road swamp, Western Australia 2006. Fresh buds and flowers of various sizes were placed in Karnovsky's fixative (2.5% glutaraldehyde, 2% paraformaldehyde in 0.05M phosphate buffer, pH 7.2), deaerated under vacuum and left in fixative overnight at 4°C. Samples were washed in 0.05M phosphate buffer before being post‐fixed in 1% buffered osmium tetroxide for two hours at room temperature, washed again in phosphate buffer, and dehydrated through a graded ethanol series to 100% ethanol. The samples were then embedded in medium‐grade LR White Resin in gelatine capsules. Sectioning was carried out using a Reichert Ultracut and a diamond histoknife. Semi‐thin (ca. 0.5 µm) sections were cut, stained with toluidine blue and examined using a Leica Diolux 20 optical microscope fitted with a Zeiss Axiocam HRc digital camera. Ultra‐thin (silver‐gold) sections were mounted onto formvar‐coated slot grids, stained with uranyl acetate and lead citrate in an LKB Ultrostainer, and examined using a JEOL JEM‐1210 transmission electron microscope (TEM) at 80 KV.
Results
Hydatellaceae
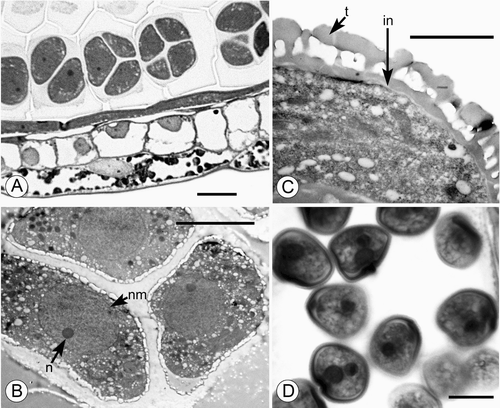
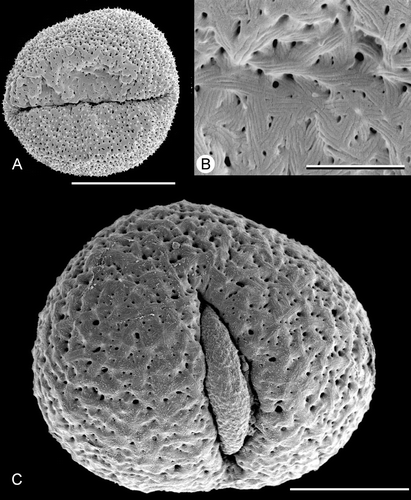
Developing microspore tetrads in young anthers of Trithuria are predominantly tetrahedral (Figure ), though other types were also occasionally observed in each sample, including irregular, linear and decussate tetrads. Tetrads rapidly fall apart during development, and pollen grains are dispersed separately. We did not observe dispersed tetrads in any species; though tetrads were found in apparently indehiscent anthers of T. inconspicua (see below). Dispersed pollen grains are two‐celled, at least in T. konkanensis (Figure ). At least in T. submersa (Figure ), the exine is tectate‐columellate, apparently lacking endexine, and the intine is very thin.
Figure 1. A–C. TEM and D. LM micrographs of Trithuria pollen. A–C. T. submersa (plants cultivated at RBG Kew): (A, B) Tetrads of developing microspores within anther locule; each tetrad ensheathed in callose; nuclear membrane (nm) and nucleolus (n) are visible; (C) Detail of pollen grain almost at anthesis showing tectate‐columellate exine with thick tectum (t) and very thin intine (in). D. T. konkanensis (Yadav s.n., 2006), binucleate male gametophytes. Scale bars – 10 µm (A, D); 5 µm (B); 2 µm (C).
Pollen grains of all Trithuria species are small. Variation in pollen length in different species is given in Table .
Table I. Measurements of pollen grains of nine species of Trithuria and two species of Cabombaceae. Measurements were taken from pollen grains seen from the distal side. At least 30 grains of each species were measured. Micro‐grains of T. filamentosa and T. inconspicua were not included, or collapsed pollen grains in tetrads in T. inconspicua.
Pollen of Trithuria is oblong or rounded (viewed from the distal pole) and monosulcate (Figures – ). The aperture has a distinct margin and extends the full length of the pollen grain. In images taken from herbarium material, the aperture appears as a slit (e.g. Figure ), whereas in fixed and critical‐point dried material the aperture membrane bulges out (e.g. Figure ). This difference is due to the preservation method, not because the bulging apertures are germinating. In most species of Trithuria we found a small percentage of pollen grains with a branched aperture (trichotomosulcate grains) interspersed among typical monosulcate grains (Figure ).
Figure 2. A–L. SEM, pollen grains of Trithuria spp. with bisexual reproductive units from southern Australia. A–J. T. submersa: (A–C) and (E–J) Macfarlane 3902; (D) Conran 961 & Rudall. (A–I) Different views of pollen grains; note that small patches of ectexine are visible on the aperture membrane in (G–I); (J) detail of an aperture with small patches of exine. K–L. T. bibracteata, different views of pollen grains. Scale bars – 10 µm (A–I, K–L); 2 µm (J).
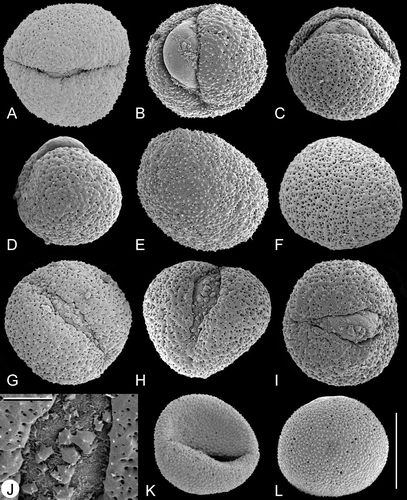
Figure 3. A–F. SEM, pollen grains of Trithuria australis, a species from south‐west Western Australia with male and female reproductive units on the same individuals: (A) Pollen grain viewed from the distal side, Macfarlane & Annels 2283; (B–F) different views of pollen grains, Macfarlane 3357 & Hearn. Scale bar common to all images – 10 µm.
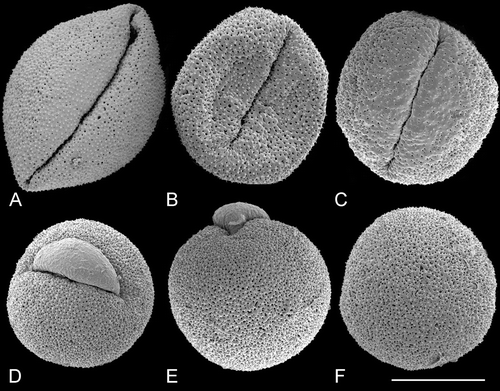
Figure 4. SEM, pollen grains of dioecious species ofTrithuria from south‐west Western Australia. A–D. T. austinensis: (A) Keighery & Gibson s.n.; (B–D) Macfarlane 4163 & Hearn. E–I. T. occidentalis: (E–H) Macfarlane 4141 & Tuckett; (I) Morrison s.n., 22 Nov. 1899. Scale bar common to all images – 10 µm.
