Abstract
An analysis was made of the protein content of pollen loads produced by the bees in a hive situated in Viana do Bolo (Ourense, north-west Spain), to establish whether or not the relative quantity of protein in the pollen of each plant species influences the preference made by the bee of the flowers that supply pollen to the hive. This analysis was performed on all types of pollen that formed more than 5% of the pollen spectrum. Pollen load samples were collected directly from the hive from March to September. Pollen loads were separated by colour, and their specific homogeneity was confirmed microscopically. The Bradford method has been used for protein extraction and spectrophotometry was used for the determination of protein content. The results show that the different pollen loads have high protein content. Pollen of the plant species that reached relatively higher percentages in the pollen spectrum are also those that have the highest protein content. These were Cytisus scoparius type, uncultivated Poaceae, Quercus robur type, Sanguisorba minor, Salix fragilis and Spergularia rubra type. The pollen of the systematic units, which had pollen loads that could be identified at the level of species, maintained a constant value of protein content independently of the date the samples were obtained. The pollen of the systematic units, which had pollen loads that could be identified at the level of pollen type, has varied in protein content in the analyses performed on samples obtained on different dates. This result is due to the fact that the different species that integrate the pollen type flower on different dates, and thus have a pollenkitt with different characteristics.
There is a close relationship between honey bees (Apis mellifera L.) and their habitat. Plants need honey bees as pollinator agents to perpetuate the plant species, and honey bees need plants to obtain the necessary nutrients for the bees' development, nectar and pollen. Multiple factors influence this relationship, including honey bee biology and characteristics of the plants species from which they obtains his food (Louveaux, Citation1958; von Frisch, Citation1969; Bolchi-Serini & Salvi, Citation1986; Fewell & Wiston, Citation1996; Armesto, Citation1998; de Sá-Otero et al., Citation2004). Honey bees visit some plants surrounding the beehive to obtain pollen and nectar, while others plants are not visited. It seems that in the floral “preference” made by the honey bee, the chemical composition of the pollen plays preponderant role in relation to the presence of volatile compounds and to the nutritional value as measured by the protein content (Dobson, Citation1987; Pacini & Juniper, Citation1979; Dobson et al., Citation1990; Bergström et al., Citation1995).
The sensory receptors in the antennae of the honey bees are associated with the taste and smell that allow the bees to detect volatile substances (Dobson, Citation1987). Studies with pollen of Brassica napus L. have shown that bees are attracted by certain volatile compounds like a-pinene, henylacetaldehyde, p-cynene, a-terpinene, linalool, 2-phenylethanol, E-E-farnesene, and 3-carene (Blight et al., Citation1997; Pham-Delegue et al. Citation1994). Honey bees are capable of perceiving odours and remember certain odours from the food source visited (Gerber et al., Citation1996).
Honey bees are sensitive to the availability of protein in the pollen, as well as the cost of foraging (collection of protein / energy cost). They forage in a manner that maximises efficiency in collecting pollen. When they collect pollen, they usually remain faithful to the plant species visited and usually visit only the flowers of one species until the pollen load is completed, producing monospecific pollen loads (Free, Citation1963; Waddington & Holden, Citation1979). Such behaviour promotes larval development and at the same time maximises the life expectancy of the foraging bees (Rasheed & Harder, Citation1997).
The aim of this of this study was to determine the diversity of plant species used as source of pollen by honey bees (Apis mellifera L.) by means of colour and microscopic analysis of pollen loads obtained form one beehive at Viana do Bolo (Ourense). We also wanted to determine if the protein content of the pollenkitt in the plant taxa used as source of pollen is a quality that influences honey bees in their foraging activity.
Material and methods
Pollen pellets were obtained from one beehive located in Viana do Bolo (Ourense, north-west Spain) 42° 11' N & 7° 06' W (). For this, a pollen trap with a grating of round holes and 10% of efficiency (Louveaux, Citation1958) was fixed to the hive entrance. The trap was active three times during April, twice during May, June, July, and August, and once during September. Pollen pellets were removed from the trap at various times but always before nightfall. Once the pollen was harvested and cleaned, it was dried in a stove for one hour, at a temperature lower than 45°C.
