Abstract
The pollen wall ultrastructure and its ontogeny in Tribulus terrestris L. are described in detail. The distinction between ectexine and endexine have been inconclusive from previous transmission electron and scanning electron microscopy observations, and periodic acid Schiff reaction (PAS) testing. It is observed that, at the beginning of the tetrad stage, the plasmalemma is wavy, with alternating evaginations and invaginations. The bi-layered primexine matrix is concentrated inside invagination, whereas pro-columellae are initiated on evaginations. At the end of the tetrad stage a primordial nexinous lamella (PNL) is formed, it is continuous all around the microspore and, at some levels a white line is observed. On the outer face of the PNL the foot layer develops; while the inner face is the base for progressive formation of the endexine lamellae. At the free microspore stage, all the exine components thicken and the primordial nexinous lamella is no longer discernible. The endexine lamellae, which are lacking in the pore region, form a network; the lumina of this network are progressively reduced and will disappear later. By the free microspore stage, the outer nexine is compact and the inner nexine is distinctly lamellar.
The genus Tribulus (Zygophyllaceae) includes 20 species (Judd et al., Citation2002). The morphology and exine structure of the mature pollen grain have been observed with light microscopy for a number of species by authors, including: Agababian (Citation1964), Huang (Citation1972), Heusser (Citation1971), Kuprianova & Alyeshina (Citation1978), Riollet (Citation1974) and Selling (Citation1947). However, only a few studies have addressed pollen exine ultrastructure using transmission electron microscopy techniques; these include Ben Mekki (Citation1982) and Praglowski (Citation1987).
The present study focuses on the pollen wall ontogeny of Tribulus terrestris L., an herbaceous plant of medicinal value which is widely distributed in Tunisia. Using transmission electron microscopy (TEM), Ben Mekki (Citation1982) observed a compact nexine in Tribulus terrestris, which is sometimes coarsely lamellar on its inner side. She considered it as a delay in endexine development. Later, Praglowski (Citation1987) described the ultrastructure of Tribulus with TEM and noted: “… Foot layer bizonal, its distal, more compact part, thinner than the proximal part consisting of a few coarse lamellae… Endexine presumably discontinuous, very thin, fibrillar, with minute globular elements…”. In other words, the foot layer forms the major part of the nexine. This type of nexine is characteristic of other genera such as Kallstroemia, Kelleronia, Tribulopis in Zygophyllaceae; it also occurs in some taxa of Amaranthaceae, Vivianiaceae as well as some taxa of Caryophyllaceae and Convolvulaceae (Borsch & Barthlott, Citation1998). However, all these results were insufficient to differentiate layers especially of the nexine (mainly ectexine and endexine). Therefore we have studied exine ultrastructure using TEM, scanning electron microscopy (SEM) and the PTA acetone histochemical test to try and distinguish the two layers. Nevertheless, our observations were inconclusive: ectexine and endexine showed the same electron density. Therefore, the aim of the present investigation has been to study the ontogeny of the pollen wall in Tribulus terrestris from the tetrad stage to the mature pollen grain, focusing mainly on the formation of the nexine layers.
Material and methods
Fresh flower buds of Tribulus terrestris, at different stages of development, were gathered in the neighbourhood of the Sciences Faculty of Tunis. For SEM, acetolysed pollen grains were mounted on stubs and sputter coated with gold palladium; a JEOL JSM 5400 SEM was used for examination. For TEM, anthers were fixed at different developmental stages with 4% glutaraldehyde in a sodium cacodylate buffer, then post fixed with buffered 1% osmium tetroxide (OsO4), dehydrated in acetone and embedded in Spurr's resin. Sections were cut with a diamond knife, collected onto copper grids, then post-stained with uranyl acetate and lead citrate (Ur/Pb) or potassium permanganate (KMnO4). To detect neutral polysaccharides, sections were stained with periodic acid-thiocarbohydrazide in acetic acid-silver proteinate ‘Thiéry test’ (Thiery, Citation1967). Subsequently the grids were examined with either a Philips EM 301 or a Jeol JEM 1010 TEM.
Terminology
In optical (light) microscopy, where staining techniques do not allow the distinction between ‘ectexine’ and ‘endexine’, the terms ‘sexine’ and ‘nexine’ are used (Erdtman, Citation1952). The sexine comprises the tectum plus the ‘infratectum’ (or ‘columellar layer’), while the nexine comprises the foot layer and the endexine. However, in transmission electron microscopy, where have been developed ectexine comprises tectum, infratectum and foot layer, while endexine is distinct – usually, in mature pollen, as a lighter staining layer (Faegri, Citation1956).
Results
The mature pollen grain (–E)
The mature pollen grain is spheroidal to subspheroidal (), with an average diameter of 49 μm; it is pantoporate. The exine is coarsely reticulate with irregular polygonal lumina (, B). It comprises a sexine (SE) and a nexine (NE) (, D). The columellae are variable in diameter. Their outer extremities are wider than their bases, and merge to form the muri (M) The nexine is thick, and the outer surface is smooth (); the compact inner surface is coarsely and densely lamellar (, D). There is a germinal pore (PO) positioned in the nexine at the centre of each lumen of the reticulum. The pore (aperture) membrane (AM) is granular (G), the ectexinous granulae (G) are variable in size and cover the pore membrane (). Nexine is not obvious in the poral regions (, D). The inter-poral intine (I) is thin () while the poral intine is thick and bi-layered (el, il) and has a different electron density (, E). Tryphine (T) is abundant (–E).
Figure 1. A–E. The mature pollen grain of Tribulus terrestris: A, B. SEM; C–E. TEM. A. Whole, pantoporate grain; note pore (PO). B. The reticulum with polygonal lumina of varying size and shape. In each lumen there is a pore (PO); ectexinous granules (G) are seen on the aperture membrane (AM) and the muri (M) have pyramidal thickenings at the intersections of the muri (*). C. A section of a mature pollen grain [pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] with a large central vacuole (V), numerous plastids (P), generative cell (GC); the sexine (SE) and nexine (NE) are interrupted by pores. D. Detail of the interporal region [material pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)]: sexine (SE), nexine (NE), ectexinous granules (G) on the pore membrane, murus (M), tryphine (T); intine (I), external layer of the intine (el), internal layer of the intine (il). E. Detail [material pre-fixed in glutaraldehyde then post-fixed in osmium tetroxide (OsO4)] of the mesopore (in Thiéry test) intine and tryphine show positive; the internal layer of the intine (il) is intensely positive, while the external layer (el) is moderately positive. Scale bars – 10 μm (A); 1 μm (B, E); 5 μm (C); 2 μm (D).