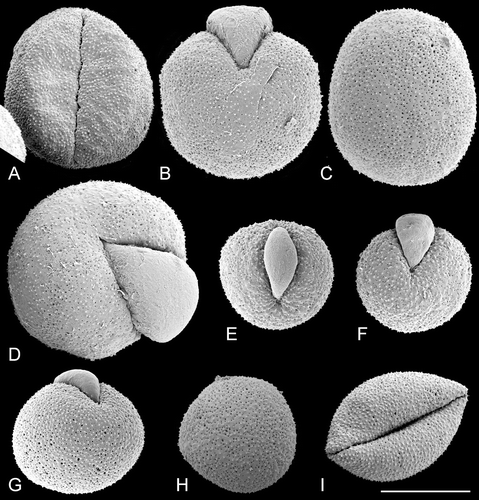
Figure 5. SEM, pollen grains ofTrithuria spp. from northern Australia. A–E. Species with bisexual reproductive units. F–I. Dioecious species. (A–C) T. lanterna (Egan 4816 & Knox); (D, E) T. cowieana (Cowie & Jacka 9995); (F, G) T. cookeana; (H, I) T. polybracteata. Scale bar common to all images – 10 µm.
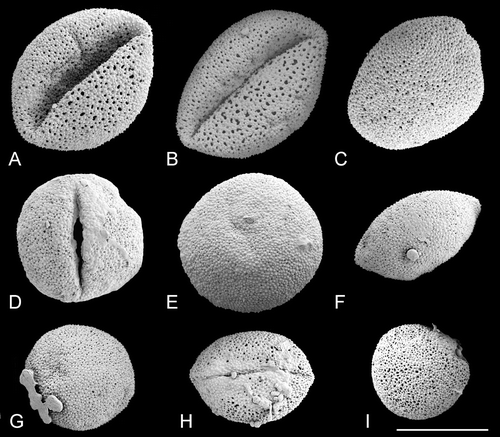
Figure 6. SEM, pollen grains ofTrithuria konkanensis, the sole Indian species of Hydatellaceae. A–D & F. Yadav s.n., 2007; E. Yadav s.n., 2006. Scale bar common to all images – 10 µm.
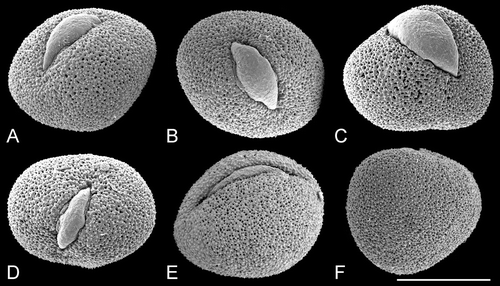
Figure 7. A–F. Light micrographs of tetrads and loose pollen grains in cross‐sections of anthers of Trithuria inconspicua, the perennial New Zealand species. (A) Loose “normal” pollen grain with aperture visible (in the upper part of the figure) and a tetrad of collapsed grains (in the lower side of the figure); (B–F) tetrads of mostly collapsed pollen grains; micrograins (arrowheads) are visible in (D). Scale bar common to all images – 20 µm.
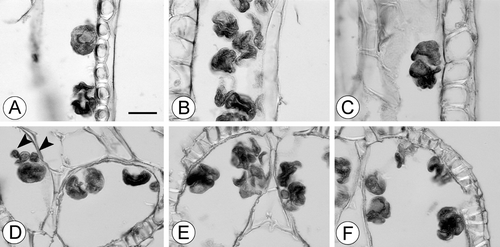
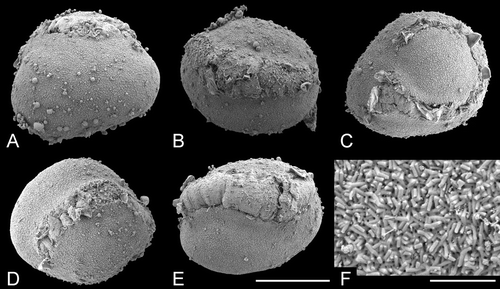
Figure 8. SEM, pollen grains ofTrithuria filamentosa, the perennial Tasmanian species A–E. Wells et al. VM9; F–H. Briggs 9862. (A) “Normal” pollen grain viewed from the distal side; (B) atypical (collapsed) pollen grain viewed from the distal pole with slightly opened very short aperture and protruding male gametophyte covered by intine; (C) pollen grain viewed from the side of the apparently normal aperture; note micrograin at lower right corner; (D) “normal” pollen grain viewed from the distal side; (E) pollen grain viewed apparently from the distal pole; the aperture shape is highly abnormal. Several micrograins are attached to this normal‐sized grain; these micrograins are much smaller than the micrograin in (C). (F) collapsed pollen grain; (G) group of three pollen grains; the left one is collapsed, the central one has a widely opened aperture with protruding male gametophyte covered by intine, the right grain has a relatively “normal” appearance. (Note that the aperture does not open so widely when pollen grains germinate on stigmas in other species of Trithuria). (H) collapsed pollen grain viewed from the side of a normal‐shaped aperture. Scale bar common to all images – 10 µm.

The tectum is discontinuous, with numerous small rounded or ovoid perforations scattered across the entire non‐apertural surface (i.e. perforate: Punt et al., Citation1994). Perforation size and density vary among the pollen grains examined, but these differences do not distinguish between pollen of different species. For example, perforations are smaller in Figure than Figure , though both pollen grains are from the same specimen of T. submersa. Figure show variation in perforation size and density among pollen grains of another specimen of T. submersa. In T. austinensis, perforations can be almost absent (Figure ), rare (Figure ), or sometimes as dense as in most grains of other Trithuria species (Figure ). Perforation density can also vary across a grain. For example, in Figure (T. australis), perforation density is greater to the right of the aperture and lower to the left of the aperture. In Figure (T. australis), there are fewer and smaller perforations near the aperture, but in some other pollen grains of the same species (Figure ), perforation density is uniform across the pollen surface. Large and small perforations can co‐occur close to each other (Figure ). In several pollen grains, extracellular material was observed just below the tectum surface within some perforations (Figure , arrowhead).
Figure 9. SEM, details of exine ornamentation inTrithuria spp. A–C. Trithuria submersa (Macfarlane 3902); D. T. bibracteata; E, F. T. occidentalis (Macfarlane 4141 & Tuckett); G. T. australis (Macfarlane 3357 & Hearn); H. T. polybracteata; I, J. T. konkanensis (I) Yadav s.n., 2007; (J) Yadav s.n., 2006; K, L. T. filamentosa (Wells et al. VM9). Scale bar common to all images – 2 µm.
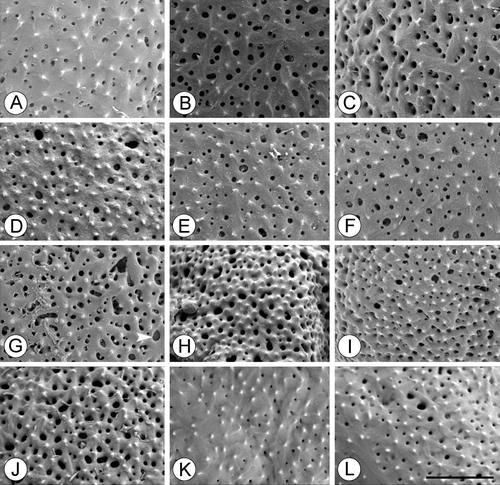
Figure 10. SEM, trichotomosulcate pollen grains ofTrithuria spp. A. T. filamentosa (Briggs 9862); B. T. austinensis (from Keighery & Gibson s.n.); C–D. T. australis (Macfarlane 3357 & Hearn); in (C) all three branches of the aperture are approximately the same length; in (D) one branch is longer than the others. E. T. konkanensis (Yadav s.n., 2006), one of the three aperture branches is very short. F. T. submersa (Conran 961 & Rudall). Scale bar common to all images – 10 µm.