Sample units consisted of pollen loads collected at each sampling day. Dried samples were weighed using a PRECISA 40SM-200A set of scales. Each sample was then split in half. Using the Pantone Guide 747XR (Pantone, Citation1989) as a reference, pollen loads from the first half of the samples were separated into groups by colour. Twenty-five pollen loads from each different colour unit were analysed (Díaz-Losada et al., Citation1998). Each pollen load was broken down on a slide, with an inoculating needle, covered with glycerol-gel, and its homogeneity was checked using an optical microscope.
The other half of the sample was used to determine the relative importance of each pollen species harvested using a percentage pollen analysis (Hodges, Citation1984; Díaz-Losada, Citation1995). The pollen loads were first homogenised and then acetolysed (Erdtman, Citation1969). The residues were mounted on phenolated glycerin and 1200 grains were counted per sample. In order to be consistent with past research and to keep analyses uniform, newer analyses techniques were not used. Pollen was identified using the pollen reference collection of the Faculty of Sciences in Ourense and the use of the palynology identification keys (Moore & Webb, Citation1978; Valdés et al., Citation1987; Ramil et al., Citation1992; Saa-Otero et al., Citation1996).
From those colour groups with enough quantity of pollen the pollenkitt protein was extracted using the method applied by Cargnello et al. (Citation1988). Pollen loads were first stirred in a solution 30 mM Tris/ EDTA, pH 8.5. The sample (0.025 g of pollen in 2 mL buffer Tris/EDTA) was spun for 20 minutes at 45000 × g. The supernatant was collected in 0.1 mL aliquots and the protein level was determined (Bradford, Citation1976). The Bradford assay works by the action of Coomassie brilliant blue G-250 (CBBG) dye.
The CBBG (100 mg) was dissolved in 50 mL 95% ethanol. To this solution 100 mL 85% (w/v) phosphoric acid was added. The resulting solution was diluted to a final volume of 1 litre. For the preparation of the standard protein, we dissolved 10 mg bovine serum albumin crystallised (Merck) in 10 mL buffer, the same used for the dissolution of the pollen samples. The pollen load protein values were determined against the absorbance at 595 nm which was calibrated from several dissolutions of control protein at different concentrations. The results are expressed in mg of protein extracted from g of pollen (mg prot./g poll.).
Results
All samples contained more than one pollen type (). Honey bees foraged on a greater diversity of plant species during 4 August and the least during 12 September (). Some pollen taxa dominated the samples, while others did not (). Cytisus scoparius L. type, occurred in all of the samples except two (16/V and 29/VII, and was dominant in two samples (30/VII and 12/ IX) (). Uncultivated Poaceae pollen was dominant in July (29/VII) and Prunus spinosa type was dominant in April (4/IV) ().
Table I. Percentages of pollen recorded for each taxon or group of taxa on each sampling day
From the pollen spectrum of each sample, it appeared that the honey bees used plant species that were common to the region (). Honey bees varied their use of the different plants species depending on what flowers were available to them at the time ().
Pollen from plants with the most protein rich in the pollenkitt, were Allium cepa L., Cytisus scoparius-type, uncultivated Poaceae, Quercus robur L. type, Ranunculus bulbosus type, Sanguisorba minor Scop., Salix fragilis L. and Spergularia rubra (L.) J. Pressl & K. Pressl type ().
Table II. Protein content for each pollen type expressed as mg protein/g pollen and μg protein/mL dissolution where 0.025 g pollen was dissolved in 2 mL Tris/EDTA buffer
Pollens containing the greatest protein content are Crocus sp. (18 April), Cytisus scoparius type (4 April, 11 April, 4 August and 12 September), Quercus robur-type (3 and 25 May), Halimium alyssoides (Lam.) C. Koch (25 May, 16 and 26 June), uncultivated Poaceae (29 July and 4 August), Prunus spinosa type (4 April), Ranunculus bulbosus type (16 June), Raphanus raphanistrum type (3 and 25 May), Salix fragilis (4 April), Sanguisorba minor (26 June) and Spergularia rubra type (18 April and 3 May) (). Often pollens harvested in larger quantities are not those with the highest levels of protein ().