![Figure 1. A–E. The mature pollen grain of Tribulus terrestris: A, B. SEM; C–E. TEM. A. Whole, pantoporate grain; note pore (PO). B. The reticulum with polygonal lumina of varying size and shape. In each lumen there is a pore (PO); ectexinous granules (G) are seen on the aperture membrane (AM) and the muri (M) have pyramidal thickenings at the intersections of the muri (*). C. A section of a mature pollen grain [pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] with a large central vacuole (V), numerous plastids (P), generative cell (GC); the sexine (SE) and nexine (NE) are interrupted by pores. D. Detail of the interporal region [material pre-fixed in glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)]: sexine (SE), nexine (NE), ectexinous granules (G) on the pore membrane, murus (M), tryphine (T); intine (I), external layer of the intine (el), internal layer of the intine (il). E. Detail [material pre-fixed in glutaraldehyde then post-fixed in osmium tetroxide (OsO4)] of the mesopore (in Thiéry test) intine and tryphine show positive; the internal layer of the intine (il) is intensely positive, while the external layer (el) is moderately positive. Scale bars – 10 μm (A); 1 μm (B, E); 5 μm (C); 2 μm (D).](/cms/asset/dcbb5c8b-ae08-4c8b-8da6-71f9eef905e7/sgra_a_391796_o_f0001g.gif)
Ontogeny (–)
Tetrad stage (–F; 3A, B)
Initially, the plasmalemma (PL) is intermittently wavy, with ‘evaginations’ and ‘invaginations’ (). The space located between the plasmalemma and the callose special wall (; 3B – CSW) is filled with the primexine matrix (PM) a loose-meshed fibrillar network. This matrix is more abundant in the invaginations than in the evaginations. The pro-columellae (pC) are initiated on the evaginations () while the future/pro-apertures (pA) are initiated on the invaginations where endoplasmic reticulum (ER) is in close contact with the plasmalemma ().
Figure 2. A–F. TEM of the Tribulus terrestris pollen tetrad stage. Early-mid tetrad stage, pre-fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with uranyl acetate and lead citrate. A. Part of a microspore, early tetrad stage: primexine matrix (PM) is fibrillar and thicker in the invaginate zones (*) than in the evaginate zones (thick arrows); note the plasmalemma (PL) and special callose wall (SCW). B. Detail of a microspore: pro-columellae (pC) initiated on the evagination; the primexine matrix increases in thickness (*) in the inter-columellar zones. C. Detail of a microspore: endoplasmic reticulum (ER, longer arrow) profile adjacent to the internal layer of the plasmalemma at a future/pro-aperture site (pA, short arrow). D. Microspore in the middle tetrad stage: the pro-murus (pM) is formed. E. Detail of (rectangle), the pro-columella (pC) and the pro-murus (pM) are heterogeneous with clear zones (arrow); the pro-columella is on the outer layer of the plasmalemma (PL). F. Aperture detail: the primexine matrix is micro-fibrillar, and comprises two layers: an outer layer (ePM), thick, with a loose mesh-like structure, which extends to the pore site (PO); and an inner layer (iPM) which fills the inter-columellar spaces but is absent at pore sites. Well-developed pro-columellae are apparent (pC) and, elements of the pro-muri (pM) of the future tectum can also be seen (arrow). Mitochondria (m), ribosomes (r) and endoplasmic reticulum (ER) are present in the cytoplasm. Scale bars – 0.5 μm (A); 0.2 μm (B, E, F); 2 μm (C, D).
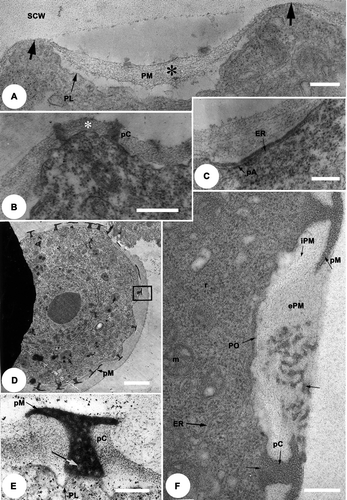
The pro-columellae (pC) are formed on the external layer of the plasmalemma (); the distal area of each pro-columella enlarges horizontally to initiate the pro-murus (pM). The composition of both the pro-columellae and the pro-murus are heterogeneous with electron translucent zones (). This suggests that they are built of several units surrounding a moderately electron dense central core zone. At this stage, the protoplasm is rich with organelles (mitochondria, ribosomes, ER, and plastids). In the invaginations (), the primexine matrix is bi-layered: the external layer (ePM) is electron dense and covers the poral (aperture) sites, while the internal layer (iPM) is less electron dense in the inter-poral zones, and is absent at the pore sites (). The external layer is moderately Thiéry positive while the internal layer is strongly Thiéry positive ().
Figure 3. TEM of the late tetrad stage [A, B] to free microspore stage [C–E] of Tribulus terrestris pollen. A. Thiéry test: the outer layer of the primexine matrix (ePM) is moderately Thiéry positive while the inner layer (iPM) is strongly Thiéry positive. B. Fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4). Callose digestion begins: primordial nexine lamellae (PNL) are initiated at the bases of the columellae; plasmalemma is present (PL), and the special callose wall (SCW) is still in place; cellular organelles are abundant. C. Part of a microspore wall fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4); the columellae and the murus have thickened within the primexine matrix (PM), the primordial endexinous lamellae branch out (PNL & arrows), plasmalemma is still present (PL). D. General view of a microspore, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with uranyl ucetate and lead citrate, showing development of endexinous lamellae. E. Detail of (box 1) at the mesoporal level: the wide spaces between the thin endexine lamellae (ENL) are filled with a fibrillar material (*), the plasmalemma (PL) is more pronounced. Sporopollenin deposition is initiated from the outside of the grain and continues towards the inner face of the endexine. The endexine is separated from the foot layer (FL) by a white line (paired arrows), the columellae (C) and muri (M) become thicker. Scale bars – 1 μm (A); 0.2 μm (B, C & E); 2 μm (D).
