Abstract
Palaua (Malvaceae) comprises 15 species endemic to the coastal deserts of Chile and Peru. Previous molecular phylogenetic analyses have shown that this genus is monophyletic and can be subdivided into three clades. In the present study, pollen morphology of all species of Palaua was examined using light and scanning electron microscopy to determine whether it provides additional data in support of the proposed infrageneric groups. The pollen grains are spheroidal, medium to large in diameter, spinose and pantocolporate – the ectocolpi are very short. The tectum is perforate and characterised by granula. In all species the nexine is of a similar thickness in all areas of the pollen grain, while the sexine thickness varies, being notably thicker in the areas where the broad spine bases are sited (‘spine cushions’). Many of the quantitative characters have conspicuous variability, but the variability shows considerable overlap between species. Nonetheless, P. guentheri, P. inconspicua, P. malvifolia and P. modesta are united as a group by having the smallest pollen grains with the smallest apertures, the shortest spines and the shortest interspinal distance. This grouping reflects only partially the suggested infrageneric clades, although it does tend to unite the species with the smallest flowers. A possible link between reproductive resources and pollen size is also considered, as well as the influence of polyploidy. However, the taxonomic utility of these quantitative characters is weakened by species with more than one cytotype.
The genus Palaua Cav. is a representative of tribe Malveae J. Presl (Malvoideae, Malvaceae) and comprises 15 species of annual to perennial herbaceous plants or sub-shrubs that are endemic to the ‘lomas formations’, a unique desert vegetation type extending along the coastal cordillera from North Peru to North Chile (Huertas et al., Citation2007). Early concepts of the tribal classification of Malvaceae (Reichenbach, Citation1828), based on the gynoecium with superimposed carpels, placed Palaua in tribe Malopeae together with the Old World genera Kitaibela Willd. and Malope L. However, chromosome numbers (Bates, Citation1968), pollen morphology (Krebs, Citation1990) and molecular data (Tate et al., Citation2005) showed that the tribe Malopeae is polyphyletic and Palaua is sister to the clade of Neotropical Fuertesimalva Fryxell and the monotypic Urocarpidium Ulbr.
Molecular phylogenetic analyses showed that the clade of Palaua is monophyletic and contains three well-supported subclades that are largely congruent with morphology (Huertas et al., Citation2007): one subclade, ‘Dissecta’, contains the species with dissected leaves and large, showy flowers: P. camananensis, P. dissecta, P. mollendoensis, P. weberbaueri, Palaua sp. nov.; another subclade, ‘Integrifolia’, includes species with entire to shallowly lobed leaves and mostly large, showy flowers: P. guentheri, P. malvifolia, P. moschata, P. rhombifolia, P. sandemanii, P. tomentosa, P. trisepala, P. velutina; although P. guentheri is an exception, because it has dissected leaves, the molecular data nevertheless support its position within the Integrifolia clade. The third subclade, ‘Inconspicua’, also has species with undivided leaves but small, white flowers: P. inconspicua and P. modesta. These three subdivisions may replace the previous infrageneric classifications of Baker (Citation1890) and Ulbrich (Citation1909), which are considered artificial (Fryxell, Citation1997; Huertas et al., Citation2007). To obtain further support for these new subdivisions of Palaua, our aim is to include additional data, for example, from pollen morphology.
Pollen of Palaua is of the Malva-type (Chaudhuri, Citation1965; Krebs, Citation1990), a type defined by spheroidal, spinose pollen which is characteristic for Malvoideae (Bayer & Kubitzki, Citation2003). Although the general pollen morphology is rather uniform in this subfamily, there are other, mainly quantitative, pollen characters that may allow discrimination between species or species groups, as has already been shown, for example, in Malvastrum (Hill, Citation1982), Hibiscus and Gossypium (Saad, Citation1960).
Previous studies describing pollen grains of Palaua (Chaudhuri, Citation1965; Krebs, Citation1990) have included too few species and specimens to evaluate whether pollen morphology may be taxonomically informative. Therefore, one of the main objectives of the present study was to examine whether pollen morphology in Palaua is sufficiently variable to be used for distinguishing species or species groups and, if so, whether the variation corroborates our previous phylogenetic hypotheses and the revised concept for a new infrageneric classification of Palaua. Our intention has also been to provide the first detailed account of the pollen morphology of Palaua, which would include all species of the genus.
To assess the taxonomic utility of pollen morphology in Palaua we have recorded pollen grain size, spine length, number and the distance between spines, aperture form, number and size, and thickness of the exine strata. We further included pollen measurements of Fuertesimalva, in order to compare the pollen characteristics of Palaua with those found in its sister group.
Material and methods
Pollen material
Pollen grains were taken from flowers collected in the field and preserved in 70% ethanol, from herbarium material in the herbaria of Frankfurt (FR) and Leipzig (LZ), as well as from plants grown in the greenhouses of the Botanical Garden of the University of Leipzig. All species of Palaua have been included in the study and the names and species circumscriptions used here follow the taxonomic revision of the genus (Huertas, unpubl.). For comparison of the pollen morphology with its sister group, Fuertesimalva, one individual of each of the following species was included: Fuertesimalva chilensis, F. echinata, F. limensis, and F. peruviana.