Figure 2. The relationship between relative contribution by weight (% Weight; ▪) and protein percentage (% Prot; □) for each plant species harvested by honey bees over the study period. Information on the relative protein content of each species in mg/g pollen (◊) is given for each day tested.
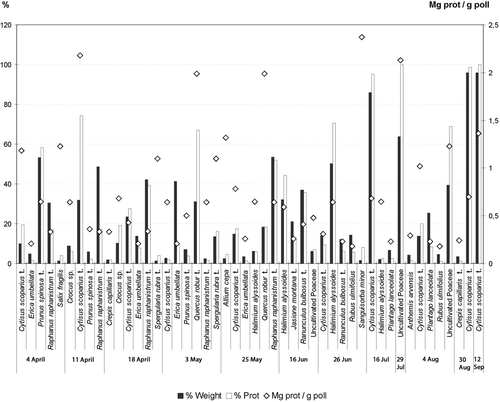
The plants with a protein content higher than 1.5 mg/g included: uncultivated Poaceae, Quercus robur type, Sanguisorba minor and Cytisus scoparius type, in any of the samples analysed (). Values between 1 to 1.5 mg/g were found in the pollen of Allium cepa, Salix fragilis and Spergularia rubra type (). Values between 1 to 0.5 mg/g were found in the pollen of Crocus sp., Halimium alyssoides, Prunus spinosa type and Raphanus raphanistrum type (). Pollen with values lower than 0.5 mg/g were found the samples of Anthemis arvensis type, Crepis capillaris type, Jasione montana type, Plantago lanceolata L., Ranunculus bulbosus-type and Rubus ulmifolius-type ().
Discussion
It is interesting to analyse the bee behaviour, the plant species used as a pollen source, and determine the individual protein content of each plant species (). Pollen taxa that occurred in the highest percentages did not always have the highest protein count. During 4 and 11 April, honey bees foraged on pollen from Prunus spinosa-type and Raphanus raphanistrum type, respectively, the most. However, Cytisus scoparium type contained the highest protein level during those sampling dates, but Prunus spinosa type and Raphanus raphanistrum type have dialipetal flowers, with easy access and with greater pollen production for each stamen (Osche, Citation1983; Currie, Citation1997; Davis, Citation1997; Armesto, Citation1998). Prunus spinosa type is an early flowering plant and was in full bloom at the beginning of the research study. Although it continued to flower through 3 May, there were not as many flowers in bloom at once as during 4 April (Chittka & Nikolas, Citation1997).
On 18 April pollen from Raphanus raphanistrum-type and Cytisus scoparius type were used by honey bees. The Crocus sp. and Cytisus scoparius type had the greatest protein level on this date. Crocus flowers provide easier access for honey bees. In addition, more pollen is produced by Crocus flowers than Cytisus scoparius type. However, Crocus is a scarce plant in this zone: flowers occur in singles, and the corollas are blues, in opposition to the yellow brooms of Cytisus scoparius type (Armesto, Citation1998; de Sá-Otero et al., Citation2004).
The 3 May harvest is principally Erica umbellate L., Quercus robur type. The Quercus robur type has greater protein content. On 18 April, honey bees did forage on the most protein rich source, although it was a secondary type. Erica umbellata may have been utilised more because of its proximity to the hive, for the population density, and for the abundance of flowers (Chittka & Nikolas, Citation1997; Armesto, Citation1998; de Sá-Otero et al., Citation2004). Also, E. umbellata was at the peak of flowering.
In the 25 May harvest, the principal pollen source was Raphanus raphanistrum type. The pollen with the greatest protein content was Quercus robur type which was second in pollen percentages. The bee preference Raphanus raphanistrum type rather than Quercus robur type; is most likely due to the yellow corolla, proximity to the hive, and number of flowers in bloom.
The 16 and 26 June harvests were mainly Ranunculus bulbosus type, and Halimium alyssoides (respectively). Halimium alyssoides contained the greatest protein content and was also utilised the most during 26 June. Although Ranunculus bulbosus type, and Cytisus scoparius type had higher protein levels, Rubus ulmifolius type was foraged on more. The bee behaviour toward the Sanguisorba minor pollen deserves a special mention; it has higher protein richness but it is a species of low density in the zone and possibly this causes the low recollection of this pollen by the honey bee.