![Figure 3. TEM of the late tetrad stage [A, B] to free microspore stage [C–E] of Tribulus terrestris pollen. A. Thiéry test: the outer layer of the primexine matrix (ePM) is moderately Thiéry positive while the inner layer (iPM) is strongly Thiéry positive. B. Fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4). Callose digestion begins: primordial nexine lamellae (PNL) are initiated at the bases of the columellae; plasmalemma is present (PL), and the special callose wall (SCW) is still in place; cellular organelles are abundant. C. Part of a microspore wall fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with potassium permanganate (KMnO4); the columellae and the murus have thickened within the primexine matrix (PM), the primordial endexinous lamellae branch out (PNL & arrows), plasmalemma is still present (PL). D. General view of a microspore, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) and stained with uranyl ucetate and lead citrate, showing development of endexinous lamellae. E. Detail of Figure 3D (box 1) at the mesoporal level: the wide spaces between the thin endexine lamellae (ENL) are filled with a fibrillar material (*), the plasmalemma (PL) is more pronounced. Sporopollenin deposition is initiated from the outside of the grain and continues towards the inner face of the endexine. The endexine is separated from the foot layer (FL) by a white line (paired arrows), the columellae (C) and muri (M) become thicker. Scale bars – 1 μm (A); 0.2 μm (B, C & E); 2 μm (D).](/cms/asset/a56475e4-345a-4e4c-81a4-6effbd2ac99a/sgra_a_391796_o_f0003g.gif)
At the end of the tetrad stage, callose digestion begins. The pro-columellae and the pro-muri become more contrasted; the primexine matrix persists and is still fibrillar. The plasmalemma is still wavy; while one primordial nexine lamella (PNL) is seen to be continuous all around the microspore and has a white line at some levels (arrow, ). The foot layer (FL) is formed on the outer layer of this primordial nexine lamella (). While on the inner layer of the primordial lamella endexinous lamellae (ENL) are formed, and become more and more numerous ().
Free microspore stage (–E; –D)
The microspores increase in size and their shape becomes more regular. The muri and the bases of the columellae increase in thickness; the primexine matrix persists; the endexine lamellae form a stratified 3-dimensional structure which has the appearance of a network in TEM thin sections (). A fibrillar, loose and slightly electron dense material fills the spaces between the lamellae ( – asterisk).
The sporopollenin is laid down on the endexine lamellae according to a gradient:
| 1. | Overall sporopollenin accumulation is greater on the outer lamellae than on the inner lamellae; | ||||
| 2. | For individual lamella, sporopollenin is more abundant on the outer face than on the inner face. | ||||
The primordial nexine lamellae are no longer observed, once the nexine is formed (). In the pore region nexine is not present. However, the pores are covered by an aperture membrane (AM) which is covered by discrete ectexinous (sporopollenin) granules (G in ). As the nexine thickens the initially large spaces between the lamellae progressively reduce in volume, and eventually they often disappear (–D). The outer nexine is compact, and the inner nexine is distinctly lamellar.
Figure 5. Schematic representation of exine development in Tribulus terrestris. Abbreviations: AM – aperture membrane, C – columella, EN – endexine, ENL – endexinous lamellae, FL – foot layer, G – ectexinous granules, L1 – ectexinous layer of the primordial nexine lamella, L2 – endexinous layer of the primordial nexine lamella, M – muri, NE – nexine, SE – sexine.
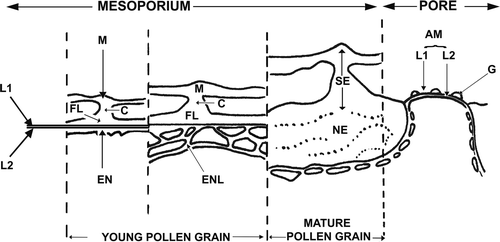
Figure 4. A–D. TEM of the development of the endexine: free microspore stage, in Tribulus terrestris pollen. A. Detail of (box 2): the pore (PO) is covered by an aperture membrane (AM) which is covered by ectexinous granules (G). Plasmalemma (PL, arrows) is continuous below the aperture membrane. B. The sporopollenin, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), deposition becomes more prominent on the endexine lamellae. C. The endexine [fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] lamellae thicken, and a more parallel alignment, together with a reduction in lumina size is apparent (arrows). D. The distal nexine, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), is now much more compact, and lamellae in the proximal/inner region are also notably compressed. Scale bars – 0.2 μm (A, B); 0.5 μm (C, D).
![Figure 4. A–D. TEM of the development of the endexine: free microspore stage, in Tribulus terrestris pollen. A. Detail of Figure 3D (box 2): the pore (PO) is covered by an aperture membrane (AM) which is covered by ectexinous granules (G). Plasmalemma (PL, arrows) is continuous below the aperture membrane. B. The sporopollenin, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), deposition becomes more prominent on the endexine lamellae. C. The endexine [fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4)] lamellae thicken, and a more parallel alignment, together with a reduction in lumina size is apparent (arrows). D. The distal nexine, fixed with glutaraldehyde, post-fixed in osmium tetroxide (OsO4) then stained with potassium permanganate (KMnO4), is now much more compact, and lamellae in the proximal/inner region are also notably compressed. Scale bars – 0.2 μm (A, B); 0.5 μm (C, D).](/cms/asset/8c91732d-3b3f-4f6d-ae23-1169d4b27e1f/sgra_a_391796_o_f0004g.gif)
, summarises the ultrastructure of the pollen wall from the immature (left) to mature (right) stages. In the immature stage, the foot layer (FL) and the endexine (EN) form simultaneously on L1 and L2. Endexine at this stage has a lamellar aspect; lamellae (ENL) form a reticulum-like structure at the mesoporal level. At the mature stage, the two layer structure (L1, L2) of the nexine (NE), are only obvious at pore level where they form the aperture membrane (AM).
Discussion
Our discussion focuses on the ontogenetic sequence of the sporoderm layers.
The primexine matrix
The primexine matrix was described first by Heslop-Harrison (Citation1963) in Silene pendula. Basically, it is a fibrillar layer which extends between the callose and the plasmalemma, with the exception of future aperture sites. Within the primexine matrix, pro-columellae are the first elements to form and subsequently the other elements of the future ectexine will develop.
There are many other species where ectexine development follows a similar pattern, for example, Teucrium flavum (Nabli, Citation1975a , Citation b ), Cosmos bipinnatus (Dickinson & Potter, Citation1976), Silene dioica (Audran & Batcho, Citation1981), Ziziphus lotus (Ben Nasri-Ayachi & Nabli, Citation1995), Scilla peruviana (Testillano et al., Citation1995), Solanum appendiculatum (Zavada & Anderson, Citation1997), Peganum harmala, Nitraria retusa and Fagonia cretica (Ben Nasri-Ayachi & Nabli, Citation2006).
In other taxa, the development of the outer pollen wall begins with the establishment of a pro-tectum. For example, in Artemisia vulgaris (Rowley & Dahl, Citation1977), the stratified tectum is initiated in a glycocalyx which covers the plasmalemma, and is formed of ‘tufts’ (Rowley et al., Citation1981) originating from the plasmalemma. These tufts which form the pro-tectum are constituted mainly of protein but also contain some neutral and some acidic polysaccharides. According to Rowley and Dahl (Citation1977), the glycocalyx in Artemisia vulgaris is equivalent to the primexine matrix since, “… it forms during the same ontogenetic interval, tapers to minimal thinness at the presumptive margins of apertures, and is absent over the pore”. The columellae, the foot layer and the endexine are differentiated later, during callose dissolution.