Pollen preparation
Three to six individuals per species, to represent their geographical and morphological variation, were used for the description of pollen (see Specimens investigated). However, for the rarely collected P. camanensis, only one collection was available for study and, for P. velutina, there were only two specimens available for pollen preparations. Since in Palaua the anthers generally open before anthesis, the pollen was removed from flower buds shortly before anthesis. For light (LM) and scanning electron microscopy (SEM), the pollen was boiled in 10% KOH for 8 – 10 minutes. Then the pollen was subjected to acetolysis following the protocol of Moore et al. (Citation1991). The pollen samples were held in the acetolysis mixture for only two minutes to minimise the risk of collapsed grains. After acetolysis the pollen samples were divided for LM and SEM. The samples for LM were mounted on glass slides in glycerine jelly under coverslips; the preparations were then sealed with nail varnish.
For SEM, the pollen was mounted on aluminium stubs, and then degassed for 24 hours in a Vacuum Plate Degasser PD3 (Edwards 1998). The samples were then coated with gold/iridium in a Bal-Tec Med 020 sputter coater and subsequently examined using a LEO 1430 vp scanning electron microscope (Carl Zeiss, Germany). In the case of large amounts of collapsed pollen grains, some pollen samples were only washed in acetone (for removal of lipids and other pollenkitt) and then observed with SEM.
Pollen grain size was measured in 30 pollen grains from a sample of three anthers from different positions along the staminal column; the pollen was then pooled in the preparation. Using at least three individuals per species, the diameter values contain the data from a minimum of 90 grains. Due to the spheroidal shape of the pollen grains, only one diameter value was taken (without spines).
Pollen aperture number and size, and the length of the spines were recorded from 30 grains per accession, whereas measurements of the thickness of pollen wall strata were based on ten pollen grains per accession; the sexine thickness was determined from the areas between the ‘spine cushions’. Additionally, the maximum height of the sexine at the ‘spine cushions’ was also recorded. Since the pollen grains are spheroidal and the spines equidistant, the number of spines N was calculated according to Hanks and Fryxell (Citation1979) using the formula N = (equatorial diameter/interspinal distance)² × Π. For the interspinal distance, three distances were measured and then averaged. All observations for LM were made with a Zeiss Axiophot microscope using a 100x oil immersion objective lens. Photographs were taken with a Leica DFC 350FX digital camera. Images were stored and calibrated using the Leica IM 50 Image Manager, Version 4.0, and measurements were made with the corresponding software module. Statistical analysis was performed with the software SPSS, Version 15. For SEM study of pollen surface detail, pollen from one individual per species was used.
Pollen terminology
Pollen terminology is based on Punt et al. (Citation2007), with the exception of the term ‘spine cushions’, which we use to describe the notably broad bases of the spines.
Results
General description for pollen of Palaua
The pollen grains of Palaua are spheroidal and pantocolporate (details of the compound aperture are shown in ), sometimes the apertures appear to be zonocolporate or spirally arranged; the ectexine is spinose. The size of the grains is medium to large, with diameters between 30.0 and 93.1 μm. The spines are morphologically closely similar in all areas of the pollen grain, with pointed (rarely obtuse) apices, ± evenly distributed, and raised on ‘cushions’ of ectexine that correspond to regions of the infratectum with larger columellae. The number of apertures varies from 4 to 14, the ectoaperture is very short and, usually, slit-shaped (), it has a maximum length of 3.5 – 8.8 μm, and a width of 0.7 – 4.9 μm; the endoaperture is circular and sometimes there is a bridge-like structure between the margins of the ectoaperture (). The sexine is generally similar in thickness to the nexine, although it can be up to three or four times as thick in the ‘spine cushion’ regions (). The tectum is usually perforate, although in some species there are no distinct perforations, and is characterised by granula (e.g. ); the inter-apertural nexine is uniformly thick (0.6 – 0.9 μm) but up to about twice as thick around the aperture.
Figure 1. Details of the colporate apertures in pollen grains of Palaua (SEM & LM): A. The inner surface (SEM); B. The outer surface (SEM) of the pollen grain with endoporus and ectocolpus in P. rhombifolia; C. Detail of the colporus in Palaua sp. nov. (LM). Scale bars – 3 μm.
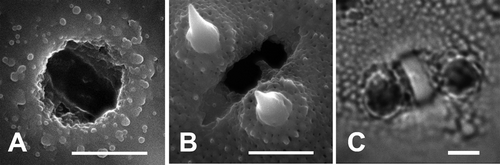
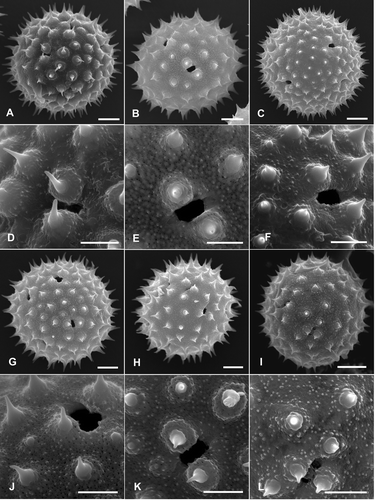
Figure 2. Pollen grains of Palaua species (Malvaceae) with detailed views of sexine sculpturing and apertures (SEM): A, D. P. dissecta; B, E. P. guentheri; C, F. P. inconspicua; G, J. P. malvifolia; H, K. P. modesta; I, L. P. mollendoensis. Scale bars – 10 μm (A–C, G–I); 5 μm (D–F, J–L).