On 16 and 29 July, 4 and 30 August, and 12 September, the pollen types harvested in the higher percentages were also the taxa with highest protein levels.
Cytisus scoparius-type has different protein values on different days of harvest. Thus it is possible to consider that this pollen type has a different assemblage of taxa during different collecting dates ().
Table III. Species that are assumed to make up the pollen types cited in this study based on the geographic distribution of species in the study area (de Sá-Otero et al. Citation2001; Valdes et al. 1987)
Similar results are observed in the pollen types of Prunus spinosa type, Ranunculus bulbosus type and Raphanus raphanistrum-type. It is remarkable that Spergularia rubra type and Crepis capillaris type show equal protein values in all the analysed samples.
If we establish a relation between the pollen percentages, and the protein quantity obtained from each group, we can determine the importance of preference of honey bees makes of the local flora in this zone. The bees have harvested the greatest relative quantities of pollen from plants with pollenkitts rich in proteins. However, honey bee “choice” is also dependent on the corolla form and colour, species abundance, and the proximity to the hive.
Acknowledgements
This investigation was carried with the support by the Consellería de Educación e Política Científica, Xunta de Galicia, D.O.G. (19 July 2001) PGIDT01PX138305PR. The authors would like to thank Dra. Cristina Pardo Martín for reviewing the paper.
References
- Armesto , S. 1998 . Evaluación de la importancia de los caracteres florales, en relación con la selección de las fuentes de polen en Apis mellifera L. a través del análisis polínico de polen corbicular , Ourense : Fac. Cie. Ourense, Univ. Vigo. Ph Lic Thes .
- Bergström , G. H. , Dobson , E. M. and Groth , I . 1995 . Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae) . Plant Syst. Evol. , 195 : 221 – 242 .
- Blight , M. M. , Le Metayer , M. , Pham-Delègue , M. H. , Picket , J. A. , Marion-Poll , F. and Wadhams , L. J. 1997 . Identification of floral volatiles involved in recognition of oilseed rape flowers, Brassica napus by honey bees, Apis mellifera . J. Chem. Ecol. , 23 : 1715 – 1727 .
- Bolchi-Serini , G. and Salvi , G. 1986 . Contributo alle conoscenza dell'etologia di Apis mellifera L. nella raccolta di polline . Apicolt. Mod. , 77 : 195 – 202 .
- Bradford , M. M. 1976 . A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding . Anal. Biochem. , 72 : 248 – 254 .
- Cargnello , G. , Gainazza , E. , Tedesco , G. , Cappella , M. and Gerola , F. M. 1988 . Wall proteins of Vitis vinifera pollen. I. Constancy of the phenotype . Vitis , 27 : 47 – 55 .
- Chittka , L. and Nikolas , W. 1997 . Why red flowers are not invisible to bees . Isr. J. Plant Sci. , 45 : 169 – 183 .
- Currie , R. W. 1997 . “ Pollination constraints and management of pollinating insects for crop production ” . In Pollen biotechnology for crop production and improvement , Edited by: Shivanna , K. R. and Sawheney , V. K. 121 – 152 . Cambridge : Univ. Press .
- Davis , A. R. 1997 . “ Pollination efficiency of insects ” . In Pollen biotechnology for crop production and improvement , Edited by: Shivanna , K. R. and Sawheney , V. K. 87 – 120 . Cambridge : Univ. Press .
- Díaz-Losada , E. 1995 . Aportación al conocimiento del origen botánico y características fisico-químicas del polen en Galicia , Ourense : Fac. Sci. Ourense, Vigo Univ. PhD Diss .
- Díaz-Losada , E. , González Porto , A. V. and Sá-Otero (Saa-Otero) , M. P. de . 1998 . Ètude de la couleur du pollen apicole recueilli par Apis mellifera L. en Espagne du nord-ouest (Galice) . Acta Bot. Gall. , 145 : 39 – 48 .
- Díaz-Losada , E. , González-Porto , A. V. , Fernández-Gómez , E. and Sá-Otero (Saa-Otero) , M. P. de . 1995 . Contribución al estudio de la utilización selectiva por Apis mellifera de la flora en un colmenar de NW de la Península Ibérica (Galicia) . Acta Bót. Malacit. , 20 : 115 – 122 .