In Poinciana (Skvarla & Rowley, Citation1987), Hibiscus syriacus (Takahashi & Kouchi, Citation1988), Caesalpinia japonica (Takahashi, Citation1989a ), Farfugium japonicum (Takahashi, Citation1989b ) and Asimina triloba (Gabarayeva, Citation1992), the early development of the wall begins with the establishment of the pro-tectum on the plasmalemma and, at the same time, the formation of the primexine matrix and the pro-columellae. Furthermore, in C. japonica it was demonstrated by freeze-fracture (Takahashi, Citation1989a ) that the plasmalemma becomes wavy and its surface has a reticulate form corresponding to the exine pattern before pro-tectum establishment.
In Tribulus terrestris, the primexine matrix is initiated at the beginning of the tetrad stage: the apertures form in the invaginations of the plasmalemma, and the pro-columellae on the evaginations. Furthermore, in T. terrestris the primexine matrix completely surrounds the microspore, and this also the case in Ulmus (Rowley & Rowley, Citation1986) and in Cercidiphyllum (Rowley et al., Citation1997); although in Cercidiphyllum the matrix covering the pore is called, ‘glycocalyx of the pore’ (Rowley et al., Citation1997).
The primexine matrix may be cellulosic as in Lilium longiflorum (Heslop-Harrison, Citation1968b ), Helleborus foetidus (Echlin & Godwin, Citation1969) and Trachymene pilosa (Roland-Heydacker & Cerceau-Larrival, Citation1975) or, it may be mucilaginous and contain some acidic polysaccharides, as in Ipomoea purpurea (Waterkeyn & Bienfait, Citation1968); while in Saxifraga cymbalaria (Abadie & Hideux, Citation1980) it contains cellulose, hemicellulose and some proteins. In T. terrestris the external layer covers the whole microspore, while the internal layer is interrupted at the future aperture sites. The Thiéry test has revealed that the internal layer in T. terrestris contains more neutral polysaccharides than the external layer.
The primexine matrix in Tribulus terrestris is heterogeneous, and comprises two very finely reticulate fibrillar layers; these two layers are apparent by the end of the tetrad stage ( & ).
The sexine
In their study of several species of Paeonia, Nowicke et al. (Citation1986) used ‘plasma ashing’, a method that causes oxidative degradation of the sexine. They found that the sexine is formed of cylindrical units which were not obvious before this treatment. Blackmore (Citation1990) showed in the ‘freeze fractured’ material of Echinops that the sexine elements are hollow during the young stages of development. El-Ghazaly & Rowley (Citation1999) also found cylindrical units in the exine of Echinodorus using the plasma ashing method. In Tribulus terrestris the sexine is heterogeneous at the middle tetrad stage, and formed of numerous units with a central core of low electron density. A similar structure was described in Triticum aestivum (El-Ghazaly & Jensen, Citation1985) and in Rondeletia odorata (El-Ghazaly et al., Citation2001). The structure is most obvious at tetrad stage during pro-tectum initiation and development. However, in T. aestivum and in T. terrestris the structure persists into free microspore stage. According to a recent synthesis of a new concept in the interpretation of the substructure of the exine (Blackmore et al., Citation2007), the sexine of Tribulus could originate through self-assembly of the micelle area in the glycocalyx, which could be constituted by amphiphobic long chain fatty acid molecules, with the low electron density central core as the hydrophobic region of the long chain fatty acid molecules, and the surrounding material as their hydrophilic ends.
The nexine
In angiosperm pollen development, the foot layer usually appears before the endexine (Blackmore & Barnes, Citation1990). In some cases it forms prior to callose digestion, as in Hibiscus syriacus (Takahashi & Kouchi, Citation1988) and Ziziphus lotus (Ben Nasri-Ayachi & Nabli, Citation1995). However, in Asimina triloba (Annonaceae; Gabarayeva, Citation1993), Lycopersicon esculentum (Polowick & Sawhney, Citation1993a , Citation b ) and Felicia muricata (Jordaan & Kruger, Citation1993), it is initiated after callose dissolution. In Artemisia vulgaris (Rowley & Dahl, Citation1977), Caesalpinia japonica (Takahashi, Citation1989a ), Uraria crinita (Liu & Huang, Citation1999), and Borago officinalis (Ben Saad-Limam et al., Citation2002), the two layers of the nexine appear simultaneously. In B. officinalis, the foot layer and endexine form at the same time but not at the same sites: the endexine first in the apertural zone and the foot layer first in the inter-apertural zone.
In Tribulus terrestris, the two layers are initiated simultaneously in the inter-apertural regions, at the end of tetrad stage, just before callose digestion.
During exine development the timing of the origin and formation of endexine and ectexine can also be distinguished by their chemical composition. Audran and Batcho (Citation1981) used a general stain (basic toluidine blue) and cytochemical tests on Silene dioica (Caryophyllaceae) in order to elucidate the chemical composition of the ectexine and the endexine. They showed that the ectexine and the endexine cannot be distinguished from each other prior to microspore vacuolisation when both regions contain carbohydrates, phospholipids and lipids but no protein. However, by the end of the vacuolisation stage the endexine also contains protein, while all the pre-vacuolisation substances, Audran & Batcho (Citation1981) concluded, are apparently no longer present in the ectexine. According to Southworth (Citation1973) the endexine contains sporopollenin and traces of polysaccharides in the apertures regions, while the ectexine contains sporopollenin associated with proteins, lipids and traces of polysaccharides (remnants of primexine matrix). Furthermore, Southworth (Citation1974) showed that the endexine is more resistant to treatment with the 2-aminoethanol and oxidation procedure than the ectexine. In the Calluna-model of Rowley (Citation2001) this difference in resistance to oxidation is interpreted thus: the endexine contains primary accumulated sporopollenin which is resistant to oxidation, whereas the ectexine, in addition to primary accumulated sporopollenin, contains a secondary accumulated polymer. The latter fills in gaps between exine units (tufts) and can be easily removed by oxidation.
In many taxa the endexine is separated from the ectexine by a very fine, electron translucent, line of discontinuity; this is visualised, with TEM, as a ‘white line’ or, a ‘commissural line’ (Simpson, Citation1983) or a ‘junction plane’ (Rowley, Citation1987–88, Citation1995) since it separates units of the ectexine from those of the endexine. This discontinuity is a common feature of angiosperm and gymnosperm pollen.
