Abstract
An unusual megaspore has been recorded from beds within the Weald Clay Group of south-east England. Described as Clockhousea capelensis gen. et sp. nov., it is characterised by having a thick outer layer of exine consisting of closely packed columnar to clavate elements with constricted bases attached to a perforated inner layer and a sculpture that ranges from having the appearance of a negative reticulum through closely spaced verrucae to a mixture of verrucate and essentially baculate elements, all of which are surface manifestations and extensions of the underlying structure. These characters do not readily indicate a systematic relationship with any known heterosporous plant genus or family. The localised occurrence and relative abundance of the spores in a few beds suggest that some of the parent plants grew close to water bodies where they were deposited and preserved. Their recovery from sediments of late Hauterivian–early Barremian age indicates that the species has potential as a biostratigraphic marker in the upper Wealden succession of southern England and perhaps elsewhere.
Introduction
The Weald Clay Group of south-east England consists largely of argillaceous sediments that accumulated in lacustrine to lagoonal conditions in the Weald Sub-basin of the Wessex-Weald Basin. In contrast to the underlying Hastings Beds and overlying Lower Greensand groups, which crop out in mostly hilly terrain, the Weald Clay is generally low-lying, with exposures largely confined to brickpits. The succession, which is considered to range from Hauterivian to early Aptian in age, contains a wide variety of fossils including bivalves, gastropods, ostracods and spinicaudatans, and remains of plants, insects, fishes and dinosaurs. Along with taxonomic studies in connection with these, the stratigraphy, depositional setting and climate have been considered in many publications during the past 50 years, a few of which are cited below in the context of the occurrence of the megaspore described in this paper. This is referred to a new genus and species because its wall structure differs significantly from that of other Mesozoic and younger megaspore taxa. Its uncertain systematic relationship is also considered along with its stratigraphic and palaeoenvironmental significance.
Material and methods
All of the specimens that have been attributed to the new taxon were recovered from two localities in the western Weald Sub-basin: two brickwork pits at Clock House, Capel, and a third at Smokejacks, Ockley, both in the county of Surrey. Stratigraphic details pertaining to these localities were provided by Batten (Citation1998), who also indicated the horizons sampled for mesofossils on two composite lithological logs (op. cit.: , ) and described the laboratory procedure for extracting them from the sedimentary matrix. In addition, a lithological log for the Upper Weald Clay section exposed more recently on the south-eastern face of the Smokejacks quarry has been presented by Nye et al. (Citation2008). Hence, discussion of the material examined and the methods used are restricted here to only the most salient points.
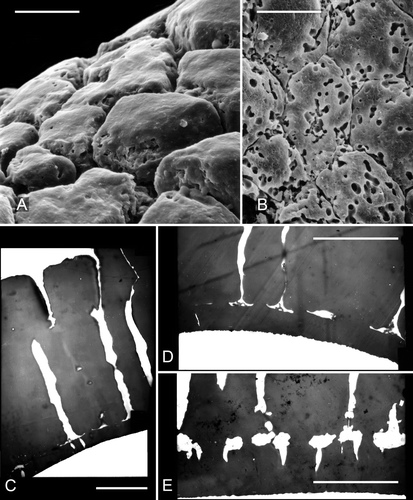
Figure 2. Clockhousea capelensis. SEM. A. Distal surface of specimen, sample DJBCl88/10, preparation MCM193, BGS reg. MPK13806. B. Lateral view of spore, part of narrow triradiate ridge visible (arrow), DJBCl88/10, MCM193, MPK13807. C. Specimen with pronounced triradiate ridge that shows evidence of damage in the form of abrasion of sculptural elements, especially towards proximal pole, DJBCl88/11, MFP166, MPK13808. D. Well-preserved specimen sculptured predominantly by closely spaced verrucae, but with scattered composite baculate elements mainly on proximal surface, especially towards triradiate ridge, DJBCl88/11, MFP166, MPK13809. E. Close-up of proximal polar area of spore depicted in C. F. Example of a spore sculptured solely by closely spaced verrucae and assumed to be an atypical, immature or aberrant form of the species, DJBCl88/10, MCM193, MPK13810. Scale bars – 100 μm (A–D, F); 10 μm (E).