- Dobson , E. M. 1987 . Role of flower and pollen aromas in host-plant recognition by solitary bees . Oecología , 72 : 618 – 623 .
- Dobson , E. M. , Bergström , G. and Groth , I . 1990 . Differences in fragance chemistry between flower parts of Rosa rugosa Thunb. (Rosaceae) . Isr. J. Bot. , 39 : 134 – 156 .
- Erdtman , G. 1969 . “ Handbook of palynology ” . In An introduction to the study of pollen grains and spores , Copenhagen : Munksgaard .
- Fewell , J. H. and Wiston , M. L. 1996 . Regulation of nectar collection in relation to honey storage levels by honey bees, Apis mellifera . Behav. Ecol. , 7 : 286 – 291 .
- Free , J. B. 1963 . The flower constancy of honeybees . J. Anim. Ecol. , 32 : 19 – 131 .
- Frisch , K. von . 1969 . Aus dem Leben der Bienen , Berlin : Springer .
- Gerber , B. , Gerbrzahn , N. , Hellstern , F. , Klein , J. , Kowalksy , O. , Wuestenberg , D. and Menzel , R. 1996 . Honey bees transfer olfactory memories established during flower visits to a proboscis extension paradigm in the laboratory . Anim. Behav. , 52 : 1079 – 1085 .
- Hodges , D. 1984 . The pollen loads of the honeybee , London : Bee Res. Assoc .
- Louveaux , J. 1958 . Recherchers sur la rècolte du pollen par les abeilles (Apis mellifera L.) . Ann. Abeille , 4 : 197 – 221 .
- Moore , P. D. and Webb , J. A. 1978 . An illustrated guide to pollen analysis , London : Hodder & Stoughton .
- Osche , G. 1983 . Optische Signale in der Koevolution von Pflanze und Tiere . Ber. Dtsch. Bot. Ges. , 96 : 1 – 27 .
- Pacini , E. and Juniper , B. E. 1979 . The ultrastructure of pollen grain development in the olive (Olea europaea). 1. Proteins in the pore . New Phytol. , 83 : 157 – 163 .
- Pantone Color Specifier , ed. 1989 . 747XR. The Pantone library of color , Carlstad, NJ : Pantone Inc .
- Pham-Delègue , M. , Loublier , I. , Ducruet , V. , Douault , P. , Marilleau , R. and Etiévant , P. 1994 . Caractérisation de signaux chimiques impliqués dans les relations plantes-abeilles domestiques . Grana , 33 : 184 – 190 .
- Ramil , P. , Aira , M. J. and Sá-Otero , M. P. de . 1992 . Clave polínica de las Ericaceae gallegas . Lazaroa , 13 : 33 – 40 .
- Rasheed , S. A. and Harder , L. D. 1997 . Foraging currencies for non-energetic resources: Pollen collection by bumblebees . Anim. Behav. , 54 : 911 – 926 .
- Sá-Otero , M. P. de , Díaz-Losada , E. and Armesto-Baztán , S. 2004 . Flower election by honeybee and floral morphology . Lazaroa , 25 : 113 – 123 .
- Sá-Otero , M. P. de , Díaz-Losada , E. and González Porto , A. V. 2001 . Relación categorizada de especies de la flora gallega (NO de España) que Apis mellifera L. utiliza como fuente de polen . Bol. R. Soc. Esp. Hist. Nat. (Sec. Biol.) , 96 : 81 – 89 .
- Saa Otero (de Sá-Otero) , M. P. de , Suárez-Cervera , M. and Gracia , V. R. , eds. 1996 . Atlas de pólen de Galicia , Ourense : Deput. Our .
- Valdés , B. , Díez , M. J. and Fernández , I. , eds. 1987 . Atlas polínico de Andalucía Occidental , Sevilla : Inst. Desarr. Reg. 43, Sevilla Univ. & Diput. Cádiz .
- Waddington , K. D. and Holden , L. R. 1979 . Optimal foraging: On flower selection by bees . Am. Nat. , 114 : 179 – 193 .