Some authors have interpreted the presence of a white line as proof of the existence of endexine in pollen of taxa where, generally, endexine is considered to be lacking, for example in the primitive angiosperms (Gabarayeva, Citation1991) and some monocotyledons (El-Ghazaly & Rowley, Citation1999). Differential staining for TEM work generally allows the distinction of the ectexine from the endexine. However, in pollen of some taxa ectexine and endexine have the same electron density. The only two ways to distinguish them are: an acetone histochemical test (PTA) and/or the presence of a white line, for example, in Saxifraga cymbalaria (Hideux & Abadie, Citation1985) and Nelumbo nucifera (Kreunen & Osborn, Citation1999) where the authors observed a white line, and in Hypecoum where the PTA test was used (Romero et al., Citation2003).
In Tribulus terrestris, although the endexine and foot layer form simultaneously, the endexine differs from the foot layer by its lamellar appearance. These lamellae appear at the plasmalemma and have similarly fine tripartite structure (Rowley & Dunbar, Citation1967; Rowley & Southworth, Citation1967; Heslop-Harrison, Citation1968a , b; Guedès, 1982; Hideux & Abadie, Citation1985). Furthermore, the endexine and foot layer in T. terrestris are separated at some levels by a white line. According to Nabli (Citation1975a , Citation b ), this white line is a discontinuity separating two layers of a nexinous primordial lamella, which forms around the microspore, at the end of the tetrad stage. It has a tripartite structure comprising: ectexinous layer Ll, endexinous layer L2 separated by a ‘white line’. The endexine forms on the inner face of layer L2 while the foot layer initiates on the outer face of L1. Therefore, in T. terrestris, the two layers (L1 and L2) demarcate the foot layer, as well as the infratectum and tectum, from the endexine.
At the mesoporium (interporal) sites, layer L2 gives rise to digitations at its inner face. The digitations branch out and form a network-like structure with loose meshes. This aspect of the network was described at endoaperture level, during tetrad stage, in Ziziphus lotus (Ben Nasri-Ayachi & Nabli, Citation1995) and in Fuchsia magellanica var. riccartonii (Horvat, Citation1992) at the microspore stage.
In angiosperms the lamellar appearance of the meso-apertural endexine, which characterises the young developmental stages, often disappears at mature, free microspore stage, following the juxtaposition and compression of the lamellae against one another. This has been shown in numerous studies of pollen ontogeny, as for example, in Lavandula dentata (Suárez-Cervera & Seoane-Camba, Citation1986), Ulmus (Rowley & Rowley, Citation1986), Olea europaea (Fernández & Rodríguez-García, Citation1989), Calluna (Dahl & Rowley, Citation1990–1991), Fuchsia magellanica var. riccartonii (Horvat, Citation1992), Felicia muricata (Jordaan & Kruger, Citation1993), Platanus acerifolia (Suárez-Cervera et al., Citation1995), Rosmarinus officinalis (Ubera Jiménez et al., Citation1996). However, this lamellar structure may persist at pollen grain maturity, for example, in Compositae: Ambrosia sp. (Larson et al., Citation1962), and in some primitive angiosperms such as Michelia fuscata, M. figo, Manglietia tenuipes, Magnolia delavayi and Liriodendron chinensis (Magnoliaceae); in Asimina triloba (Annonaceae) and Anaxogorea brevipes (Nymphaeaceae: Gabarayeva, Citation1991, 1992, 1993). The lamellar structure also persists to maturity in some gymnosperms such as, Cunninghamia lanceolata (Taxodiaceae: Kurmann, Citation1990) where the endexine is formed with eight to ten anastomosing lamellae, in Chamaecyparis lawsoniana (Cupressaceae: Lugardon, Citation1995) where the endexine is formed of regular and compressed layers.
In Tribulus terrestris endexinous lamellae are characterised by an unequal sporopollenin deposit. This situation has previously been described by Dickinson (Citation1971) in Pinus banksiana and by Lugardon (Citation1995) in Chamaecyparis lawsoniana where a “… asymmetrical thickening of the laminae, larger on the inner side of the electron-translucent line…” was described. In T. terrestris the contrary is observed, with the deposition of the corresponding lamella (lamina) being more prominent on the outer face than on the inner face of the electron translucent white line. Furthermore, for the lamellae as a whole, sporopollenin deposition follows a centripetally decreasing gradient, during which the large spaces between the lamellae (which in TEM thin section look like lumina) gradually decrease, and the outer endexine becomes compact, while some of inner endexinous lamellae persist so that instead of a foot layer, as described by Praglowski (Citation1987), there is a bizonal endexine. Other ontogenetic studies of exines with a thick nexine have shown that the nexine may be one-layered, for example, in Heliotropium europaeum (Boraginaceae) where the foot layer is also lacking (Ben Saad et al., Citation2005).
The aperture
In angiosperms the future aperture sites are usually delimited during the tetrad stage. The mechanism seems to involve lack, or reduction of, the primexine matrix following the proximity of the plasmalemma against the callosic special wall (Ehrlich, Citation1958; Heslop-Harrison, Citation1963, Citation1968a , b; Skvarla & Larson, Citation1966; Christensen & Horner, Citation1974). Endoplasmic reticulum (ER) profiles are often observed, near or against the plasmalemma. However, the sequence of aperture pattern development varies between angiosperm taxa. In Tribulus terrestris the future aperture sites are well-delimited at tetrad stage in the invaginations caused by interruption of the internal layer of the primexine matrix (cf. glycocalyx) where the endoplasmic reticulum is in close contact with the plasmalemma. While in Epilobium (Rowley, Citation1975) glycocalyx is abundant on the future aperture sites. In Campanula rapunculoides (Dunbar, Citation1973) the primexine matrix covers future pores, folding inside; in Nelumbo (Flynn & Rowley, Citation1971; Rowley, Citation1975), apertural areas do not exist at the tetrad stage, but by young free microspore stage three apertures are in place. According to Rowley (Citation1975) the formation of the apertures in Nelumbo may result from a ‘focal autolysis’ or ectexine dissolution in the apertural site at young microspore stage.
The intine
According to Heslop-Harrison and Heslop-Harrison (Citation1991) at apertural sites the intine consists of three layers: a homogeneous external layer containing pectic substances, a middle layer containing proteins incorporated in lamellae, tubules or columns, and a microfibrillar cellulosic internal layer. In the inter-apertural regions the intine is less developed.