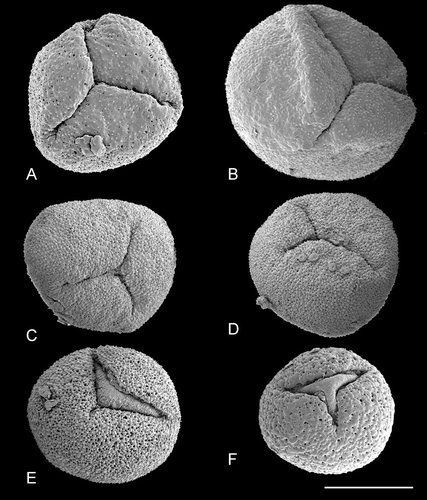
The exine surface is microechinate, i.e., covered with small spines shorter than 1 um (Figure ; this is also clear under LM). In all species, the aperture membrane is typically psilate (i.e., with a smooth surface). The only exception was one specimen of T. submersa (Macfarlane 3902), in which most pollen grains possessed small patches of ectexine on the aperture membrane with sculpturing similar to that of the non‐apertural region (Figure ).
Some pollen grains illustrated here possess a small amount of associated tapetal material that remained attached to the grains (e.g., Figures , ). This material was present only in immature anthers, not in anthetic anthers containing abundant pollen. It lacks a clear shape, and is therefore probably not composed of sporopollenin, and cannot be interpreted as orbicules or Ubisch bodies.
We found considerable variation in pollen morphology within the perennial New Zealand species T. inconspicua (Figure ). In this species, pollen grains are often gathered in tetrads, collapsed and lack any visible content. Loose pollen grains of relatively normal size and morphology are rare in T. inconspicua and considerably larger than degenerating pollen in tetrads. In another perennial species, T. filamentosa, one of two specimens studied (Wells et al. VM9) showed considerable variation in aperture morphology (Figure ). In this specimen, as well as in T. inconspicua (Figure ), we found several very small, clearly abnormal and sterile pollen grains, though the exine sculpturing (studied only in T. filamentosa) resembled that of normal‐sized pollen. Another specimen of T. filamentosa (Briggs 9862) lacked abnormal aperture structure and micro‐grains, but the pollen was mostly collapsed and apparently sterile (Figure ).
Atypical pollen grains with abnormal striate sculpturing were present in T. submersa (several grains in one specimen) and T. australis (a single grain). In atypical grains of T. submersa, striate sculpturing was less pronounced but covered the entire non‐apertural surface (Figure ). In the atypical grain of T. australis, striate sculpturing was more conspicuous than in those grains of T. submersa that possess striate surface, but covered a relatively small area of the exine surface near one side of the aperture (Figure ); otherwise this grain had microechinate sculpturing typical for Trithuria. Perforations similar to those of typical pollen grains of Trithuria were present in these abnormal grains with striate sculpturing (Figure ).
Figure 11. SEM, unusual pollen grains with(partially) striate ornamentation in Trithuria australis and T. submersa. A, B. T. australis (Macfarlane 3357 & Hearn). (A) Pollen grain viewed from distal side; a small region just above the aperture has striate ornamentation; (B) detail of striate region of the same pollen grain. C. T. submersa (Conran 961 & Rudall), pollen grain viewed from the distal side. Scale bars – 10 µm (A); 2 µm (B); 5 µm (C).
Cabombaceae
In Cabomba aquatica, each anther develops relatively few pollen grains; these are very large (Table ), elongate and monosulcate, with the aperture extending almost the full length of the grain (Figure ). The aperture has a distinct margin, and some pollen grains are operculate, though this feature is variable; in some (trichotomosulcate) pollen grains the aperture was branched. The tectum lacks visible perforations. The sculptural elements consist of parallel rods of two types: large rods (macrostriae sensu Gabarayeva et al., Citation2003), which are large single ridges along the grain, and small rods (microstriae sensu Gabarayeva et al., Citation2003), which are hidden between large rods. In non‐apertural regions, large rods form long pronounced ridges interspersed with small rods. In the operculum, both large rods and small rods are short and arranged in a relatively chaotic pattern at different angles. In the apertural region there are smaller, randomly oriented cylindrical elements similar to large rods and small rods.
Figure 12. A–I. SEM, pollen grains of Cabomba aquatica: (A, B) Pollen grains viewed from distal side; aperture without operculum; (C) pollen grain from proximal side; (D) pollen grain from distal side; aperture without operculum; (E) trichotomosulcate pollen grain; (F) detail of a grain with an operculum; (G) exine sculpturing in non‐apertural region on the distal side of the same pollen grain as in (A); (H) exine sculpturing in non‐apertural region on proximal side; (I) sculpturing of the aperture of the same pollen grain as in (A). Scale bars – 30 µm (A–E); 10 µm (F); 4 µm (G); 5 µm (H); 4 µm (I).
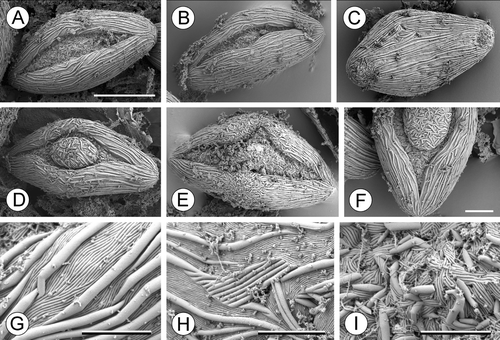
In Brasenia schreberi, each anther contains numerous pollen grains. Pollen grains of Brasenia schreberi are elliptic, monosulcate and scabrate (Figure ). They are larger than grains of Trithuria, but smaller than those of Cabomba aquatica (Table ). The aperture lacks an operculum, has a distinct margin and is the same length as the pollen grain; in aberrant grains the aperture is twisted and almost encircling. The tectum is discontinuous, though discontinuities are very small and visible only at high magnifications. The non‐apertural surface is covered with small cylindrical elements (rodlets) scattered chaotically and densely over the non‐apertural region, whereas the apertural surface is almost smooth (irregular and weak sculpturing observed on the aperture membrane is probably due to the presence of a secretion).
Figure 13. A–F. SEM, pollen grains of Brasenia schreberi. (A) Pollen grain viewed from the proximal side; (B, D, E) pollen grains in which only a portion of very long aperture visible; (C) pollen grain with horseshoe‐shaped aperture; (F) detail of exine ornamentation. Scale bars – 20 µm (A–E), 2 µm (F).
Discussion
Pollen of Hydatellaceae is relatively uniform between species; grains are oblong or rounded, monosulcate and microechinate with a well‐defined smooth (psilate) and elongated aperture. The tectum is discontinuous; tectal perforations are rounded or ovoid in outline and randomly scattered over the entire non‐apertural surface. Pollen grains are very small (though not as small as in some other early‐divergent angiosperms – Smith & Stockey, Citation2007), and the anther contains copious pollen. Our observations contradict an earlier description of Trithuria konkanensis (Gaikwad & Yadav, Citation2003), which suggested that pollen of this species lacked spines. Similarly, in contrast to Hamann's report (Citation1975), pollen of Trithuria australis resembles that of other Hydatellaceae, and does not possess unusual features of the aperture. We did not observe tetrads in any species, except in T. inconspicua, which (together with T. filamentosa) is possibly apomictic (Hamann, Citation1975; Rudall et al., Citation2008a ). In support of an apomictic interpretation for these two species, their pollen grains are frequently abnormal and probably at least partially sterile.