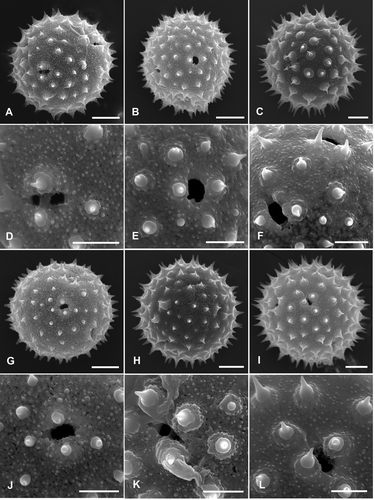
Figure 4. Pollen grains of Palaua and Fuertesimalva species (Malveae, Malvaceae) with detailed views of sexine sculpturing and apertures (SEM): A, D. P. weberbaueri; B, E. Palaua sp. nov.; C, F. Fuertesimalva peruviana; G. F. echinata; H. F. limensis; I. Palaua rhombifolia, interior of pollen grain and section of exine. Scale bars – 10 μm (A–C, G–I); 5 μm (D–F).
Species level pollen descriptions for Palaua
Palaua camanensis
Pollen grains 50.6 – 70.2 μm in diameter. Apertures 5 – 7, ectoaperture 3.9 – 6.8 μm long, 1.7 – 3.1 μm wide. Spines 85 – 255, pointed, 3.5 – 6.3 μm long, at base 2.0 – 3.4 μm wide, distance between spines 6.8 – 11.0 μm. The sexine 0.8 – 1.0 μm thick, at the ‘spine cushions’ to 1.7 – 2.1 μm, the tectum is inconspicuously perforate or without perforations.
Palaua dissecta (, D)
Pollen grains 49.5 – 83.0 μm in diameter. Apertures 5 – 12, ectoaperture 2.8 – 8.8 μm long, 2.7 – 4.9 μm wide. Spines 66 – 231, pointed, 2.8 – 6.2 μm long, at base 1.5 – 3.6 μm wide, distance between spines 6.6 – 14.9 μm. The sexine 0.8 – 1.3 μm thick, at the ‘spine cushions’ to 2.1 – 3.0 μm; tectum perforate.
Palaua guentheri (, E)
Pollen grains 37.1 – 48.3 μm in diameter. Apertures 5 – 9, ectoaperture 2.1 – 4.6 μm long, 1.0 – 2.6 μm wide. Spines 61 – 179, pointed, 2.0 – 3.7 μm long, at base 1.6 – 2.9 μm wide, distance between spines 5.6 – 8.6 μm. The sexine 0.9 – 1.1 μm thick, at the ‘spine cushions’ to 1.6 – 2.2 μm; tectum perforate.
Palaua inconspicua (, F)
Pollen grains 30.0 – 45.8 μm in diameter. Apertures 4 – 8, ectoaperture 2.2 – 5.4 μm long, 0.9 – 3.2 μm wide. Spines 50 – 269, pointed, 1.8 – 3.9 μm long, at base 1.3 – 2.8 μm wide, distance between spines 4.5 – 7.8 μm. The sexine 0.8 – 1.1 μm thick, at the ‘spine cushions’ 1.7 – 2.2 (very rarely to 2.5) μm; tectum inconspicuously perforate or without perforations.
Palaua malvifolia (, J)
Pollen grains 36.9 – 50.6 μm in diameter. Apertures 4 – 10, ectoaperture 2.1 – 4.3 μm long, 0.8 – 2.4 μm wide. Spines 58 – 194, blunt, 2.7 – 4.7 μm long, at base 1.4 – 3.1 μm wide, distance between spines 5.3 – 9.6 μm. The sexine 0.9 – 1.2 μm thick, at the ‘spine cushions’ to 1.7 – 2.2 μm; tectum perforate.
Palaua modesta (, K)
Pollen grains 31.7 – 51.5 μm in diameter. Apertures 4 – 9, ectoaperture 2.3 – 5.5 μm long, 0.9 – 2.9 μm wide. Spines 83 – 203, pointed, 2.1 – 4.2 μm long, at base 1.3 – 3.0 μm wide, distance between spines 4.5 – 9.3 μm. The sexine 0.8 – 1.2 μm thick, at the ‘spine cushions’ to 1.5 – 2.0 μm; tectum perforate.
Palaua mollendoensis (, L)
Pollen grains 48.1 – 93.1 μm in diameter. Apertures 5 – 14, ectoaperture 2.9 – 8.7 μm long, 0.9 – 4.8 μm wide. Spines 48 – 241, pointed, 2.7 – 6.6 μm long, at base 1.1 – 3.7 μm wide, distance between spines 6.2 – 14.9 μm. The sexine 0.8 – 1.3 μm thick, at the ‘spine cushions’ to 1.8 – 2.8 μm; tectum inconspicuously perforate or without perforations.
Palaua moschata (, D)
Pollen grains 39.2 – 82.3 μm in diameter. Apertures 5 – 9, ectoaperture 2.6 – 6.4 μm long, 0.8 – 3.9 μm wide. Spines 59 – 239, pointed, 2.9 – 8.3 μm long, at base 1.7 – 3.7 μm wide, distance between spines 5.8 – 17.3 μm. The sexine 0.8 – 1.2 μm thick, at the ‘spine cushions’ to 2.0 – 2.5 μm; tectum inconspicuously perforate or without perforations.