In Tribulus terrestris the intine consists of two layers; similar cases have been described in a wide range of angiosperm families: from early evolving dicotyledons, for example, Asimina triloba (Annonaceae) by Gabarayeva (Citation1993); and the monocotyledons, for example, Amphibolis griffithii (Cymodoceaceae) by McConchie et al. (Citation1982), Gibasis karwinskyana and G. venustula (Commelinaceae) by Owens & Dickinson (Citation1983), and Amaryllidaceae, Liliaceae, Bromeliaceae, Dianellaceae, Dracaenaceae, Funkiaceae, Hyacinthaceae, Iridaceae, Melanthiaceae, Velloziaceae (Halbritter & Hesse, Citation1993); to the eudicotyledons, for example, Nelumbo nucifera and N. lutea (Nelumbonaceae) by Kreunen & Osborn (Citation1999) and Rosmarinus officinalis (Lamiaceae) by Ubera Jiménez et al. (Citation1996). In R. officinalis the outer layer of the intine is electron lucent and thick at the aperture level, while the inner layer is electron dense and has a uniform thickness around the microspore. In T. terrestris the outer layer is strongly electron dense (Thiéry positive) while the inner layer is moderately electron dense (Thiéry positive). Both intine layers are thick at the pore level and very thin elsewhere.
Conclusions
The exine ontogeny of Tribulus terrestris shows many features commonly observed in other taxa, such as the heterogeneity of columellate substructure during development, and the early lamellar aspect of the endexine. However, some ontogenetic aspects are less commonly observed, these include: the mode of foot layer initiation and the simultaneous occurrence of endexine on both faces of the primordial nexine lamella, giving rise to the nexine (foot layer and endexine), but with no foot layer discernible later in the mature pollen grain. We plan to study the pollen of further species of Tribulus in order to provide a wider view of exine and aperture development within the genus, with particular emphasis on the nexine.
Acknowledgements
We would like to thank our referees their valuable comments and advice with regard to our manuscript; we also extend our thanks to M. Harley, A. Le Thomas, J. R. Rowley, A. Audran, and C. Clément for their advice and assistance in various ways.
References
- Abadie , M. and Hideux , M. 1980 . L'anthère de Saxifraga cymbalaria L. ssp. huetiana (Boiss.) Engl. et Irmsch. en microscopie électronique (M.E.B. et M.E.T.). 2. Ontogenèse du sporoderme . Ann. Sci. Nat. Bot. (Paris) 14 Ser. , 1 : 237 – 281 .
- Agababian , V. C. 1964 . Morphologie des types de pollen et systématique de la famille de Zygophyllaceae . Izv. Akad. Nauk. Arm. SSR. Biol. Nauki , 17 : 39 – 45 . in Russian, with Armenian summary
- Audran , J.-C. and Batcho , M. 1981 . Cytochemical and infrastructural aspects of pollen and tapetum ontogeny in Silene dioica (Caryophyllaceae) . Grana , 20 : 65 – 80 .
- Ben Mekki , N. 1982 . Contribution à l’étude de l'exine de quelques Zygophyllacées tunisiennes. Fac. Sci. Univ. Tunis . Dipl. Etud. Approf. ,
- Ben Nasri-Ayachi , M. and Nabli , M. A. 1995 . Pollen wall ultrastructure and ontogeny in Ziziphus lotus L. (Rhamnaceae) . Rev. Palaeobot. Palynol. , 85 : 85 – 98 .
- Ben Nasri-Ayachi , M. and Nabli , M. A. 2006 . Comparative exine ontogeny in some members of the family Zygophyllaceae sensu lato . Protoplasma , 228 : 49 – 53 .
- Ben Saad-Limam , S. , Nabli , M. A. and Rowley , J. R. 2002 . Exine ontogeny in Borago officinalis pollen . Grana , 41 : 1 – 11 .
- Ben Saad-Limam , S. , Nabli , M. A. and Rowley , J. R. 2005 . Pollen wall ultrastructure and ontogeny in Heliotropium europaeum L. (Boraginaceae) . Rev. Palaeobot. Palynol. , 133 : 135 – 149 .
- Blackmore , S. 1990 . Sporoderm homologies and morphogenesis in land plants, with a discussion of Echinops sphaerocephala (Compositae) . Plant Syst. Evol. Suppl. , 5 : 1 – 12 .
- Blackmore , S. and Barnes , S. H. 1990 . “ Pollen wall development in angiosperms ” . In Microspores: Evolution and ontogeny , Edited by: Blackmore , S. and Knox , B. R. 173 – 192 . London : Acad. Press .
- Blackmore , S. , Wortley , A. H. , Skvarla , J. J. and Rowley , J. R. 2007 . Pollen wall development in flowering plants . N. Phytol. , 174 : 483 – 498 .
- Borsch , T. and Barthlott , W. 1998 . Structure and evolution of metareticulate pollen . Grana , 37 : 68 – 78 .
- Christensen , J. and Horner , H. T. Jr. 1974 . Pollen pore development and its spatial orientation during microsporogenesis in the grass Sorghum bicolor . Am. J. Bot. , 61 : 604 – 623 .
- Dahl , A. O. and Rowley , J. R. 1990–1991 . Microspore development in Calluna (Ericaceae). Exine formation . Ann. Sci. Nat. Bot. (Paris) 13 Ser. , 11 : 155 – 176 .
- Dickinson , H. G. 1971 . “ The role played by sporopollenin in the development of pollen in Pinus banksiana ” . In Sporopollenin , Edited by: Brooks , J. , Grant , P. R. , Muir , M. , van Gijzel , P. and Shaw , G. 31 – 67 . London : Acad. Press .
- Dickinson , H. G. and Potter , U. 1976 . The development of patterning in the alveolar sexine of Cosmos bipinnatus . N. Phytol. , 76 : 543 – 550 .
- Dunbar , A. 1973 . Pollen ontogeny in some species of Campanulaceae. A study by electron microscopy . Bot. Not. , 126 : 277 – 315 .
- Echlin , P. and Godwin , H. 1969 . The ultrastructure and ontogeny of the pollen in Helleborus foetidus L. III. The formation of the pollen grain wall . J. Cell Sci. , 5 : 459 – 477 .
- Ehrlich , H. G. 1958 . Electron microscope studies of Saintpaulia ionantha Wendl. pollen walls . Exp. Cell Res. , 15 : 463 – 474 .
- El-Ghazaly , G. and Jensen , W. A. 1985 . Studies of the development of wheat (Triticum aestivum) pollen. III. Formation of microchannels in the exine . Pollen Spores , 27 : 5 – 14 .
- El-Ghazaly , G. and Rowley , J. R. 1999 . Microspore and tapetal development in Echinodorus cordifolius (Alismataceae) . Nord. J. Bot. , 19 : 101 – 120 .
- El-Ghazaly , G. , Huysmans , S. and Smets , E. F. 2001 . Pollen development of Rondeletia odorata (Rubiaceae) . Am. J. Bot. , 88 : 14 – 30 .
- Erdtman , G. 1952 . Pollen morphology and plant taxonomy. Angiosperms , Stockholm : Almqvist & Wiksell .