Comparison between acetolysed and non‐acetolysed pollen
Previous palynological studies of Hydatellaceae were based on acetolysed pollen. In particular, Linder and Ferguson (Citation1985) published a detailed LM, SEM, and TEM study of Trithuria occidentalis. Our SEM images of non‐acetolysed pollen grains taken from herbarium specimens of T. occidentalis are almost identical to the images published by Linder and Ferguson (Citation1985) and by P. Linder in Hamann (Citation1998). Details of the microechinate sculpturing are closely similar in images taken from acetolysed and non‐acetolysed pollen grains, so SEM images taken from herbarium specimens can be used for comparison with fossil pollen.
The TEM section illustrated by Linder and Ferguson (Citation1985) shows the absence of ectexine and obscure traces of endexine in the aperture of T. occidentalis. The protruding aperture membrane that we observed in fixed and critical‐point dried pollen grains of this species (Figure ) is therefore probably composed of both intine and weakly developed endexine.
Pollen morphology and life‐strategy in sexual reproduction
Species of Hydatellaceae can be grouped into four distinct assemblages according to their sexual life‐strategy, though these groups are not necessarily monophyletic (Sokoloff et al., Citation2008a ; see also Iles et al., Citation2008). (1) The first group is represented by annuals with bisexual reproductive units (T. submersa, T. bibracteata, T. lanterna, T. cowieana, T. konkanensis). These plants possess no more than five (usually 1–2) stamens per reproductive unit, each with short anthers. (2) The second group (T. occidentalis, T. austinensis, T. cookeana, T. polybracteata) are dioecious annuals, with male plants bearing reproductive units that are large relative to entire plant size. Each male reproductive unit consists of several (up to 17) stamens with long anthers. (3) Trithuria australis represents the only species that is a monoecious annual with unisexual reproductive units; it possesses 3–8 stamens per male unit. (4) Finally, two species that are perennial and possibly apomictic – T. filamentosa and T. inconspicua – usually (T. inconspicua) or often (T. filamentosa) produce only female reproductive units. When these plants develop stamens, they are in separate units or sometimes in bisexual units; stamens are only very rarely present in T. inconspicua. Pollen grains are at least partially sterile in perennial species. The apparently apomictic nature of T. inconspicua could contradict Friedman's (Citation2008) hypothesis on the phylogenetic significance of starch accumulation before fertilization in ovules of this species.
It is noteworthy that species with clearly different sexual strategies possess the same pollen type, without any significant variation even in pollen size (apart from obviously abnormal micrograins and collapsed grains in T. filamentosa and T. inconspicua). The only difference is that, in general, species with bisexual reproductive units show a wider range of pollen grain length than species with unisexual reproductive units, including T. filamentosa and T. inconspicua (Table ). Among the two dioecious species measured here, T. occidentalis has smaller pollen grains than T. austinensis (Table ); this could represent a potentially significant taxonomic difference between these two species, in which male plants are rather similar (Sokoloff et al., Citation2008a ), though more data are needed to test whether the distinction is stable. From the perspective of pollination biology, a given reproductive unit of a male plant in a dioecious species produces much more pollen, as it possesses more stamens with significantly longer anthers. Future investigations will compare the pollen/ovule ratios in dioecious and cosexual species at the population level. According to preliminary field observations by one of us (TDM), large populations of T. austinensis contain apparently similar numbers of male and female individuals. If the sexes are of similar frequency, then species with large anthers and unisexual reproductive units would produce vastly more pollen than ones with few small anthers in bisexual reproductive units. This could be related to differences in breeding system. In south‐western Western Australia, species with bisexual units tend to occur in damp or temporarily wet places. These populations often contain relatively few plants and possess short windows of opportunity in terms of season length or number of years, and hence have to cater for small population sizes and often small plants; perhaps these species have a greater tendency to self pollination. The species with unisexual reproductive units tend to occur in large populations in deeper water in stable environments, i.e. appearing most years over very long periods in distinct wetlands rather than semi‐ to fully terrestrial (though damp) sites. It is possible that pollen of the large deeper‐water populations is partly dispersed on the water surface (T.D. Macfarlane, personal observations). Field observations suggest that the Indian species with bisexual reproductive units is self‐pollinated (S.R. Yadav, unpubl. data).
Apomictic perennial species presumably produce some fertile pollen (at least in T. filamentosa), so cross‐pollination could occur from time to time. Normal‐sized pollen of T. filamentosa is similar in morphology to that of other species of the genus. Apomixis in T. filamentosa and T. inconspicua could have developed as a consequence of their fully submerged habitat in deep water. In contrast to other Hydatellaceae, these two species are perennials and can propagate vegetatively. More embryological work is needed to document apomixis in perennial Trithuria, and more fieldwork is essential to understand their biology. For example, there could be occasions when plants flower at the water edges and undergo sexual reproduction there.
Pollen size and chromosome number
To our knowledge, only two chromosome counts are currently available for Hydatellaceae. The New Zealand species T. inconspicua is diploid with 2n = ca. 20 (de Lange et al., Citation2004), whereas the Indian species T. konkanensis is tetraploid with 2n = 40 (Gaikwad & Yadav, Citation2003). From our data, pollen grains of T. inconspicua are 20.0–27.0×14.1–21.9 µm (mean 23.3×17.5 µm), which extends the range reported by Bortenschlager et al. (Citation1966) of 26×22 µm for this species. In T. konkanensis, our data show pollen size of 16.1–21.1×14.6–18.1 µm, a similar but wider range than the 21.0–22.5×16.5–19.5 µm reported by Gaikwad & Yadav (Citation2003). These observations suggest that there is no correlation between ploidy and pollen size in Trithuria, though more data on chromosome numbers in Hydatellaceae are needed to confirm this.
Comparison with other Nymphaeales
Pollen of Hydatellaceae differs considerably in size and surface patterning from that of its close relatives Cabombaceae and Nymphaeaceae, but resembles them in possessing a single distinct and elongated aperture. Monosulcate (or monsulcate‐derived) pollen is a well‐conserved feature of many non‐eudicot angiosperms (e.g. Furness & Rudall, Citation2004). Our data on pollen of Cabombaceae largely agree with earlier descriptions (Snigirevskaya, Citation1955; Meyer, Citation1964; Batygina & Shamrov, Citation1983; Osborn et al., Citation1991; Taylor & Osborn, Citation2006; Taylor et al., Citation2008).
We confirm the earlier report by Linder & Ferguson (Citation1985) of the presence of tectate‐columellate pollen in Hydatellaceae, though the inter‐columellar spaces are narrower in our material of T. submersa than in the material of T. occidentalis examined by Linder & Ferguson. Cabombaceae and most Nymphaeaceae also have tectate‐columellate pollen (Osborn et al., Citation1991; Gabarayeva & Rowley, Citation1994; Gabarayeva et al., Citation2001, Citation2003; Taylor & Osborn, Citation2006; Taylor et al., Citation2008). Sampson (Citation2000) referred to the unique “cupulate” tectum of Amborella as “columellar‐like”, and Doyle (Citation2005) reinterpreted it as columellate, even though Hesse (Citation2001) considered that Amborella lacks “genuine” columellae. Since tectate‐columellate pollen is common in flowering plants, including early‐divergent angiosperms, this feature could be a synapomorphy of angiosperms as a whole, as suggested by Doyle (Citation2005). Thus, the presence of columellae adds little to the debate surrounding placement of Hydatellaceae in Nymphaeales.