Palaua rhombifolia (, E and 4I)
Pollen grains 44.8 – 58.9 μm in diameter. Apertures 5 – 9, ectoaperture 3.2 – 5.7 μm long, 1.1 – 3.7 μm wide. Spines 52 – 183, pointed, 2.9 – 6.1 μm long, at base 2.1 – 3.7 μm wide, distance between spines 6.9 – 12.0 μm. The sexine 0.7 – 1.2 μm thick, at the ‘spine cushions’ to 2.1 – 2.7 μm; tectum perforate.
Palaua sandemanii (, F)
Pollen grains 49.8 – 69.3 μm in diameter. Apertures 6 – 9, ectoaperture 1.9 – 5.3 μm long, 1.1 – 3.2 μm wide. Spines 74 – 284, pointed, 2.8 – 5.4 μm long, at base 1.9 – 3.9 μm wide, distance between spines 6.0 – 13.6 μm. The sexine 0.7 – 1.2 μm thick, at the ‘spine cushions’ to 1.8 – 2.1 μm; tectum inconspicuously perforate or without perforations.
Palaua tomentosa (, J)
Pollen grains 34.5 – 74.1 μm in diameter. Apertures 4 – 9, ectoaperture 1.6 – 6.2 μm long, 0.8 – 3.9 μm wide. Spines 63 – 245, pointed, 2.5 – 5.9 μm long, at base 1.7 – 3.8 μm wide, distance between spines 5.3 – 12.6 μm. The sexine 0.8 – 1.2 μm thick, at the ‘spine cushions’ to 1.8 – 2.3 μm; tectum perforate.
Palaua trisepala (, K)
Pollen grains 47.3 – 84.9 μm in diameter. Apertures 6 – 12, ectoaperture 2.6 – 6.4 μm long, 1.0 – 4.1 μm wide. Spines 63 – 263, pointed, 3.1 – 6.8 μm long, at base 1.8 – 3.9 μm wide, distance between spines 7.4 – 13.5 (very rarely to 15.6) μm. The sexine 0.9 – 1.3 μm thick, at the ‘spine cushions’ to 2.5 – 3.0 μm; tectum perforate.
Palaua velutina (, L)
Pollen grains 42.4 – 66.9 μm in diameter. Apertures 7 – 10, ectoaperture 2.5 – 5.8 μm long, 1.0 – 4.8 μm wide. Spines 45 – 171, pointed, 3.8 – 7.2 μm long, at base 1.2 – 3.8 μm wide, distance between spines 6.0 – 14.2 (very rarely to 15.4) μm. The sexine 1.1 – 1.5 μm thick, at the ‘spine cushions’ to 2.8 – 2.9 μm; tectum inconspicuously perforate or without perforations.
Palaua weberbaueri (, D)
Pollen grains 49.7 – 77.4 μm in diameter. Apertures 4 – 11, ectoaperture 3.4 – 6.3 μm long, 0.8 – 4.3 μm wide. Spines 74 – 178, pointed, 3.1 – 6.3 μm long, at base 1.8 – 4.3 μm wide, distance between spines 8.3 – 14.1 μm. The sexine 0.8 – 1.4 μm thick, at the ‘spine cushions’ to 2.5 – 3.0 μm; tectum inconspicuously perforate or without perforations.
Palaua sp. nov. (, E)
Pollen grains 43.2 – 75.9 μm in diameter. Apertures 5 – 10, ectoaperture 2.5 – 5.3 μm long, 0.7 – 3.2 μm wide. Spines 49 – 181, pointed, 2.9 – 7.0 μm long, at base 1.6 – 3.1 μm wide, distance between spines 6.7 – 12.9 μm. The sexine 0.8 – 1.2 μm thick, at the ‘spine cushions’ to 2.1 – 2.8 μm; tectum inconspicuously perforate or without perforations.
General description for pollen of Fuertesimalva
The pollen grains of the four investigated species of Fuertesimalva are spheroidal, pantocolporate, spinose, and medium to large-sized, with diameters between 38.7 and 56.4 μm. The spines are morphologically closely similar in all areas of the pollen grain, pointed, ± evenly distributed and raised on cushions as in Palaua. The number of apertures varies between four and 11, the ectoaperture is usually slit-shaped, has a maximum length of 5.0 – 6.0 μm, a width of 1.1 – 3.3 μm, and the endoaperture is circular. The sexine is slightly thicker than the nexine or up to about three times as thick at the ‘spine cushions’, and the tectum is perforate or without distinct perforations and characterised by granula; the nexine is 0.6 – 0.8 μm thick.
Species level pollen descriptions for Fuertesimalva
Fuertesimalva chilensis
Pollen grains 38.7 – 50.3 μm in diameter. Apertures 7 – 10, ectoaperture 2.5 – 5.0 μm long, 1.3 – 3.0 μm wide. Spines 144 – 422, pointed, 1.9 – 3.7 μm long, at base 1.2 – 2.5 μm wide, distance between spines 3.6 – 6.1 μm. The sexine 0.8 – 1.1 μm thick, at the ‘spine cushions’ to 1.6 – 1.8 μm; tectum inconspicuously perforate or without perforations.