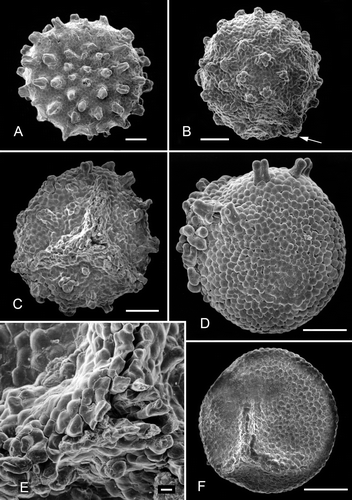
Figure 3. Clockhousea capelensis. SEM. A. Detail of spore depicted in showing ‘negative reticulum’ and composite baculate process, sample DJBCl88/10, preparation MCM193, BGS reg. MPK13811. B–F. Details of specimen broken in half, DJBCl88/10, MCM193, MPK13812: B. Components of ‘negative reticulum’ become narrower towards interior surface of wall, giving them a clavate appearance; they are attached to a thin inner, perforated layer; C. Detail of part of inner surface of perforated layer; D. General view of part of broken specimen; E. Perforations of another part of inner layer viewed more obliquely than in C; F. Part of wall showing an exinal extension that consists of single baculate and spinose elements that are not fused together. Scale bars – 10 μm.
The section that was exposed at Clock House at the time of logging during the 1970s and 1980s revealed approximately 35 m of gently dipping beds within what was regarded as the upper part of the Lower Division of the Weald Clay Formation, with the base of the Upper Division, British Geological Survey Bed 3a, being exposed at the top of the section. Worssam (Citation1978) considered this bed also to delineate the Hauterivian/Barremian boundary, although later work (e.g. Horne, Citation1995) has suggested that it is slightly lower than this.
The Smokejacks succession, as logged in 1988 (DJB, unpublished field notes) and recorded by Ross and Cook (Citation1995) and Nye et al. (Citation2008), comprises up to 29 m of somewhat younger sediments in the lower part of the Upper Division of the Weald Clay Formation (terminology of Batten, Citation1998). This part of the succession is considered to be of early Barremian age.
It is necessary to note here that the Weald Clay has been regarded as both a Formation (e.g. Sumbler, Citation1996) and a Group (e.g. Horne, Citation1995), if its status has been mentioned at all (commonly not in the older, pre-1980, literature). When considered to be a formation, or when this is implied, its two main lithological components are referred to in terms of lower and upper divisions, applied either formally, as in Batten (Citation1998), or informally, as in Worssam (Citation1978). When regarded as a group, the divisions become the Lower and Upper Weald Clay formations, as in Rasnitsyn et al. (Citation1998). This terminology has been used more widely in recent years and is accepted here.
Most of the specimens recorded by Batten (Citation1998), and referred to informally as ‘Clockhousea’, were recovered from the Clock House locality. Seven of the 33 samples processed for mesofossils (‘large’ plant microfossils) yielded specimens. They proved to be relatively common in four of these, with abundances in two comprising ca. 20% and 25% of the megaspore assemblages (ca. 65 and 45 specimens recovered from ca. 400 and 450 g of sample respectively). By contrast, only one sample from the Smokejacks section yielded any. Recently however, a few more specimens have been recovered from both localities, and were also reported from the Smokejacks pit by Nye et al. (Citation2008). The new taxon is based on Clock House material. Fifteen samples from other sites in the Weald Sub-basin, many from the Wessex and Vectis formations on the Isle of Wight, and a few from other locations exposing beds of similar age have also been examined, but have not yielded any specimens so far.
Spores selected for study under scanning and transmission electron microscopes (SEM and TEM) were handled using standard procedures. Those for SEM analysis were mounted on exposed photographic film glued to stubs 12 mm in diameter and coated with gold-palladium before being examined. Specimens intended for transmission microscopy were dehydrated in ethanol followed by the solvent propylene oxide before being embedded in Agar 100 resin prior to sectioning. Ultrathin sections were collected on formvar-coated slot grids and examined under a Jeol JEM-100CX TEM.
All of the specimens illustrated in will be deposited in the collections of the British Geological Survey (BGS), Keyworth, Nottingham, UK and have accordingly been allocated BGS reference numbers, which are prefixed by MPK. Other number prefixes in this paper refer to Clock House rock samples (DJBCl) and laboratory preparations (MCM and MFP).
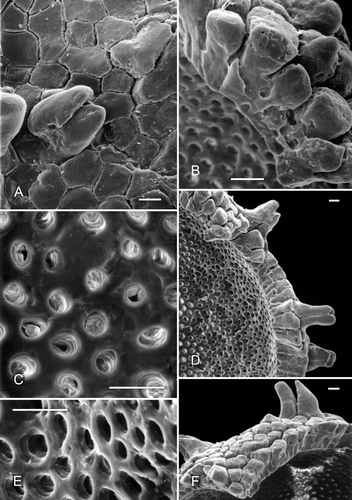
Figure 1. Clockhousea capelensis gen. et sp. nov. LM. A, B. Holotype, sample DJBCl88/10, preparation MCM193, BGS reg. MPK13804, photographed under reflected light: A. Proximal view, triradiate ridge unclear owing to the profusion of bacula, which mask it; B. Distal surface; C, D. A second, smaller specimen, DJBCl88/10, MCM193, MPK13805: C. Proximal view, triradiate ridge faintly discernible; D. Distal surface. Scale bar – 100 μm.