Pollen of Trithuria has some characters in common with that of Brasenia, possibly correlated with wind pollination. In Cabomba, which is fly‐pollinated (Osborn et al., Citation1991), each anther develops relatively few pollen grains, which are very large, elongate, monosulcate and often operculate, all features associated with insect pollination (Figure ). By contrast, in Brasenia, which is predominantly wind‐pollinated (Osborn & Schneider, Citation1988), the polymerous androecium releases a copious amount of relatively small pollen grains, though these are larger than those of Trithuria (Table ). Cavities in the tectum reduce the amount of wall material, making pollen lighter and allowing more effective wind‐pollination. The small microechinate pollen of Hydatellaceae, with a discontinuous tectum, could indicate wind pollination (anemophily), though spinules are frequently present in insect‐pollinated species. Furthermore, our observations of material of T. submersa cultivated at Kew suggest that pollen is retained in anthers after dehiscence and is not readily released when the anther is manipulated, features that would not be expected in a wind‐pollinated plant. In the Indian species T. konkanensis, the inflorescences are at ground level and stamens are barely 5 mm above ground level. The central erect stamen of an inflorescence dehisces laterally, and pollen grains fall downwards onto the stigmatic hairs by gravitational force (S.R. Yadav, pers. obs.). As plants grow in close vicinity to each other, cross pollination could be also expected in this process. Since the Australian species T. lanterna is morphologically similar to T. konkanensis, we predict that its reproductive biology is also similar. A further possibility is hydrophily, at least in some Hydatellaceae, through their pollen grains show no obvious features of reduction in exine, which is characteristic of specialised aquatics.
Most Hydatellaceae are annual semi‐aquatic plants of temporary pools; they commence flowering half‐submerged with their reproductive units exposed in the air. Production of copious amounts of viable seeds suggests that pollination is highly effective. At anthesis, stigmatic hairs of annual Trithuria species are covered by adhered pollen and growing pollen tubes (Rudall et al., Citation2008a ). By contrast, plants of perennial species remain fully submerged and are possibly apomictic. In these species, pollen is often abnormal and apparently sterile. We have not documented pollen‐tube growth in the perennial species, though female reproductive units with developing fruits were examined carefully (Rudall et al., Citation2008a and unpubl. data).
In addition to production of relatively small abundant pollen grains, Trithuria and Brasenia share pollen characters associated with wind pollination, including small sculptural elements and perforated tectum. In contrast, the fly‐pollinated Cabomba differs from both Trithuria and Brasenia in pollen size, frequent presence of an operculum and ornamentation: finely elaborated striae in the non‐apertural region and small rodlets on the apertural surface. The tectum contains an extensive system of microchannels filled with pollenkitt (Gabarayeva et al., Citation2003; Osborn et al., Citation1991; Taylor & Osborn, Citation2006; Taylor et al., Citation2008). Microchannels develop secondarily at the free microspore stage. Pollenkitt is deposited over the entire surface of the pollen grain. However, we observed sculptural elements similar to the small rods of Cabomba in some atypical pollen grains of Trithuria submersa and T. australis, in which groups of striae were present in several parallel elements, situated at different angles and surrounding tectal perforations, with spines absent from this region (Figure ). Interestingly, in T. australis, this unusual sculpturing extended along only one side of the aperture, the other side resembling a typical grain of T. australis. Small patches of ectexine on the aperture membrane observed in one specimen of T. submersa could be compared with an operculum. Among Nymphaeales, infraspecific variation in exine sculpturing is not unique for Trithuria; it has also been documented in Nymphaea (e.g., Snigirevskaya, Citation1955; Johnes & Clarke, Citation1981; Volkova & Shipunov, Citation2008).
The pollinating vectors of most Nymphaeaceae are insects. The showy giant flowers of most extant Nymphaeaceae produce large quantities of pollen, of which a significant part serves to attract pollinators that feed on it. Pollen grains of Nymphaea are unusual in possessing two equal or unequal hemispherical parts – a psilate or ornamented distal region and an ornamented proximal region – with a thin transitional zone between them (Snigirevskaya, Citation1955; Meyer, Citation1964; Kupriyanova, Citation1976; Gabarayeva & Rowley, Citation1994; Gabarayeva & El‐Ghazaly, Citation1997).
The aperture type of Nymphaea has long been the subject of discussion. Some authors (Jones & Clarke, Citation1981 and references cited therein) have described the aperture as monoporate with a large operculum, in which case the entire distal hemisphere represents a broad aperture. Others regard pollen of Nymphaea as possessing an encircling aperture (e.g., Snigirevskaya, Citation1955; Meyer, Citation1964), though some authors (e.g., Meyer, Citation1964) accepted that this is derived from an operculate‐anasulcate condition. Finally, the entire distal part of the pollen grain could be regarded as a very wide aperture (e.g., Kupriyanova, Citation1976; Meyer‐Melikian & Diamandopulu, Citation1996). Pollen grains with an encircling or almost encircling aperture have also been described in Barclaya (Batygina & Shamrov, Citation1983), Victoria and Euryale (Snigirevskaya, Citation1955; Meyer, Citation1964). However, according to Meyer (Citation1964) the encircling aperture of Euryale could be nonhomologous with that of Nymphaea, and is better compared with the almost encircling aperture of some grains of Brasenia. Pollen grains of Nuphar are monosulcate and ornamented with prominent spines (Snigirevskaya, Citation1955; Meyer, Citation1964; Johnes & Clarke, Citation1981; Batygina & Shamrov, Citation1983); Johnes and Clarke (Citation1981) illustrated the presence of an operculum in their material. The tectum is continuous in both Nymphaea and Nuphar, superficially without perforations, but actually containing a network of microchannels, as in Cabomba. The presence of microchannels is not unique to Nymphaeaceae and Cabomba, since they also occur in many other angiosperm species, both wind‐ and insect‐pollinated (Gabarayeva & Rowley, Citation1994). Gabarayeva & Rowley (Citation1994) suggested that formation of microchannels is physiologically necessary in an exine with a thick tectum.
Friis et al. (Citation2001) described an Early Cretaceous fossil related to Nymphaeaceae, and noted that the only feature of the fossil that is not present in extant Nymphaeales is the reticulate tectum of the pollen wall. They hypothesized that the reticulate tectum is the plesiomorphic condition for angiosperms as a whole, and that a pollen wall with a closed tectum probably evolved independently in Cabombaceae and Nymphaeaceae. Our data showing a perforated tectum in Hydatellaceae supports the hypothesis that a closed tectum is derived in Cabombaceae and Nymphaeaceae.
Microsporogenesis and aperture variation
Our observations of predominantly tetrahedral tetrads in developing anthers of Trithuria submersa indicate that microsporogenesis is simultaneous, since tetrad shape is often a good indicator of microsporogenesis type, especially in monocots (Furness & Rudall, Citation1999). However, this interpretation requires more comparative data, because patterns of microsporogenesis are relatively labile in early‐divergent angiosperms, and several species possess intermediate forms that do not fit readily into simultaneous or successive categories, making it difficult to predict microsporogenesis type from tetrad configuration in these species (Furness et al., Citation2002).