- Faegri , K. 1956 . Recent trends in palynology . Bot. Rev. , 22 : 639 – 664 .
- Fernández , M. C. and Rodríguez-García , M. I. 1989 . Developmental changes in the aperture during pollen grain ontogeny in Olea europaea L . N. Phytol. , 111 : 717 – 723 .
- Flynn , J. J. and Rowley , J. R. 1971 . Primexine of Nelumbo nucifera . Experientia , 27 : 227 – 228 .
- Gabarayeva , N. I. 1991 . “ Pattern of development in primitive angiosperm pollen ” . In Pollen and spores: Patterns of diversification , Edited by: Blackmore , S. and Barnes , S. H. 257 – 268 . Oxford : Clarendon Press. Syst. Assoc. Sp. Vol. 44 .
- Gabarayeva , N. I. 1992 . Sporoderm development in Asimina triloba (Annonaceae). I. The developmental events before callose dissolution . Grana , 31 : 213 – 222 .
- Gabarayeva , N. I. 1993 . Sporoderm development in Asimina triloba (Annonaceae). II. The developmental events after callose dissolution . Grana , 32 : 210 – 220 .
- Guèdes , M. 1982 . Exine stratification, ectexine structure and angiosperm evolution . Grana , 21 : 161 – 170 .
- Halbritter , H. and Hesse , M. 1993 . Sulcus morphology in some monocot families . Grana , 32 : 87 – 99 .
- Heslop-Harrison , J. 1963 . An ultrastructural study of pollen wall ontogeny in Silene pendula . Grana, Palynol. , 4 : 7 – 24 .
- Heslop-Harrison , J. 1968a . Pollen wall development . Science , 161 ( 3836 ) : 230 – 237 .
- Heslop-Harrison , J. 1968b . Wall development within the microspore tetrad of Lilium longiflorum . Can. J. Bot. , 46 : 1185 – 1192 .
- Heslop-Harrison , J. and Heslop-Harrison , Y. 1991 . “ Structural and functional variation in pollen intines ” . In Pollen and spores: Patterns of diversification , Edited by: Blackmore , S. and Barnes , S. H. 331 – 343 . Oxford : Clarendon Press . Syst. Assoc. Sp. Vol. 44
- Heusser , C. J. 1971 . Pollen and spores of Chile , Tucson, AZ : Univ. Arizona Press .
- Hideux , M. and Abadie , M. 1985 . Cytologie ultrastructurale de l'anthère de Saxifraga. I. Période d'initiation des précurseurs des sporopollénines au niveau des principaux types exiniques . Can. J. Bot. , 63 : 97 – 112 .
- Horvat , F. 1992 . Développement du pore germinatif des grains de pollen et plus spécialement de l'endexine d'un Fuchsia . Belg. J. Bot. , 125 : 80 – 88 .
- Huang , T. C. 1972 . Pollen flora of Taiwan , Taipei : Bot. Dept. Natl Taiwan Univ. Press .
- Jordaan , A. and Kruger , H. 1993 . Pollen wall ontogeny of Felicia muricata (Asteraceae, Astereae) . Ann. Bot. , 71 : 97 – 105 .
- Judd , W. S. , Cambell , C. S. , Kellogg , E. A. and Stevens , P. 2002 . “ Zygophyllacées ” . In (Intr. & Rev.), Botanique systématique. Une perspective phylogénétique , Edited by: Judd , W. S. , Cambell , C. S. , Kellogg , E. A. , Stevens , P. , Bouharmont , J. and Evrard , C. M. 264 – 266 . Issy-les-Moulineaux : De Boeck Univ .
- Kreunen , S. S. and Osborn , J. M. 1999 . Pollen and anther development in Nelumbo (Nelumbonaceae) . Am. J. Bot. , 86 : 1662 – 1673 .
- Kuprianova , L. A. and Alyeshina , L. A. 1978 . Pollen dicotyledonearum florae partis europaeae URSS. 3. Lamiaceae – Zygophyllaceae , Leningrad (St. Petersburg) : V. L. Komarov Inst. Bot. USSR Acad. Sci. /Nauka Publ. H. . in Russian
- Kurmann , M. H. 1990 . Exine formation in Cunninghamia lanceolata (Taxodiaceae) . Rev. Palaeobot. Palynol. , 64 : 175 – 179 .
- Larson , D. A. , Skvarla , J. J. and Lewis , C. W. 1962 . An electron microscope study of exine stratification and fine structure . Pollen Spores , 4 : 233 – 246 .
- Liu , C.-C. and Huang , T.-C. 1999 . Microsporogenesis and exine substructure in Uraria crinata (Fabaceae) . Grana , 38 : 277 – 283 .
- Lugardon , B. 1995 . Exine formation in Chamaecyparis lawsoniana (Cupressaceae) and a discussion on pteridophyte exospore and gymnosperm exine ontogeny . Rev. Palaeobot. Palynol. , 85 : 35 – 51 .
- McConchie , C. A. , Knox , R. B. , Ducker , S. C. and Pettitt , J. M. 1982 . Pollen wall structure and cytochemistry in the seagrass Amphibolis griffithii (Cymodoceaceae) . Ann. Bot. , 50 : 729 – 732 .
- Nabli , M. A. 1975a . Contribution à l’étude ultrastructurale et ontogénique de l'exine de quelques Labiatae , Marseille : Fac. Sci. C. Luminy Univ. Aix - Marseille II. D. Sc. Thes .
- Nabli , M. A. 1975b . Mise en évidence de deux lamelles primordiales ectexinique et endexinique, dans l'exine de quelques Labiatae . C. R. Acad. Sci., Sér. D (Paris) , 281 : 251 – 254 .
- Nowicke , J. W. , Bittner , J. L. and Skvarla , J. J. 1986 . “ Paeonia, exine substructure and plasma ashing ” . In Pollen and spores: Form and function , Edited by: Blackmore , S. and Ferguson , I. K. 81 – 94 . London/New York : Acad. Press. Linn. Soc. Symp. Ser. 12 .
- Owens , S. J. and Dickinson , H. G. 1983 . Pollen wall development in Gibasis (Commelinaceae) . Ann. Bot. , 51 : 1 – 15 .
- Polowick , P. L. and Sawhney , V. K. 1993a . An ultrastructural study of pollen development in tomato (Lycopersicon esculentum). I. Tetrad to early binucleate microspore stage . Can. J. Bot. , 71 : 1039 – 1047 .
- Polowick , P. L. and Sawhney , V. K. 1993b . An ultrastructural study of pollen development in tomato (Lycopersicon esculentum). II. Pollen maturation . Can. J. Bot. , 71 : 1048 – 1055 .
- Praglowski , J. 1987 . Pollen morphology of Tribulaceae . Grana , 26 : 193 – 211 .