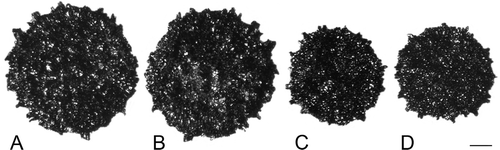
Figure 4. A–D, F. Clockhousea capelensis. SEM. A–C. Broken specimen, sample DJBCl88/10, preparation MCM193, BGS reg. MPK13813: A. Close-up of inner surface of exine in vicinity of triradiate suture, and possibly also a thin layer beneath it within suture (arrow); B. Detail of inner suface of exine; unlike the perforations shown in , E, these are less clearly delineated because the surface is much more uneven; C. General view of whole specimen. D. Detail of top of clavate element of exine that has suffered from extensive microbial degradation, same specimen as that in . F. Cross-section of spore that has been broken in half, showing a complex of baculate and other exinal elements bordering triradiate suture and a more or less smooth inner surface of what appears to be a thin inner layer of exine, DJBCl88/10, MCM193, MPK13814. E. An example of what may or may not be an ‘inner body’ (the inner separable layer) of a specimen of C. capelensis that has lost its outer wall, DJBCl88/10, MCM193, MPK13815. Scale bars – 100 μm (A, C, E, F); 10 μm (B); 1 μm (D).
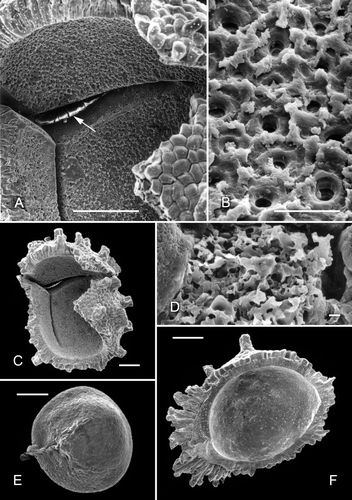
Figure 5. A, B. Scanning electron micrographs of parts of the ‘negatively reticulate’ surface of two examples of Clockhousea capelensis showing the beginnings of, and more serious, microbial degradation respectively: A. Same specimen as that in B. Sample DJBCl88/10, preparation MCM193, BGS reg. MPK13816. C–E. Transmission electron micrographs of parts of the exine of two specimens of C. capelensis showing its structure. The columnar elements become much narrower just above their points of attachment to the inner perforated layer. The specimens were difficult to cut and none of the sections passes through the centre of a perforation: C, D. Cross-sections of same specimen; E. Slightly oblique cross-section of a second specimen. Scale bars – 10 μm.
Systematic palaeontology
Genus Clockhousea gen. nov.
Derivation of name
After the Clock House Brickworks, Capel, Surrey, south-east England.
Type species
Clockhousea capelensis sp. nov.
Diagnosis
Triradiate, spherical to subspherical, medium-sized megaspore. The triradiate ridge is commonly difficult to discern under reflected light, especially in strongly sculptured specimens. The exine consists of a thick outer layer of closely packed columnar to clavate elements with constricted bases attached to a thin, perforated, inner zone, beneath which a further thin layer of exine that has a smooth inner surface may be present. The sculpture ranges from having the appearance of a negative reticulum through closely spaced verrucae to a mixture of verrucate and essentially baculate elements, all of which are surface manifestations and extensions of the underlying structure. The bacula are simple or composite structures and concentrated around the triradiate ridge, with only scattered elements elsewhere, or fairly evenly distributed over the surface of the spore. Simple bacula are single extensions of the outer wall; composite bacula, which are more common, usually consist of 2–5, but sometimes more, elements, most if not all of which are partially to entirely fused together.
Remarks
Previous use of the informal name ‘Clockhousea’ (Batten, Citation1998; Nye et al., Citation2008) is formalised here to accommodate a new genus, the erection of which is merited because the spores concerned differ in both overall appearance and wall structure from the general aspect and construction of all other verrucate, baculate and spinose fossil megaspores that have been described hitherto (see Discussion). Closest superficially are the genera Bacutriletes van der Hammen, Citation1955 ex Potonié, Citation1956 emend. Banerji et al. Citation1984 and Echitriletes van der Hammen, Citation1955 ex Potonié, Citation1956, both represented by Cretaceous species, and perhaps also older (Triassic) Narkisporites Kannegieser and Kozur, Citation1972, and some baculate and echinate megaspores of extant Isoetes Linnaeus (e.g. Berthet & LeCocq, Citation1978).