Occasional trichotomosulcate pollen, as we observed in Trithuria, is frequently present in species with otherwise monosulcate pollen, and is also often a good indicator of simultaneous microsporogenesis (e.g. Furness et al., Citation2002). However, trichotomosulcate pollen (with a short additional aperture branch) occurs in the elongated grains of Cabomba aquatica, but microsporogenesis is reportedly successive in Cabomba (Batygina et al., Citation1980; Batygina & Shamrov, Citation1983; Taylor et al., Citation2008), casting doubt on the simultaneous/trichotomosulcate correlation, at least in some early‐divergent angiosperms. Microsporogenesis type and tetrad configuration – normally relatively consistent within families – are unusually variable within some early‐branching angiosperm families (e.g. Furness et al., Citation2002, see also Taylor et al., Citation2008). For example, the magnoliid family Aristolochiaceae is highly variable in tetrad configuration (González et al., Citation2001). Batygina et al. (Citation1980) reported variation in microsporogenesis type within a single genus, Ceratophyllum and interpreted Khanna's (Citation1965) data as evidence of an intermediate type of microsporogenesis in Brasenia.
The highly abnormal grains often found in Brasenia could be sterile due to chromosomal abnormalities. Khanna (Citation1965) found that in about 45% of cases of microsporogenesis in B. schreberei, during the first meiotic division some of the chromosomes fail to reach the poles and subsequently organize into micronuclei which ultimately become included in ca 5% of microspores. Trichotomosulcate pollen was absent from our material of Brasenia, but we found several unusual pollen grains with a twisted, circular or almost circular horseshoe‐shaped aperture (but no such abnormal grains in Cabomba). The presence of similar pollen grains with the aperture extending to the proximal side and almost encircling was reported in Brasenia by Meyer (Citation1964). Such instances of developmental instability are not uncommon in angiosperm pollen, including early‐divergent groups. For example, Meyer (Citation1964) discussed high variation in number, shape and position of apertures in Euryale (Nymphaeaceae) and Banks et al. (Citation2007) found considerable instability in pollen of the early‐divergent eudicot Nelumbo. A surprising degree of aperture variation was also documented in the early‐divergent angiosperm Trimenia (Austrobaileyales) (Sampson, Citation2007).
Conclusions
The presence of anasulcate pollen and tectate‐columellate exine does not contradict placement of Hydatellaceae within Nymphaeales, but these characters are also common among other early‐divergent angiosperms and monocots. Exine sculpturing differs between Cabombaceae, Hydatellaceae and Nymphaeaceae, but this character also differs between the two genera of Cabombaceae and among genera of Nymphaeaceae.
Compared with other families of Nymphaeales, Hydatellaceae are relatively uniform in pollen morphology. The primary interspecific difference lies in the fact that apparently sterile pollen grains of abnormal morphology are common in the two perennial species (T. filamentosa and T. inconspicua) but not observed in annual species. This supports the view that perennial species of Trithuria could be apomictic. The two dioecious species of Trithuria from south‐west Western Australia show very similar gross morphology of male plants, but differ in pollen size. However, pollen size ranges in these two species overlap strongly with those of other species of Trithuria. No other pollen feature shows any clear interspecific difference within Hydatellaceae.
By contrast, we found significant infraspecific variation in Trithuria. In addition to occasional trichotomosulcate pollen in several species, we found rare atypical pollen grains with striate exine sculpturing in two species. To some extent, the striate pattern in atypical pollen grains of Trithuria resembles striate exine sculpturing of Cabomba, but it is remarkably similar to the striate exine sculpturing found in another early‐divergent angiosperm, Gymnotheca (Saururaceae, Piperales), though the abnormal grains of Trithuria differ from Gymnotheca in overall size and aperture membrane sculpturing (Smith & Stockey, Citation2007). Such variation in exine sculpturing in Hydatellaceae is an example of “Krenke's rule” (Meyen, Citation1973), which states that a character state found as the normal one in a given taxon can be present as teratology in a related taxon. This phenomenon is especially problematic for performing cladistic analyses, because as more data is accumulated on a given taxon, more polymorphic character states are inserted in the matrix.
Specimens investigated
Brasenia schreberi J.F. Gmel. Uganda. Drummond & Hemsley 4628 (Kew spirit collection No. 15395).
Cabomba aquatica Aubl. Brazil. Philcox 4636 (Kew spirit collection No. 7405).
Trithuria austinensis D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall. (1) Australia: Western Australia, c. 55 km E of Manjimup, 19 Nov. 2007. Macfarlane 4163 & Hearn (fixed material, voucher at PERTH); (2) Australia: Western Australia, Lake Pindicup, 23 Feb. 1999. Keighery & Gibson s.n. (herbarium specimen, PERTH).
Trithuria australis (Diels) D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall. (1) Australia: Western Australia, c. 50 km E of Manjimup, 15 Dec. 1999. Macfarlane 3357 & Hearn (fixed material, vouchers at NSW and PERTH); (2) Australia: Western Australia, 4.3 km S of Tonebridge townsite, 27 Oct. 1994. Macfarlane & Annels 2283 (herbarium specimen, PERTH).
Trithuria bibracteata Stapf ex D.A. Cooke. Australia: Western Australia, 1 km S of Tambellup to Cranbrook, 20 Oct. 1983. Keighery 6719 (herbarium specimen, PERTH).
Trithuria cookeana D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall. Australia: Northern Territory, 24 km SE of Maningrida, 22 Aug. 1995. Cowie 5934 (herbarium specimen, DNA).
Trithuria cowieana D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall. (1) Australia: Northern Territory, Kakadu NP, 08 Apr. 2003. Cowie & Dixon s.n. (herbarium specimen, DNA); (2) Australia: Northern Territory, Wangi Road, near Finniss River crossing, 11 May 2004. Cowie & Jacka 9995 (herbarium specimen, DNA).
Trithuria filamentosa Rodway. (1) Australia: Tasmania, Lake Dobson, 27 Jan. 1997. Wells et al. VM9 (herbarium specimen, HO); (2) Australia: Tasmania, Dove Lake, 16 Jan. 2008. Briggs 9862 (herbarium specimen, K).
Trithuria inconspicua Cheesem. New Zealand: North Island, Lake Waiparera, 1964, 1966. Butcher s.n. (microtome sections from this material are used from the collection of U. Hamann).
Trithuria konkanensis Yadav & Janarthanam. India, near Kolhapur, 2006 and 2007. Yadav s.n. (fixed material).
Trithuria lanterna D.A. Cooke. (1) Australia: Northern Territory, 18 Mar. 1978. Dunlop 4740A (Kew spirit collection No. 47115); (2) Australia: Northern Territory, Kakadu NP, 26 Apr. 1995. Egan 4816 & Knox (herbarium specimen, DNA).
Trithuria occidentalis Benth. (1) Australia: Western Australia, c. 16 km N of Midland, 6 Nov. 2007. Macfarlane 4141 & Tuckett (fixed material, voucher at PERTH); (2) Australia: Western Australia, Midland Junction, 22 Nov. 1899. Morrison s.n. (herbarium specimen, K); (3) Western Australia, Midland Junction, 16 Nov. 1898. Morrison s.n. (herbarium specimen, PERTH).