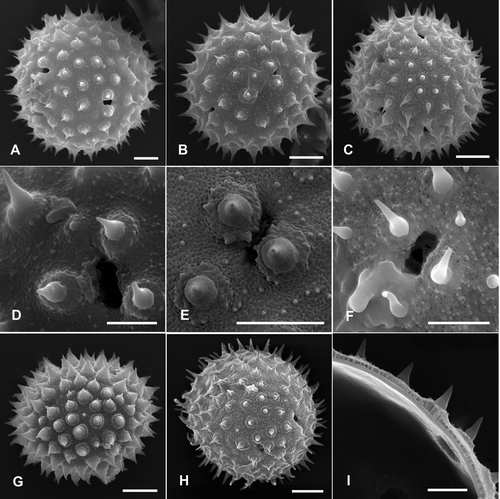
Fuertesimalva echinata ()
Pollen grains 47.3 – 55.3 μm in diameter. Apertures 4 – 11, ectoaperture 3.4 – 6.0 μm long, 1.4 – 4.1 μm wide. Spines 96 – 259, pointed, 2.9 – 4.3 μm long, at base 1.8 – 3.6 μm wide, distance between spines 6.0 – 9.3 μm. The sexine 0.8 – 1.1 μm thick, at the ‘spine cushions’ to 2.1 – 2.3 μm; tectum inconspicuously perforate or without perforations.
Fuertesimalva limensis ()
Pollen grains 40.8 – 56.4 μm in diameter. Apertures 6 – 8, ectoaperture 2.5 – 5.0 μm long, 1.6 – 3.0 μm wide. Spines 53 – 188, pointed, 3.2 – 5.7 μm long, at base 2.5 – 3.0 μm wide, distance between spines 5.5 – 10.6 μm. The sexine 1.0 – 1.1 μm thick, at the ‘spine cushions’ to 1.7 – 2.3 μm, the tectum inconspicuously perforate or without perforations.
Fuertesimalva peruviana (, F)
Pollen grains 48.7 – 55.1 μm in diameter. Apertures 4 – 10, ectoaperture 3.0 – 5.3 μm long, 1.6 – 3.1 μm wide. Spines 106 – 256, pointed, 3.8 – 6.1 μm long, at base 1.8 – 4.4 μm wide, distance between spines 5.8 – 8.9 μm. The sexine 0.9 – 1.3 μm thick, at the ‘spine cushions’ to 1.7 – 2.5 μm; tectum perforate.
Discussion
Infra- and intergeneric variability
Our data show that there is conspicuous variation in the majority of the assessed pollen characters in Palaua, but there is also considerable overlap in these characters between the species, rendering it difficult to provide a clear-cut grouping of the taxa based on these characters alone. The most promising feature is pollen grain size, which varies from 30 – 45.8 μm in P. inconspicua to 48.1 – 93.1 μm in P. mollendoensis. Four species (, , ) show substantially smaller pollen grains than the rest of the genus: P. guentheri, P. inconspicua, P. malvifolia and P. modesta. Their grain diameters are mainly well below 50 μm and their means range between 36.4 and 41.9 μm. This allows us to distinguish them from the bulk of the other species, where the grains are predominantly > 50 μm with mean diameters of between 49.2 and 66.3 μm. The four species with the smallest pollen also possess the smallest apertures (average length 3.1 – 3.7 μm), the shortest spines (2.9 – 3.5 μm) and the shortest distance between the spines (5.9 – 7.3 μm).
Figure 5. Pollen grains (LM) of representative specimens to illustrate size variation between species of Palaua: A. P. mollendoensis; B. P. dissecta; C. P. malvifolia; D. P. guentheri; E. P. modesta; F. P. inconspicua. All at same magnification; scale bars – 20 μm.
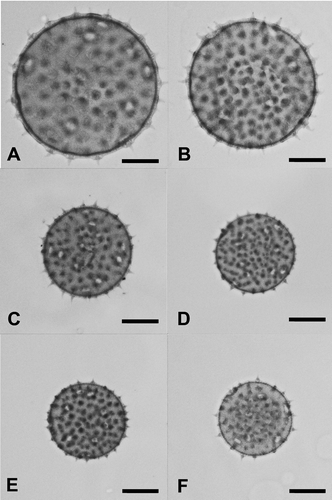
Figure 6. Boxplot of pollen grain characters for species of Palaua, including outliers (circles) and extreme outliers (asterisks). Species are arranged according to the three principal molecular phylogenetic clades in Huertas et al. (Citation2007): Integrifolia clade (IntC), Dissecta clade (DC) and Inconspicua clade (IncC). The displayed boxplots of the sister group Fuertesimalva are from pooled values of the four investigated species.
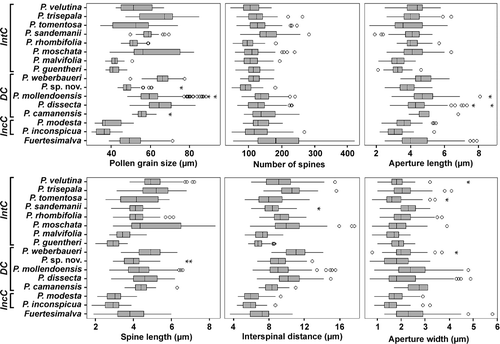
Table I. Pollen grain characteristics of Palaua.
Other species groups based on shared pollen characters are hard to identify because of overlapping values, particularly when the extreme values are included (e.g., outliers; ).
The pollen type of Palaua fits the general pattern encountered in closely related genera of tribe Malveae, sensu Bayer and Kubitzki (Citation2003). The grain size, the low number of apertures, their size, the spine length and shape, the presence of ± prominent ‘spine cushions’, the sexine that is as thick or thicker than the nexine, the tectum with granula, are all features that also characterise most genera of Malveae (Saad, Citation1960; Chaudhuri, Citation1965; Hanks & Fryxell, Citation1979; Hill, Citation1982; Christensen, Citation1986; Krebs, Citation1990). This is also true for the studied species of the sister group Fuertesimalva, whose pollen type is indistinguishable from that of Palaua (, ). In general, the variability of pollen morphology seems to be more pronounced within Palaua than between Palaua and its closest relatives, especially in terms of the generic level size ranges for the quantitative pollen characters.
