Abstract
The pollen grains of 111 species of Lessingianthus were analysed. Almost all the species belong to the type “B” pollen, characteristic of the genus. These grains are oblate-spheroidal, spheroidal to prolate-spheroidal (P/E 0.93–1.04), echinolophate, 3-colporate, with lacunae in a regular pattern, without polar lacunae. However, some species show variations of this basic type. The type “B-1”, “B-2” and “B-3” grains are respectively 3-porate lacking equatorial lacunae, 3-porate with equatorial lacunae and 3-colporate, psilolophate. Also, one species showed type “D” pollen, a palynomorph present in other genera of the tribe but not reported until now for Lessingianthus. The analysis confirms that the species of the genus constitute well-defined groups based on the pollen characteristics. The results ascertain that pollen morphology is a significant feature in the identification and delimitation of species and genera of Vernonieae.
Keywords:
The tribe Vernonieae Cass. is one of the largest groups of Asteraceae with about 1700 species, which are concentrated in two major centres of diversification, the central region of Africa and southern Brazil (Robinson, Citation1999). Members of the tribe are grouped into 21 subtribes based on inflorescence pattern, persistence of the phyllaries, pollen morphology, chemical composition and chromosome number (Keeley & Robinson, Citation2009). Subtribe Lepidaploinae Keeley & H. Rob. constitutes the largest group within the Vernonieae, including more than 300 species with almost all species previously placed into the huge section Lepidaploa (Cass.) DC. of the genus Vernonia Schreb., the majority of which has been segregated to different genera by Robinson (Citation1988a , Citation b , Citation c , Citation1992b , Citation1993).
The genus Lessingianthus H. Rob. was initially established to recognise the species originally arranged under Vernonia Schreb. sect. Lepidaploa subsect. Macrocephalae Benth. (Baker, Citation1873). As presently delimited (Robinson, Citation2007), the genus comprises about 110 species occurring within campo cerrado and campo rupestre habitats (Bremer, Citation1994). Lessingianthus is widely distributed in tropical South America, with a concentration in eastern Brazil (Robinson, Citation1988a ; Dematteis, Citation2006). The species are perennial herbs or shrubs with xylopodia, having medium or large-sized heads and seriate-cymose inflorescences (Robinson, Citation1988a ). The genus is distinguished from related groups by pollen type, anther appendages, chromosome number and the shape of the raphids in the achene wall (Dematteis, Citation2006).
Pollen morphology is one of the characters, other than style bases and anther appendages, that is useful in delineating natural groups in the Vernonieae (Robinson, Citation1992a ; Bolick & Keeley, Citation1994). Several authors have analysed the pollen grains of species of the tribe, mainly belonging to the genus Vernonia s.l. (Wodehouse, Citation1928; Stix, Citation1960; Keeley & Jones, Citation1977, 1979; Jones, Citation1979, Citation1981; Robinson, Citation1988a , Citation b , Citation c ; Skvarla et al., Citation2005; Dematteis & Pire, Citation2008). Wodehouse (Citation1928) studied the pollen grains of the Vernonieae and recognised three patterns of ornamentation: psilate, lophate, and echinate. Stix (Citation1960) suggested 42 pollen types for the Asteraceae on the basis of the exine structure, including some species of Vernonieae.
The current terminology applied for pollen types in Vernonieae was initially suggested by Keeley & Jones (Citation1979) and includes six pollen types for the tribe (from A to F) based on the pollen aperture and surface morphology. Robinson (Citation1992a ) recognised some additional features and currently, ten main pollen types are recognised within the Vernonieae. The genus Lessingianthus has type “B” pollen grains (Robinson, Citation1988a ), which are characterised by being tricolporate, echinolophate, with a discontinuous tectum, very long germinal furrows that converge at the poles and lacunae disposed in a regular pattern, and by the lack of polar lacuna (Dematteis & Pire, Citation2008). This pollen type is also characterised by crests with distinct baculae discernible from the point attachment on the foot layer to the point above where they join the ridge of the crest (Robinson, Citation1988a ).
The pollen morphology of some Lessingianthus species has been discussed previously (Robinson, Citation1988a ; Dematteis, Citation2006; Dematteis & Pire, Citation2008), but these studies were focused on comparative analysis among genera of the tribe Vernonieae. A recent analysis of the genus pollen morphology undertaken by Mendonça et al. (Citationin press) includes 30 species of Lessingianthus. This study also includes an analysis of the taxa previously placed in the subgenus Oligocephalus H. Rob., which have recently been transferred to the genus Chrysolaena H. Rob. on the basis of its pollen morphology and chromosome number (Dematteis, Citation2007, Citation2009).