Clockhousea capelensis sp. nov. (––D, F and )
Holotype
Specimen from Clock House Brickworks pit, sample DJBCl88/10, preparation MCM193, BGS registration MPK13804; , ).
Other specimens
All of the specimens on which this species is based are from samples DJBCl88/10 and DJBCl88/11.
Diagnosis
As for genus.
Description
Triradiate spore, body spherical to subspherical (–, ); maximum diameter 310–(425)–550 μm (35 specimens). The triradiate ridge may not be much wider than the diameter of the surface bacula (, arrow) but is usually more pronounced than this (), being raised up to varying degrees. It is commonly difficult to discern under a reflected-light microscope, especially when partially obscured by sculptural elements (, ), but it extends to about three-quarters of the equatorial diameter. The line of suture, if visible under a scanning electron microscope, may also appear irregular owing to the distribution of the surrounding sculpture (, ), but is clearly straight when viewed from the underside (, ).
The sculpture varies from being dominated by surface manifestations of the underlying structure in having the appearance of a negative reticulum ( in part) or closely spaced verrucae () through to a mixture of verrucate and essentially baculate elements (e.g. , –); the polygonal components of the ‘negative reticulum’ and the verrucae are mainly between 8 and 28 μm in maximum diameter. The bacula are typically 20–80 μm across and 35–60 μm high; they may be concentrated near the triradiate ridge, with only scattered elements elsewhere, mainly on the proximal surface (, ), to fairly evenly distributed over the entire surface of the spore (e.g. , ). Although occasionally consisting of single elongate, exinal extensions with flat to gently rounded tops, most bacula comprise 2–5 elements that are partially to entirely fused together (e.g. –, ); a small number may be unfused and merely closely spaced. Some components have pointed tips (e.g. ) and are, therefore, more appropriately termed spines. A few consist of more irregular elements (), especially adjacent to the triradiate suture where they may also be up to 100 μm high.
Most undamaged specimens are black and tend to glisten in reflected light (). Cross-sections of the wall of broken spores show that it is generally 20–30 μm thick. They also emphasise the ‘negatively reticulate’ to closely packed verrucate to baculate outer surface. The diameter of these elements becomes narrower towards the inner surface; as a result they take on a clavate appearance, with their commonly constricted bases fused to a perforated layer ca. 2.5–3 μm thick (e.g. , , , –). The perforations on the inner surface of this layer are 20–40 μm in diameter and may penetrate well into the outer wall (e.g. , ). Distances between them may vary but they tend to be remarkably evenly spaced (, ). In some specimens, the perforations are surrounded by a more irregular, ‘rougher’ surface (–). A few broken spores reveal what may be a very thin inner separable layer that has a more or less smooth inner surface (, just visible within part of the triradiate suture; , lining the whole of the inner surface and triradiate suture); under high magnification (×5000) this surface has a more uneven, scabrate appearance.
Remarks
Commonly associated with the typical spores described above are somewhat smaller, weakly sculptured specimens. Some of these, which have been taken into account in the description, may be incompletely developed, aberrant, forms (e.g. ). It is possible that a few (e.g. , not considered in the description) are ‘inner bodies’ that have been released from broken specimens, in which case they would be composed solely of an inner separable layer of exine (cf. Moore et al., Citation2006). So far, however, it has not been possible either to prove or to refute this suggestion.
Although some examples of Clockhousea capelensis are slightly flattened, little folding is apparent, fracturing and fragmentation having been the more usual response to compression of the sediments in which they are preserved. This must reflect, to some extent, both the structure and the composition of the exine, but fire may also have played a part, brittleness being characteristic of charred plant matter. Weald Clay mesofossil assemblages commonly contain charcoalified phytoclasts and other organic remains that are considered to reflect a strongly seasonal climate, with the vegetation of the region prone to fire during periods of drought (Batten, Citation1988). Almost all of the specimens of C. capelensis recovered hitherto are black and shiny, and commonly associated with them are many black and degraded fragments that are attributable to this species and perhaps also to other taxa. This might indicate that the parent plants grew in drier parts of the lowland, the megaspores having been washed into the sites of deposition during rainy periods where they were subsequently preserved along with other spores that are various shades of brown and have clearly not been affected by fire.
Some specimens show evidence of varying degrees of microbial degradation ranging from simple rounded to elongate perforations () through to complex ‘structures’ in the form of meandering openings, irregular rosettes () and more complete breakdown of the exine ().