Trithuria polybracteata D.A. Cooke ex D.D. Sokoloff, Remizowa, T.D. Macfarl. & Rudall. Australia: Western Australia, North Kimberley, Vansittart Bay, 26 May 1984. Willis s.n. (herbarium specimen, MEL).
Trithuria submersa Hook. f. (1) Australia: Western Australia, 15.1 km N of Manjimup, 24 Oct. 2006. Macfarlane 3902 (fixed material, voucher at PERTH); (2) Australia: South Australia, near Bangham Conservation Park, 3 Nov. 1998. Conran 961 & Rudall (fixed material, voucher at ADU); (3) Australia: Tasmania, 5 km NNE of Waddamana, 1 Dec. 1990. Moscal 20272 (herbarium specimen, HO); (4) Australia: Tasmania, 10 miles W of Ross, 11 Apr. 1971. Curtis s.n. (herbarium specimen, HO); (5) Australia, Victoria, 18 miles SW of Kaniva, 22 Sep. 1952. Melville et al. 1201 (herbarium specimen, K); (6) Plants cultivated at RBG Kew from seeds collected by Tuckett at Mersea Road swamp, Western Australia, 2006.
Acknowledgements
We thank the curators of several Herbaria (DNA, HO, MEL, NT, PERTH) for loan of specimens to Kew and permission to use material, Thomas Stutzel for loan of Hamann's slides of Trithuria inconspicua, Barbara Briggs for providing a specimen of Trithuria filamentosa, Renee Tuckett for providing seeds of Trithuria submersa, Roger Hearn for help with monitoring and sampling populations of Trithuria species near Manjimup, and Hannah Banks and Carol Furness for helpful discussion. We are grateful to two anonymous reviewers for helpful comments. We acknowledge funding from the CoSyst program, which is funded by the UK research councils BBSRC and NERC and administered by the Systematics Association and the Linnean Society of London.
References
- Angiosperm Phylogeny Group (APG) . 2003 . An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. . Bot. J. Linn. Soc. , 141 : 399 – 436 .
- Banks , H. , Stafford , P. and Crane , P. R. 2007 . Aperture variation in the pollen of Nelumbo (Nelumbonaceae). . Grana , 46 : 157 – 163 .
- Batygina , T. B. , Kravtsova , T. I. and Shamrov , I. I. 1980 . The comparative embryology of some representatives of the orders Nymphaeales and Nelumbonales. . Bot. Zh. , 65 : 1071 – 1086 .
- Batygina , T. B. and Shamrov , I. I. 1983 . Embryology of the Nelumbonaceae and Nymphaeaceae. Pollen grain structure (some peculiar features of correlated development of the pollen grain and anther wall). . Bot. Zh. , 68 : 1177 – 1182 .
- Bortenschlager , S. , Erdtman , G. and Praglowski , J. 1966 . Pollenmorphologische Notizen über einige Blütenpflanzen incertae sedis. . Bot. Not. , 119 : 160 – 168 .
- Brenner , G. J. 1996 . “ Evidence for the earliest stage of angiosperm pollen evolution: A paleoequatorial section from Israel. ” . In Angiosperm Origin, Evolution, and Phylogeny , Edited by: Taylor , D. W and Hickey , L. J . 91 – 115 . New York : Chapman & Hall .
- Cooke , D. A. 1987 . “ Hydatellaceae. ” . In Flora of Australia, vol. 45 , Edited by: George , A. S . 1 – 5 . Canberra : Australian Gov. Publ. Serv .
- Cronquist , A. 1981 . An integrated system of classification of flowering plants , New York : Columbia Univ. Press .
- Dahlgren , R. M. T. , Clifford , H. T. and Yeo , P. F. 1985 . The families of the monocotyledons , Berlin : Springer .
- Doyle , J. A. 2005 . Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. . Grana , 44 : 227 – 251 .
- Edgar , E. 1966 . The male flowers of Hydatella inconspicua (Cheesem.) Cheesem. (Centrolepidaceae). . N. Z. J. Bot. , 4 : 153 – 158 .
- Friedman , W. E. 2008 . Hydatellaceae are water lilies with gymnospermous tendencies. . Nature , 453 : 94 – 97 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2001 . Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. . Nature , 410 : 357 – 360 .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2006 . Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction. . Paleogeogr., Paleoclim., Paleoecol. , 232 : 251 – 293 .
- Furness , C. A. and Rudall , P. J. 1999 . Microsporogenesis in monocotyledons. . Ann. Bot. , 84 : 475 – 499 .
- Furness , C. A. and Rudall , P. J. 2004 . Pollen aperture evolution – a crucial factor for eudicot success? . Trends Plant Sci. , 9 : 1360 – 1385 .
- Furness , C. A. , Rudall , P. J. and Sampson , F. B. 2002 . Evolution of microsporogenesis in angiosperms. . Int. J. Pl. Sci. , 163 : 235 – 260 .
- Gabarayeva , N. I. and El‐Ghazaly , G. 1997 . Sporoderm development in Nymphaea mexicana (Nymphaeaceae). . Pl. Syst. Evol. , 204 : 1 – 19 .
- Gabarayeva , N. I. , Grigorjeva , V. V. and Rowley , J. R. 2003 . Sporoderm development in Cabomba aquatica (Cabombaceae). . Rev. Palaeobot. Palynol. , 127 : 147 – 173 .
- Gabarayeva , N. I. and Rowley , J. R. 1994 . Exine development in Nymphaea colorata (Nymphaeaceae). . Nord. J. Bot. , 14 : 671 – 691 .
- Gabarayeva , N. I. , Walles , B. , El‐Ghazaly , G. and Rowley , J. R. 2001 . Exine and tapetum development in Nymphaea capensis (Nymphaeaceae): A comparative study. . Nord. J. Bot. , 21 : 529 – 548 .
- Gaikwad , S. P. and Yadav , S. R. 2003 . Further morphotaxonomical contribution to the understanding of family Hydatellaceae. . J. Swamy Bot. Club , 20 : 1 – 10 .
- González , F. , Rudall , P. J. and Furness , C. A. 2001 . Microsporogenesis and systematics of Aristolochiaceae. . Bot. J. Linn. Soc. , 137 : 221 – 242 .
- Hamann , U. 1975 . Neue Untersuchungen zur Embryologie und Systematik der Centrolepidaceae. . Bot. Jahrb. , 96 : 154 – 191 .
- Hamann , U. 1976 . Hydatellaceae – a new family of Monocotyledoneae. . N. Z. J. Bot. , 14 : 193 – 196 .
- Hamann , U. 1998 . “ Hydatellaceae. ” . In The families and genera of vascular plants. IV. Flowering plants – Monocotyledons – Alismatanae and Commelinanae , Edited by: Kubitzki , K . 231 – 234 . Berlin : Springer .
- Hesse , M. 2001 . Pollen characters of Amborella trichopoda (Amborellaceae): A reinvestigation. . Int. J. Pl. Sci. , 162 : 201 – 208 .
- Hieronymus , G. 1888 . “ Centrolepidaceae. ” . In Die Natürlichen Pflanzenfamilien. II, 4 , Edited by: Engler , A and Prantl , K . 11 – 16 . Leipzig : W. Engelmann .
- Hughes , N. F. 1976 . Palaeobiology of Angiosperm Origins , Cambridge : Cambridge Univ. Press .