Infrageneric variability and taxonomic utility
Structural differences of the pollen grains among the species of Palaua are slight, and are confined to minor variations in the degree of perforation in the tectum. Thus, the infrageneric variability of pollen morphology is largely confined to the quantitative characters measured. Despite the considerable overlap between species for all quantitative characters used, we can tentatively segregate a group of four species that share the smallest pollen grains, the smallest apertures, and the shortest spines with the shortest distance between them: P. inconspicua, P. modesta, P. guentheri, and P. malvifolia. The first two of these four species coincide with the Inconspicua clade (), one of the clades suggested in Huertas et al. (Citation2007). The other two species, Palaua guentheri and P. malvifolia, are independent lineages within the Integrifolia clade and morphologically distinct from each other (Huertas et al., Citation2007). Therefore, the small size of the grains and the associated characteristics in these four species, although containing some taxonomically useful information, do not serve to discriminate between the major clades within Palaua. The reason may be that pollen size differences are probably less reliable indicators of phylogenetic relatedness than structural characteristics because there are a number of factors which can influence pollen grain size, such as: cytology (ploidy level), ecology, floral morphology or physiological constraints (e.g. Muller, Citation1979; Lau & Stephenson, Citation1993; Vonhof & Harder, Citation1995; Harder, Citation1998; Torres, Citation2000). Interestingly, the four species in Palaua with the smallest grains are also the species with the smallest flowers (Huertas, unpubl.), an observation that may point to an alternative explanation for the pollen-based grouping. A relationship between flower size and pollen size has been noted previously by a number of authors, including Muller (Citation1979), López et al. (Citation2006), and Tate and Simpson (Citation2004), and it may reflect a purely morphological constraint on pollen characteristics, such as a trend towards an overall reduced size or, perhaps more likely, it is linked to the reproductive strategy of the species. For example, the small-flowered species of Palaua have shorter pistils, which encourages the plant to allow pollen grains less energy resources, and thus smaller grains are produced because the shorter distance the pollen tubes have to grow to reach the ovules requires less cell growth. This rationale is based on the assumption that pollen grain size is related to their resource content (e.g., Vonhof & Harder, Citation1995; Harder, Citation1998; Torres, Citation2000; but see also Wolff & Liede-Schumann, Citation2007). Whether this rationale applies to Palaua has not yet been tested and needs to be addressed in future investigations.
In relation to size differences of pollen grains, chromosome data should also be analysed, since larger pollen grains are often a result of polyploidy, following the tenet that polyploidy generates larger cells and therefore larger pollen grains (Bronckers, Citation1963; Stebbins, Citation1971; Muller, Citation1979; Southworth & Pfahler, Citation1992). A detailed chromosome survey of Palaua is still wanting; however, preliminary data (Schneider & Huertas, unpubl.) suggest that polyploidy plays a significant role in generating size differences between pollen grains among species of Palaua. So far five species have been found to contain tetraploids: P. dissecta, P. mollendoensis, P. moschata, P. tomentosa, P. trisepala, and these species have the largest pollen grains (maximum values; ) and the greatest variation in the pollen characteristics (). The other species so far investigated are diploid (). The existence of species with mixed cytotypes (e.g. the diploid and tetraploid populations of P. dissecta, P. mollendoensis, P. moschata, P. tomentosa; Schneider & Huertas, unpubl.) notably weakens the taxonomic utility of quantitative pollen characters, since the mixed ploidy levels extend the size ranges and lead to stronger overlapping of the values.
Conclusions
Structural differences in pollen morphological characters are scarce among species of Palaua.
In spite of the uniform pollen type observed in Palaua species, there are some quantitative characters that are variable enough to provide a means to distinguish some species groups. Nonetheless, the taxonomic utility of pollen morphology to support an infrageneric classification of Palaua is limited. The congruence in pollen characters is only partially due to phylogenetic relatedness – it also appears to reflect independently inherited cytological constraints and/or constraints related to the reproductive system. In particular, species with different ploidy races count against the taxonomic value of quantitative pollen characters.
Specimens investigated
Palaua camanensis Ferreyra & Chanco. Peru: Dept. Arequipa, Camaná. Ferreyra 11554 (FR).
P. dissecta Ulbr. Peru: Dept. Tacna, Sama Grande, Schneider et al. 2829 (FR). Chile: I. Región, Punta Gruesa. Schneider & Huertas 2836 (FR). Chile: II. Región, Qda. Miguel Diaz. Schneider & Huertas 2888 (FR). Peru: Dept. Arequipa, Camaná. Schneider 3093 (LZ).
P. guentheri Bruns. Peru: Dept. Tacna, Lomas de Camiara. Huertas 42 (FR). Peru: Dept. Tacna, Lomas de Camiara. Schneider et al. 2788 (FR). Peru: Dept. Tacna, between Ite and Ilo. Schneider et al. 2816 (FR).
P. inconspicua I. M. Johnst. Peru: Dept. Arequipa, Tintayani, FLSP 16/1161 (FR). Peru: Dept. Arequipa, Mollendo, FLSP 1427–1428 (FR). Peru: Dept. Arequipa, Lomas de Cachendo. Schneider et al. 3016 (FR).