The characters of the two specimens from the Smokejacks Brickworks pit referred to ‘Clockhousea’ that were illustrated by Nye et al. (Citation2008: figure 9H, I) are entirely comparable to the material described here.
Discussion
The ultrastructure of spores and pollen grains is commonly taken into account in investigations of systematic relationships of extant plant species. It has also been shown to be of value in determining affinities of dispersed fossil material, although only a small proportion has been investigated in this way, and as demonstrated by an analysis of four species of Triassic megaspores from Australia (Hemsley & Scott, Citation1989), it does not guarantee determination of taxonomic position. Kovach (Citation1994) noted that of the 343 publications cited in the catalogue of Batten and Kovach (Citation1990) concerned with Mesozoic and Tertiary megaspores, only 26 contain descriptions of wall structure, and for many of the 78 species involved, these are very brief. Nevertheless, this was sufficient for him to be able to review the wall structure of fossil megaspores and assess its value for determining likely relationships between extinct taxa and the two main groups of extant heterosporous plants, the aquatic ferns and the lycopsids. More recent studies have demonstrated similar connections.
Modern lycopsids belong to either the Selaginellales or the Isoetales. Although both the appearance of the whole plant and the morphology of their microspores differ, their megaspores are less easy to tell apart. The exines of Isoetes Linnaeus tend to be composed of a porous, three-dimensional network of loosely anastomosing sporopollenin elements that are mainly orientated parallel to the surface of the spore, whereas the arrangement in those of Selaginella Beauvois is more variable and usually more compact (e.g. Kempf, Citation1970; Tryon & Lugardon, Citation1978, Citation1990; Minaki, Citation1985; Taylor, Citation1989, Citation1993, Citation1994; Kovach, Citation1994; Morbelli, Citation1995; Korall & Taylor, Citation2006). This also applies to Cretaceous megaspores that have been referred to the Isoetales and Selaginellales (e.g. Batten, Citation1988; Kovach & Dilcher, Citation1988; Archangelsky & Villar de Seoane, Citation1990; Batten & Koppelhus, Citation1993; Wilde & Hemsley, Citation2000) as well as to older taxa (e.g. Palaeozoic selaginellalean megaspores; Cottnam et al., Citation2000).
Within the aquatic fern order Salviniales, the megaspores of both fossil (e.g. Batten & Collinson, Citation2001; Collinson et al., Citation2009) and extant (e.g. Kempf, Citation1969; Fowler & Stennett-Willson, Citation1978) Azolla Lamarck are especially complex. They differ markedly from the megaspores of extant Regnellidium Lindman of the Marsileales and morphologically similar species of the Cretaceous–Tertiary spore genus Molaspora Schemel, Citation1950 emend. Hall Citation1963.
Megaspores known only as fossils that are considered to be derived from aquatic ferns include species of the genera Arcellites Miner, Citation1935 emend. Ellis and Tschudy, Citation1964, Ariadnaesporites Potonié, 1956 emend. Tschudy, Citation1966, Bohemisporites Knobloch, Citation1984, Capulisporites Potonié, Citation1956, Glomerisporites Potonié, Citation1956 and Parazolla Hall, Citation1969. These attributions are based on overall morphology and wall structure, which differs from that of lycophyte megaspores, and also on a tendency for relatively large numbers to be recovered from sediments at least occasionally, if not commonly, suggesting that their occurrence reflects the presence of parent plant communities living adjacent to, or within, bodies of water close to the site of deposition.
Significantly, none of the studies on the wall structure of fossil and modern megaspores has demonstrated a morphology that is comparable to that of Clockhousea capelensis. The closest is that of species of Molaspora and Regnellidium in which the outer wall (exospore, solid perine) includes a thick alveolate layer with, in common with C. capelensis, the majority of the elements orientated normal to the surface (e.g. Lupia et al., Citation2000; Schneider & Pryer, Citation2002; Gardenal et al., Citation2007). However, this layer is otherwise very different, as are all of the other morphological characters of these genera.
It is possible, therefore, that C. capelensis represents another, hitherto unknown, group of heterosporous plants. Unfortunately, unlike the situation in connection with the much more common younger Cretaceous megaspore Balmeisporites Cookson and Dettmann, Citation1958, which was referred by Krassilov and Golovneva (Citation2000) to a new fossil order of heterosporous plants following the recovery of unusual sporophyll fragments, further evidence for this possibility is wanting. No specimens of C. capelensis have been encountered with microspores attached.