- Hutchinson , J. 1959 . The families of flowering plants , Oxford : Clarendon Press . Ed. 2
- Iles, W., Rudall, P. J., Sokoloff, D. D., Remizowa, M. V., Macfarlane, T. D., Yadav, S. R., Goh, J., Harris, P., Feild, T. S., Bateman, R.M. & Graham, S. W. (2008). Phylogenetics of Hydatellaceae. In Botanical Society of America (Ed.), Botany 2008 (Abstract 473). http://www.botanyconference.org .
- Jones , M. R. and Clarke , G. C. S. 1981 . The Northwest European pollen flora. Nymphaeaceae. . Rev. Paleobot. Palynol. , 33 : 57 – 67 .
- Khanna , P. 1965 . Morphological and embryological studies in Nymphaeaceae. II. Brasenia schreberei Gmel. and Nelumbo nucifera Gaertn. . Austr. J. Bot. , 13 : 379 – 387 .
- Kupriyanova , L. A. 1976 . Pollen morphology of Nymphaea species in the European part of the USSR. . Bot. Zh. , 61 : 1558 – 1563 .
- Ladd , P. G. 1977 . Pollen morphology of some members of the Restionaceae and related families, with notes on the fossil record. . Grana , 16 : 1 – 14 .
- Lange , P. J. de. , Murray , B. G. and Datson , P. M. 2004 . Contributions to a chromosome atlas of the New Zealand flora – 38. Counts for 50 families. . New Zealand J. Bot. , 42 : 873 – 904 .
- Linder , H. P. and Ferguson , I. K. 1985 . On the pollen morphology and phylogeny of the Restionales and Poales. . Grana , 24 : 65 – 76 .
- Meyen , S. V. 1973 . Plant morphology in its nomothetical aspects. . Bot. Rev. , 39 : 205 – 260 .
- Meyer , N. R. 1964 . Palynological studies in Nymphaeaceae. . Bot. Zh. , 49 : 1421 – 1429 .
- Meyer‐Melikian , N. R. and Diamondopulu , N. 1996 . Ultrastructure of pollen grains of the order Nymphaeales. . Bot. Zh. , 81 : 1 – 9 .
- Osborn , J. M. and Schneider , E. L. 1988 . Reproductive biology of freshwater aquatic angiosperms. Morphological studies of the Nymphaeaceae sensu lato. XVI. The floral biology of Brasenia schreberi. . Ann. Mo. Bot. Gard. , 75 : 778 – 794 .
- Osborn , J. M. , Taylor , T. N. and Schneider , E. L. 1991 . Pollen morphology and ultrastructure of the Cabombaceae: Correlations with pollination biology. . Am. J. Bot. , 78 : 1367 – 1378 .
- Punt , W. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 1994 . Glossary of pollen and spore terminology , Utrecht : LPP Univ. Utrecht . LPP Contrib, Ser. 1
- Rudall , P. J. , Remizowa , M. V. , Beer , A. S. , Bradshaw , E. , Stevenson , D. W. , Macfarlane , T. D. , Tuckett , R. E. , Yadav , S. R. and Sokoloff , D. D. 2008a . Comparative ovule and megagametophyte development in Hydatellaceae and water lilies reveal a mosaic of features among the earliest angiosperms. . Ann. Bot. , 101 : 941 – 956 .
- Rudall , P. J. , Remizowa , M. V. , Prenner , G. , Prychid , C. J. , Tuckett , R. and Sokoloff , D. D. 2008b . Non‐flowers near the base of extant angiosperms? Spatiotemporal arrangement of organs in reproductive units of Hydatellaceae, and its bearing on the origin of the flower. . Am. J. Bot. , in press
- Rudall , P. J. , Sokoloff , D. D. , Remizowa , M. V. , Conran , J. G. , Davis , J. I. , Macfarlane , T. D. and Stevenson , D. W. 2007 . Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early‐divergent angiosperm lineage. . Am. J. Bot. , 94 : 1073 – 1092 .
- Saarela , J. M. , Rai , H. S. , Doyle , J. A. , Endress , P. K. , Mathews , S. , Marchant , A. D. , Briggs , B. G. and Graham , S. W. 2007 . Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. . Nature , 446 : 312 – 315 .
- Sampson , F. B. 2000 . Pollen diversity in some modern magnoliids. . Int. J. Pl. Sci. , 161 ( Suppl. 6 ) : 129 – 137 .
- Sampson , F. B. 2007 . Variation and similarities in pollen features in some basal angiosperms, with some taxonomic implications. . Pl. Syst. Evol. , 263 : 59 – 75 .
- Smith , S. Y. and Stockey , R. A. 2007 . Pollen morphology and ultrastructure of Saururaceae. . Grana , 46 : 250 – 267 .
- Snigirevskaya , N. S. 1955 . To the pollen morphology in Nymphaeales. . Bot. Zh , 40 : 108 – 115 .
- Sokoloff , D. D. , Remizowa , M. V. , Macfarlane , T. D. and Rudall , P. J. 2008a . Classification of the early‐divergent angiosperm family Hydatellaceae: One genus instead of two, four new species, and sexual dimorphism in dioecious taxa. . Taxon , 57 : 1 – 22 .
- Sokoloff , D. D. , Remizowa , M. V. , Macfarlane , T. D. , Tuckett , R. E. , Ramsay , M. M. , Beer , A. S. , Yadav , S. R. and Rudall , P. J. 2008b . Seedling diversity in Hydatellaceae: Implications for the evolution of angiosperm cotyledons. . Ann. Bot. , 101 : 153 – 164 .
- Stevens, P. F. (2007) Angiosperm Phylogeny Website. Vers. 8, June 2007. http://www.mobot.org/MOBOT/research/APweb/ .
- Stevenson , D. W. , Davis , J. , Freudenstein , J. , Hardy , C. , Simmons , M. and Specht , C. 2000 . “ A phylogenetic analysis of the monocotyledons based on morphological and molecular character sets, with comments on the placement of Acorus and Hydatellaceae. ” . In Monocots: Systematics and evolution , Edited by: Wilson , K and Morrison , D . 17 – 24 . Melbourne : CSIRO .
- Takhtajan , A. L. 1980 . Outline of the classification of flowering plants (Magnoliophyta). . Bot. Rev. , 46 : 225 – 359 .
- Taylor , M. L. , Gutman , B. L. , Melrose , N. A. , Ingraham , A. M. , Schwartz , J. A. and Osborn , J. M. 2008 . Pollen and anther ontogeny in Cabomba caroliniana (Cabombaceae, Nymphaeales). . Am. J. Bot. , 95 : 399 – 413 .
- Taylor , M. L. and Osborn , J. M. 2006 . Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). . Am. J. Bot. , 93 : 344 – 356 .
- Tillich , H.‐J. , Tuckett , R. and Facher , E. 2007 . Do Hydatellaceae belong to the monocotyledons or basal angiosperms? Evidence from seedling morphology. . Willdenowia , 37 : 399 – 406 .
- Yadav , S. R. and Janarthanam , M. K. 1994 . Hydatellaceae: A new family to the Indian flora with a new species. . Rheedea , 4 : 17 – 20 .
- Yadav , S. R. and Janarthanam , M. K. 1995 . Trithuria konkanensis (Hydatellaceae), eine neue Art aus Indien. . Aqua‐Planta , 3 : 91 – 97 .
- Volkova , P. A. and Shipunov , A. B. 2007 . Morphological variation of Nymphaea (Nymphaeaceae) in European Russia. . Nord. J. Bot. , 26 (available online: www.interscience.wiley.com/journal… etc.; in print)