P. malvifolia Cav. Peru: Dept. Lima, Panamericana Norte km 114–115. Huertas 17 (FR). Peru: Dept. Lima, Quebrada Verde. Schneider & Huertas 2712 (FR). Peru: Dept. Lima, Panamericana Km 114–118. Schneider & Huertas 2745 (FR).
P. modesta (Phil.) Reiche. Chile: II. Región, N of Paposo. Schneider & Huertas 2969 (FR). Chile: II. Región, between Paposo and El Cobre. Schneider & Huertas 3032 (FR). Chile: II. Región, between Paposo and El Cobre. Schneider & Huertas 3033 (FR). Chile: II. Región, Quebrada Rinconada. Schneider & Huertas 3057 (FR).
P. mollendoensis Ulbr. Peru: Dept. Moquegua, 13 km S de Ilo. Huertas 56 (FR). Peru: Dept. Moquegua, Lomas de Tacahuay. Schneider & Huertas 2813 (FR). Peru: Dept. Arequipa, Lomas de Camaná. Schneider & Huertas 2995 (FR). Peru: Dept. Arequipa, Lomas de Camaná. Schneider & Huertas 2999 (FR). Peru: Dept. Arequipa, Lomas de Camaná. Schneider & Huertas 3005 (FR). Peru: Dept. Moquegua, South of Ilo. Schneider 3094 (LZ).
P. moschata Cav. Peru: Dept. Tacna, 50 km N of Tacna, Ferreyra 11657 (FR). Peru: Dept. Lima, Morro Solar. Schneider & Huertas 2759 (FR). Chile: II. Región, between Paposo and El Cobre, Qda. Rinconada. Schneider & Huertas 3056 (FR).
P. rhombifolia Graham. Peru: Dept. Lima, Lachay. Ferreyra 17753 (FR). Peru: Dept. Lima, Lomas de Lachay. Müller et al. 755 (LZ). Peru: Dept. Lima, Valle Hermoso. Schneider & Huertas 2736 (FR). Peru: Dept. Lima, Lomas de Chilca. Schneider & Huertas 2754 (FR).
P. sandemanii (Sandwith) Fryxell. Peru: Dept. Arequipa, entre Nazca y Chala. Ferreyra 1526 (FR). Peru: Dept. Arequipa, Lomas de Jahuay. Ferreyra 14015 (FR). Peru: Dept. Arequipa, Lomas de Jahuay. Ferreyra 18687 (FR).
P. tomentosa Hochr. Peru: Dept. Arequipa, Atiquipa. Schneider & Huertas 2763 (FR). Peru: Dept. Arequipa, Atiquipa. Schneider & Huertas 2766 (FR). Peru: Dept. Arequipa, Lomas de Cachendo. Schneider 3091 (LZ).
P. trisepala Hochr. Peru: Dept. Ica, Punta de los Ingleses. Ferreyra 13406 (FR). Peru: Dept. Arequipa, Mollendo. FLSP 1430 (FR). Peru: Dept. Arequipa, Lomas de Camaná. Schneider & Huertas 2998 (FR).
P. velutina Ulbr. & Hill. Peru: Dept. Arequipa, Pampas El Alto Tambo. FLSP 896 (FR). Peru: Dept. Arequipa, Lomas de Mejía. Schneider et al. 2794 (FR).
P. weberbaueri Ulbr. Peru: Dept. Moquegua, Lomas de Ilo. Ferreyra 11602 (FR). Peru: Dept. Tacna, Lomas de Morro Sama. Schneider et al. 2778 (FR). Peru: Dept. Tacna, Lomas de Ite. Schneider et al. 2824 (FR).
Palaua sp. nov. Peru: Dept. Moquegua, entre Ilo e Ite, 13 km al sur de Ilo. Huertas 53 (FR). Peru: Dept. Tacna, Lomas de Morro Sama. Schneider et al. 2777 (FR). Peru: Dept. Moquegua, Lomas de Tacahuay. Schneider et al. 2814 (FR).
Fuertesimalva chilensis (A. Braun & C. D. Bouché) Fryxell. Chile: II. Región, Paposo. Schneider & Huertas 3038 (FR).
F. echinata (C. Presl) Fryxell. Bolivia: Dept. La Paz, Charazani. Gutte 129 (LZ).
F. limensis (L.) Fryxell. Peru: Dept. Cajamarca, surroundings of Cajamarca. Becker & Terrones 992 (LZ).
F. peruviana (L.) Fryxell. Peru: Dept. Arequipa, Lomas de Cachendo. Schneider 3097 (LZ).
Acknowledgements
The authors are grateful to the Deutsche Forschungsgemeinschaft for providing funds to J. V. Schneider (DFG Schn 714/1-1) within the SPP 1127 “Radiations – origin of biodiversity”. The Friedrich-Ebert Foundation is acknowledged for a Ph.D. studentship to M. L. Huertas. The authors thank also two anonymous reviewers for their helpful comments. Manfred Ruppel is acknowledged for providing some additional SEM photographs. We are grateful to INRENA for permission to collect specimens in Peru, to César Cáceres, Asunción Cano, Magda Chanco, Leoncio Mariño, Mélica Muñoz-Schick, José Roque, Eduardo Ruíz and Abundio Sagástegui for assisting our work in Peru and Chile, and to the Directors of the Frankfurt (FR) and Leipzig (LZ) herbaria for permission to remove pollen material from herbarium specimens.