The relative abundance of C. capelensis at just a few horizons in the Weald Clay Group of south-east England suggests that it is derived from plants that grew near water bodies, but probably not in them. Indeed, they may have inhabited drier parts of the lowland, as noted earlier. The lack of any association with megaspores that are commonly taken to indicate the presence of temporary or semi-permanent water bodies in the Wealden succession, such as Arcellites, Minerisporites Potonié, Citation1956 and Paxillitriletes Hall and Nicolson, Citation1973, supports this observation. Standard palynological preparations of the samples in which C. capelensis is relatively common suggest that deposition occurred in either fresh or slightly saline conditions, with most of the very few algal palynomorphs present (e.g. Schizophacus parvus (Cookson & Dettmann, 1959) Nakoman, Citation1966, Schizosporis reticulatus Cookson & Dettmann, 1959 emend. Pierce, Citation1976 and Tetranguladinium conspicuum Yu, Guo & Mao, Citation1983 ex Chen et al. Citation1988) being freshwater indicators.
Records of the species are confined at present to deposits of late Hauterivian–early Barremian age, suggesting that it has potential as a biostratigraphic marker within the Wealden of the Weald Sub-basin and possibly elsewhere. So far, however, specimens have only been recovered from the Capel and Ockley quarries. Four other brickpits in the region in which beds of similar age are exposed have not yielded any specimens, and they have not been recovered hitherto from Hauterivian–Barremian strata on the Isle of Wight and in Dorset.
Conclusions
A thick outer exine that consists of closely packed columnar to clavate elements with constricted bases attached to a perforated inner layer, and a sculpture that ranges from having the appearance of a negative reticulum through closely spaced verrucae to a mixture of verrucate and essentially baculate elements, characterise Clockhousea capelensis. This morphology distinguishes it from all other megaspores, both fossil and those of extant plants, that have been described hitherto. It has potential as a biostratigraphic marker within the Wealden succession of south-east England, and perhaps also further afield. Its relative abundance in a few beds suggests that the plants from which it was derived lived in a lowland habitat close to, but not in, bodies of water. The evolutionary implications of the possibility that it is the product of a hitherto unknown, heterosporous plant order or family are intriguing and require further investigation.
Acknowledgements
I thank Mrs Lorraine Morrison (Aberystwyth University) for sample processing, Dr Peter Morris (MicroStrat Services, Devon) for photographing the specimens depicted in , and Mr Richard Hartley (Manchester University) for help in preparing the figures. I am grateful for the thoughtful comments on the manuscript provided by two anonymous referees.
References
- Archangelsky , S. and Villar de Seoane , L. 1990 . Morfología y estructura de megasporas Cretácicas de Patagonia, Republica Argentina . Rev. Esp. Micropaleontol. , 22 : 419 – 450 .
- Banerji , J. , Jana , B. N. and Maheshwari , H. K. 1984 . The fossil floras of Kachchh. II. – Mesozoic megaspores . Palaeobotanist , 33 : 190 – 227 .
- Batten , D. J. 1988 . Revision of S. J. Dijkstra's Late Cretaceous megaspores and other plant microfossils from Limburg, The Netherlands. Utrecht: Geol. Surv. Neth . Meded. Rijks Geol. Dienst , 41-3
- Batten , D. J. 1998 . Palaeoenvironmental implications of plant, insect and other organic-walled microfossils in the Weald Clay Formation (Lower Cretaceous) of southeast England . Cretac. Res. , 19 : 279 – 315 .
- Batten , D. J. and Collinson , M. E. 2001 . Revision of species of Minerisporites, Azolla and associated plant microfossils from deposits of the Upper Palaeocene and Palaeocene/Eocene transition in the Netherlands, Belgium and the USA . Rev. Palaeobot. Palynol. , 115 : 1 – 32 .
- Batten , D. J. and Koppelhus , E. B. 1993 . Morphological reassessment of some zonate and coronate megaspore genera of mainly post-Palaeozoic age . Rev. Palaeobot. Palynol. , 78 : 19 – 40 .
- Batten , D. J. and Kovach , W. L. 1990 . Catalog of Mesozoic and Tertiary megaspores. Dallas, TX: Am. Assoc. Stratigr. Palynol . AASP Contrib. Ser. , 24
- Berthet , P. and LeCocq , M. 1978 . Morphologie sporale des espèces françaises du genre Isoetes L . Pollen Spores , 19 : 329 – 359 . for 1977
- Chen , Y. Y. , Harland , R. , Stover , L. E. and Williams , G. L. 1988 . Fossil dinoflagellate taxa by Chinese authors, 1978–1984. Ottawa: DFO/CHS . Can. Tech. Rep. Hydrogr. Ocean Sci. , 103
- Collinson , M. E. , Barke , J. , van der Burgh , J. and van Konijnenburg-van Cittert , J. H. A. 2009 . A new species of freshwater Azolla (Azollaceae) from the Eocene Arctic Ocean . Rev. Palaeobot. Palynol. , 155 : 1 – 14 .