- Riollet , G. 1974 . “ Acanthaceae, Aizoaceae, Amaranthaceae, Boraginaceae, Burseraceae, Capparidaceae, Combretaceae, Convolvulaceae, Zygophyllaceae ” . In Pollen et spores d'Afrique tropicale , Edited by: Caratini , C. and Guinet , P. 40 – 49 . 72 – 77 . 86 106 – 107 . 266 – 267 . Talence : CEGET Univ. Bordeaux/CNRS/APLF . Trav. Doc. Géogr. Trop. 16
- Riollet , G. and Bonnefille , R. 1974 . “ Acanthaceae ” . In Pollen et spores d'Afrique tropicale , Edited by: Caratini , C. and Guinet , P. 38 – 40 . Talence : CEGET Univ. Bordeaux/CNRS/APLF . Trav. Doc. Géogr. Trop. 16
- Roland-Heydecker , F. and Cerceau-Larrival , M. T. 1975 . Ontogénie du pollen de Trachymene pilosa (Ombellifères) au M.E.T . Bull. Soc. Bot. Fr. (Coll. Palynol.) , 122 : 125 – 127 .
- Romero , A. T. , Salinas , M. J. and Fernandez , M. C. 2003 . Pollen wall development in Hypecoum imberbe Sm. (Fumariaceae) . Grana , 42 : 91 – 101 .
- Rowley , J. R. 1975 . Germinal apertural formation in pollen . Taxon , 24 : 17 – 25 .
- Rowley , J. R. 1987–88 . Substructure within the endexine, an interpretation . J. Palynol. , 23–24 : 29 – 42 .
- Rowley , J. R. 1995 . Are the endexines of pteridophytes, gymnosperms and angiosperms structurally equivalent? . Rev. Palaeobot. Palynol. , 85 : 13 – 34 .
- Rowley , J. R. 2001 . Why the endexine and ectexine differ in resistance to oxidation. Calluna as a model system . Grana , 40 : 159 – 162 .
- Rowley , J. R. and Dahl , A. O. 1977 . Pollen development in Artemisia vulgaris with special reference to glycocalyx material . Pollen Spores , 19 : 169 – 284 .
- Rowley , J. R. and Dunbar , A. 1967 . Sources of membranes for exine formation . Sv. Bot. Tidskr. , 61 : 49 – 64 .
- Rowley , J. R. and Rowley , J. S. 1986 . “ Ontogenetic development of microspores of Ulmus (Ulmaceae) ” . In Pollen and spores: Form and function , Edited by: Blackmore , S. and Ferguson , I. K. 19 – 33 . London/New York : Acad. Press . Linn. Soc. Symp. Ser. 12
- Rowley , J. R. and Southworth , D. 1967 . Deposition of sporopollenin on lamellae of unit membrane dimension . Nature , 213 : 703 – 704 .
- Rowley , J. R. , Dahl , A. O. and Rowley , J. S. 1981 . Substructure in exines of Artemisia vulgaris (Asteraceae) . Rev. Palaeobot. Palynol. , 35 : 1 – 38 .
- Rowley , J. R. , Huynh , K. L , Qureshi , S. Z. and Rowley , J. S. 1997 . Pollen wall development in Cercidiphyllum. A review . Jpn. J. Palynol. , 43 : 113 – 121 .
- Selling , C. H. 1947 . Studies in Hawaiian pollen statistics. P. II. The pollens of the Hawaiian phanerogams , Goeteborg/Honolulu, HI : B.P. Bishop Mus. Sp. Publ. 38 .
- Simpson , M. G. 1983 . Pollen ultrastructure of the Haemodoraceae and its taxonomic significance . Grana , 22 : 79 – 103 .
- Skvarla , J. J. and Larson , D. A. 1966 . Fine structural studies on Zea Mays pollen. I. Cell membranes and exine ontogeny . Am. J. Bot. , 53 : 1112 – 1125 .
- Skvarla , J. J. and Rowley , J. R. 1987 . Ontogeny of the pollen in Poinciana (Leguminosae). 1. Development of exine template . Rev. Palaeobot. Palynol. , 50 : 293 – 311 .
- Southworth , D. 1973 . Cytochemical reactivity of pollen walls . J. Histochem. Cytochem. , 21 : 73 – 80 .
- Southworth , D. 1974 . Solubility of pollen exines . Am. J. Bot. , 61 : 36 – 74 .
- Suárez-Cervera , M. and Seoane-Camba , J. A. 1986 . Ontogenèse des grains de pollen de Lavandula dentata L. et évolution des cellules tapétales . Pollen Spores , 28 : 5 – 28 .
- Suárez-Cervera , M. , Marquez , J. and Seoane-Camba , J. A. 1995 . Pollen grain and Ubisch body development in Platanus acerifolia . Rev. Palaeobot. Palynol. , 85 : 63 – 84 .
- Takahashi , M. 1989a . Pattern determination of the exine in Caesalpinia japonica (Leguminosae: Caesalpinioideae) . Am. J. Bot. , 76 : 1615 – 1626 .
- Takahashi , M. 1989b . Development of the echinate pollen wall in Farfugium japonicum (Compositae: Senecioneae) . Bot. Mag. (Tokyo) , 102 : 219 – 234 .
- Takahashi , M. and Kouchi , J. 1988 . Ontogenetic development of spinous exine in Hibiscus syriacus (Malvaceae) . Am. J. Bot. , 75 : 1549 – 1558 .
- Testillano , P. S. , Fadón , B. and Risueño , M. C. 1995 . Ultrastructure localization of the polysaccharidic component during the sporoderm ontogeny of the pollen grain of Scilla peruviana (Liliaceae) and Capsicum annuum (Solanaceae) . Rev. Palaeobot. Palynol. , 85 : 53 – 62 .
- Thiéry , J. P. 1967 . Mise en évidence des polysaccharides sur coupes fines en microscope électronique . J. Microsc. , 6 : 987 – 1018 .
- Ubera Jiménez , J. L , Hidalgo-Fernández , P. , Schlag , M. G. and Hesse , M. 1996 . Pollen and tapetum development in male fertile Rosmarinus officinalis L. (Lamiaceae) . Grana , 34 : 305 – 316 .
- Waterkeyn , L. and Bienfait , A. 1968 . La paroi spéciale callosique et les premiers dépôts de l'exine chez Ipomoea purpurea L., Roth . C. R. Acad. Sci. (Paris) , 267 : 56 – 58 .
- Zavada , M. S. and Anderson , G. J. 1997 . The wall and aperture development of pollen from dioecious Solanum appendiculatum: What is inaperturate pollen? . Grana , 36 : 129 – 134 .