References
- Baker , E. G. 1890 . Synopsis of genera and species of Malveae . J. Bot. Br. Foreign , 28 : 15 – 18 .
- Bates , D. M. 1967 . Chromosome numbers in the Malvales. I . Gentes Herb. , 10 : 39 – 46 .
- Bates , D. M. 1968 . Generic relationships in the Malvaceae, tribe Malveae . Gentes Herb. , 10 : 117 – 135 .
- Bayer , C. and Kubitzki , K. 2003 . “ Malvaceae ” . In The families and genera of vascular plants, Vol. 5. Flowering plants, dicotyledons: Malvales, Capparales, and non-betalain Caryophyllales , Edited by: Kubitzki , K. and Bayer , C. 225 – 311 . Berlin : Springer .
- Bronckers , F. 1963 . Variations polliniques dans une série d'autopolyploïdes artificiels d’Arabidopsis thaliana (L.) Heynh . Pollen Spores , 5 : 233 – 238 .
- Chaudhuri , S. K. 1965 . Pollen morphological studies of the order Malvales. II . Bull. Bot. Soc. Bengal , 19 : 147 – 158 .
- Christensen , P. B. 1986 . Pollen morphological studies in the Malvaceae . Grana , 25 : 95 – 117 .
- Fryxell , P. A. 1997 . The American genera of Malvaceae – II . Brittonia , 49 : 204 – 269 .
- Hanks , S. and Fryxell , P. A. 1979 . Palynological studies of Gaya and Herissantia (Malvaceae) . Am. J. Bot. , 66 : 494 – 501 .
- Harder , L. D. 1998 . Pollen-size comparisons among animal-pollinated angiosperms with different pollination characteristics . Biol. J. Linn. Soc. , 64 : 513 – 525 .
- Hill , S. R. 1982 . A monograph of the genus Malvastrum A. Gray (Malvaceae: Malveae) . Rhodora , 84 : 1 – 83 .
- Huertas , M. L. , Schneider , J. V. and Zizka , G. 2007 . Phylogenetic analysis of Palaua (Malveae, Malvaceae) based on plastid and nuclear sequences . Syst. Bot. , 32 : 157 – 165 .
- Krebs , G. 1990 . Zur Monophylie der Tribus Malopeae Reichenb . Catal. Herb. Lipsiensis. Plantae Neotrop. , 3 : 45 – 51 .
- Krapovickas , A. 1969 . Notas citotaxonómicas sobre Malváceas . Bonplandia , 2 : 9 – 24 .
- Lau , T.-C. and Stephenson , A. G. 1993 . Effects of soil nitrogen on pollen production, pollen grain size, and pollen performance in Cucurbita pepo (Cucurbitaceae) . Am. J. Bot. , 80 : 763 – 768 .
- López , H. A. , Anton , A. M. and Galetto , L. 2006 . Pollen-pistil size correlation and pollen size-number trade-off in species of Argentinian Nyctaginaceae with different pollen reserves . Plant Syst. Evol. , 256 : 69 – 73 .
- Moore , P. D. , Webb , J. A. and Collinson , M. E. 1991 . Pollen analysis , Oxford : Blackwell .
- Muller , J. 1979 . Form and function in angiosperm pollen . Ann. Mo. Bot. Gard. , 66 : 593 – 632 .
- Punt , W. , Hoen , P. P. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 2007 . Glossary of pollen and spore terminology . Rev. Palaeobot. Palynol. , 143 : 1 – 81 .
- Reichenbach , H. G. L. 1828 . Conspectus regni vegetabilis , Leipzig : Cnobloch .
- Saad , S. I. 1960 . The sporoderm stratification in the Malvaceae . Pollen Spores , 2 : 11 – 40 .
- Southworth , D. and Pfahler , P. 1992 . The effects of genotype and ploidy level on pollen surface sculpturing in Maize (Zea mays L.) . Am. J. Bot. , 79 : 1418 – 1422 .
- Stebbins , G. L. 1971 . Chromosomal evolution in higher plants , London : E. Arnold .
- Tate , J. A. , Fuertes Aguilar , J. , Wagstaff , S. J. , La Duke , J. C , Slotta , T. A. B. and Simpson , B. B. 2005 . Phylogenetic relationships within the tribe Malveae (Malvaceae, subfamily Malvoideae) as inferred from ITS sequence data . Am. J. Bot. , 92 : 584 – 602 .
- Tate , J. A. and Simpson , B. B. 2004 . Breeding system evolution in Tarasa (Malvaceae) and selection for reduced pollen grain size in the polyploid species . Am. J. Bot. , 91 : 207 – 213 .
- Torres , C. 2000 . Pollen size evolution: Correlation between pollen volume and pistil length in Asteraceae . Sex. Plant Reprod. , 12 : 365 – 370 .
- Ulbrich , E. 1909 . Malvaceae austro-americanae imprimis andinae . Bot. Jahrb. Syst. , 42 : 104 – 124 .
- Vonhof , J. J. and Harder , L. D. 1995 . Size-number trade-offs and pollen production by papilionaceous legumes . Am. J. Bot. , 82 : 230 – 238 .
- Wolff , D. and Liede-Schumann , S. 2007 . Evolution of flower morphology, pollen dimorphism, and nectar composition in Arcytophyllum, a distylous genus of Rubiaceae . Org. Div. Evol. , 7 : 106 – 123 .