- Cookson , I. C. and Dettmann , M. E. 1958 . Cretaceous “megaspores” and a closely associated microspore from the Australian region . Micropaleontology , 4 : 39 – 49 .
- Cottnam , C. F. , Hemsley , A. R. , Rössler , R. , Collinson , M. E. and Brain , A. P. R. 2000 . Diversity of exine structure in Upper Carboniferous (Westphalian) selaginellalean megaspores . Rev. Palaeobot. Palynol. , 109 : 33 – 44 .
- Dijkstra , S. J. 1951 . Wealden megaspores and their stratigraphical value . Meded. Geol. Sticht., NS , 5 : 7 – 22 .
- Ellis , C. H. and Tschudy , R. H. 1964 . The Cretaceous megaspore genus Arcellites Miner . Micropaleontology , 10 : 73 – 79 .
- Fowler , K. and Stennett-Willson , J. 1978 . Sporoderm architecture in modern Azolla . Fern Gazette , 11 : 405 – 412 .
- Gardenal , P. , Morbelli , M. A. and Giudice , G. E. 2007 . Morphology and ultrastructure of heterosporous Filicophyta spores from north-west Argentina . Grana , 46 : 65 – 77 .
- Hall , J. W. 1963 . Megaspores and other fossils in the Dakota Formation (Cenomanian) of Iowa, (U.S.A.) . Pollen Spores , 5 : 425 – 443 .
- Hall , J. W. 1969 . Studies on fossil Azolla: Primitive types of megaspores and massulae from the Cretaceous . Am. J. Bot. , 56 : 1173 – 1180 .
- Hall , J. W. and Nicolson , D. H. 1973 . Paxillitriletes, a new name for fossil megaspores hitherto invalidly named Thomsonia . Taxon , 22 : 319 – 320 .
- van der Hammen , T. 1955 . Principios para la nomenclatura palinológica sistematica . Bol. Geol. , 2 : 3 – 21 . for 1954
- Hemsley , A. R. and Scott , A. C. 1989 . The ultrastructure of four Australian Triassic megaspores . Pollen Spores , 31 : 133 – 154 .
- Horne , D. J. 1995 . A revised ostracod biostratigraphy for the Purbeck–Wealden of England . Cretac. Res. , 16 : 639 – 663 .
- Kannegieser , E. and Kozur , H. 1972 . Zur Mikropaläontologie des Schilfsandsteins (Karn) . Geologie , 21 : 185 – 215 .
- Kempf , E. K. 1969 . Elektronenmikroskopie der Sporodermis von känozoischen Megasporen der Wasserfarn-Gattung Azolla . Paläontol. Z. , 43 : 95 – 108 . pls 11–13
- Kempf , E. K. 1970 . Elektronenmikroskopie der Sporodermis von Megasporen der Gattung Selaginella (Pteridophyta) . Rev. Palaeobot. Palynol. , 10 : 99 – 116 .
- Knobloch , E. 1984 . Megasporen aus der Kreide von Mitteleuropa . Sb. Geol. Věd, Paleontol. , 26 : 157 – 195 . pls 1–12
- Korall , P. and Taylor , W. A. 2006 . Megaspore morphology in the Selaginellaceae in a phylogenetic context: A study of the megaspore surface and wall structure using scanning electron microscopy . Grana , 45 : 22 – 60 .
- Kovach , W. L. 1994 . “ A review of Mesozoic megaspore ultrastructure ” . In Ultrastructure of fossil spores and pollen , Edited by: Kurmann , M. H. and Doyle , J. A. 23 – 37 . Kew : R. Bot. Gard . Edspp.
- Kovach , W. L. and Dilcher , D. L. 1988 . Megaspores and other dispersed plant remains from the Dakota Formation (Cenomanian) of Kansas, U.S.A . Palynology , 12 : 89 – 119 .
- Krassilov , V. A. and Golovneva , L. B. 2000 . New order of heterosporous plants from the Late Cretaceous of Kem’ River, Western Siberia . Palaeontol. J. (Moscow) , 34 : 84 – 92 . In Russian; Transl. English
- Lupia , R. , Schneider , H. , Moeser , G. M. , Pryer , K. M. and Crane , P. R. 2000 . Marsileaceae sporocarps and spores from the Late Cretaceous of Georgia, U.S.A . Int. J. Plant Sci. , 161 : 975 – 988 .
- Minaki , M. 1985 . Macrospore morphology and taxonomy of Selaginella (Selaginellaceae) . Pollen Spores , 26 : 421 – 479 . for 1984
- Miner , E. L. 1935 . Paleobotanical examinations of Cretaceous and Tertiary coals . Am. Midl. Nat. , 16 : 585 – 625 .
- Moore , S. E. M. , Hemsley , A. R. and Borsch , T. 2006 . Micromorphology of outer exospore coatings in Selaginella megaspores . Grana , 45 : 9 – 21 .
- Morbelli , M. A. 1995 . Megaspore wall in Lycophyta – ultrastructure and function . Rev. Palaeobot. Palynol. , 87 : 1 – 12 .
- Nakoman , E. 1966 . Contribution à l’étude palynologique des formations tertiaires du Bassin de Thrace . Ann. Soc. Géol. Nord , 86 : 65 – 107 . pls 6–11
- Nye , E. , Feist-Burkhardt , S. , Horne , D. J. , Ross , A. J. and Whittaker , J. E. 2008 . The palaeoenvironment associated with a partial Iguanodon skeleton from the Upper Weald Clay (Barremian, Early Cretaceous) at Smokejacks Brickworks (Ockley, Surrey, UK), based on palynomorphs and ostracods . Cretac. Res. , 29 : 417 – 444 .
- Pierce , S. T. 1976 . Morphology of Schizosporus reticulatus Cookson and Dettman 1959 . Geosci. Man. , 15 : 25 – 33 .
- Potonié , R. 1956 . Synopsis der Gattungen der Sporae dispersae. T I. Sporites. Hannover: Amt f. Bodenforsch. Geol. Jahrb. Beih. , 23
- Rasnitsyn , A. P. , Jarzembowski , E. A. and Ross , A. J. 1998 . Wasps (Insecta: Vespida = Hymenoptera) from the Purbeck and Wealden (Lower Cretaceous) of southern England and their biostratigraphical and palaeoenvironmental significance . Cretac. Res. , 19 : 329 – 391 .
- Ross , A. J. and Cook , E. 1995 . The stratigraphy and palaeontology of the Upper Weald Clay (Barremian) at Smokejacks Brickworks, Ockley, Surrey, England . Cretac. Res. , 16 : 705 – 716 .
- Schemel , M. P. 1950 . Cretaceous plant microfossils from Iowa . Am. J. Bot. , 37 : 750 – 754 .
- Schneider , H. and Pryer , K. M. 2002 . Structure and function of spores in the aquatic heterosporous fern family Marsileaceae . Int. J. Plant Sci. , 163 : 485 – 505 .
- Sumbler , M. G. 1996 . British Regional Geology. London and the Thames Valley , 4th , London : HMSO/BGS .
- Taylor , W. A. 1989 . Megaspore wall ultrastructure in Selaginella . Pollen Spores , 31 : 251 – 288 .
- Taylor , W. A. 1993 . Megaspore wall ultrastructure in Isoetes . Am. J. Bot. , 80 : 165 – 171 .
- Taylor , W. A. 1994 . “ Test and applications of a method of quantitative analysis of fossil and extant lycopsid megaspore walls ” . In Ultrastructure of fossil spores and pollen , Edited by: Kurmann , M. H. and Doyle , J. A. 39 – 52 . Kew : R. Bot. Gard . Edspp.
- Tryon , A. F. and Lugardon , B. 1978 . Wall structure and mineral content in Selaginella spores . Pollen Spores , 20 : 315 – 340 .
- Tryon , A. F. and Lugardon , B. 1990 . Spores of the Pteridophyta , Berlin : Springer .
- Tschudy , R. H. 1966 . Associated megaspores and microspores of the Cretaceous genus Ariadnaesporites Potonié, 1956, emend . Washington DC: USGS Prof. Pap. 550-D , : D76 – D82 .
- Wilde , V. and Hemsley , A. R. 2000 . Morphology, ultrastructure and affinity of Barremian (Lower Cretaceous) megaspores Dijkstraisporites and Paxillitriletes from Brilon-Nehden, Germany . Palynology , 24 : 217 – 230 .
- Worssam , B. C. 1978 . The stratigraphy of the Weald Clay , Edited by: Morter] , A. A. 19 – 23 . London : NERC/UK Inst. Geol. Sci. Rep.78/11 . Appendix 2, Weald Clay mollusca
- Yu , J.-X. , Guo , Z.-Y. and Mao , S.-Z. 1983 . Cretaceous palynological assemblages from the district south of the Songhua River , Beijing : Ed. Comm. Chin. Acad. Geol. Sci. Prof. Pap. Stratigr. Palaeontol. 10 .