The aim of this study was to conduct a comprehensive examination of pollen morphology in the genus Lessingianthus with almost complete species coverage to determine its possible taxonomic relevance at the specific and generic levels.
Materials and methods
Pollen samples were obtained by removing one or two florets from herbarium specimens of each species. Voucher specimens are mainly deposited at the Herbarium of the Instituto de Botánica del Nordeste (CTES) in Corrientes. The source of the specimens examined is detailed in the ‘Specimens investigated’ list, where the taxa are arranged alphabetically.
The pollen grains were acetolysed according to the methodology suggested by Erdtman (Citation1966). For light microscopy, the pollen samples were mounted in glycerine-jelly on glass slides and then examined with a Zeiss Axioplan microscope. Permanent slides were deposited at the Palynological Laboratory of the Universidad Nacional del Nordeste (PAL-CTES). The different pollen measurements such as polar axis (P), equatorial diameter (E), exine thickness, lacunae diameter, and spine length were measured from at least of 50 grains per sample.
For scanning electron microscopy, acetolysed pollen grains were first washed in 96%, and later in 100% ethanol. Grains were sputter-coated with gold-palladium and observed using a JEOL 5800 LV scanning electron microscope. The terminology proposed by Erdtman (Citation1966), Keeley and Jones (Citation1979) and Punt et al. Citation(2007) was used to describe pollen structure. Final classification of the different pollen types was determined over the basis of SEM observations .
Table I. List of examined species of Lessingianthus. P – polar axis, E – equatorial diameter, P/E – ratio; P, E, exine thickness, spinae length, and lacunae diameter – all in μm
A basic matrix based on eight pollen characters () was formed with the different average values of each species (), which have been considered as operative taxonomic units (OTUs). The qualitative variable (H: Aperture type) does not constitute average, but represents codes for the character states observed in each species. This was transformed in “dummy” variable by transforming the possible states of each variable into double state variables (presence/absence).
Table II. List of palynological characters used in the statistical analysis of Lessingianthus species
Table III. Data matrix showing the values found for the 8 characters (columns) in the 111 species (rows) of Lessingianthus included in this study. (Characters designated according to Table II)
Additionally, to evaluate the contribution of each pollen parameter to the affiliation of species, the data was subjected to a principal component analysis (PCA) based on the data matrix mentioned above. In order to establish the degree of statistical significance of the variable among the species, a multivariate analysis of the variance (MANOVA) was carried out using three variables (polar axis, equatorial diameter and number of lacunae). The analysis was carried out with the Infostat software, version 2008.
Results
The pollen grains of a total of 111 species belonging to the genus Lessingianthus were analysed ().
General pollen morphology
The pollen grains are radially symmetric, oblate-spheroidal, spheroidal or prolate-spheroidal (P/E = 0.93–1.04), 3-colporate or 3-porate, echinolophate or rarely psilolophate. Colpi long, with the apices visible in polar view. Pollen size: P = 40.8 (55.4) 74.8 μm, E = 38.0 (54.7) 73.4 μm. Exine thickness, excluding the spines, ranging from 3.4 to 13.6 μm. Tectum discontinuous, comprising lacunae surrounded by lophae, outline of lacunae more or less regular; total number of lacunae 30, or less commonly 27. Tectum surface densely microperforate, commonly spinose; spines with a linear distribution along the ridges of the lophae.
Type “B” (–C and –D)
Pollen grains are radially symmetric, oblate-spheroidal, spheroidal or prolate-spheroidal (P/E = 0.93–1.04), 3-colporate, echinolophate. Colpi long, with apices visible in polar view. Pollen size P = 40.8 (55.4) 74.8 μm, E = 38.0 (54.7) 73.4 μm. Exine thickness, excluding the spines ranging from 3.4 to 14.9 μm; L. spicatus have the lowest average (4.4 μm), while the highest value was observed in L. zuccarinianus with a mean of 12.7 μm. Tectum discontinuous. Total number of lacunae 30: three poral, six abporal, three equatorial (central mesocolpium), 12 paraporal and six interporal, polar lacunae absent. Tectum surface densely microperforate and spinose; the spine length ranges from 1.3 to 5.4 μm and the diameter ranges between 0.68 and 1.38 μm; spines apex acute, with the exception of L. farinosus which has a spine diameter smaller than the others species, i.e. 0.50–0.86 μm.
Species included: almost all the species of the genus, excluding the taxa with type “D” or modified “B” types pollen grains described below.
Type “B-1” (–F and , F)
The pollen grains are radially symmetric, oblate-spheroidal, spheroidal or prolate-spheroidal (P/E = 0.95–1.04), 3-porate, showing lophae across the colpi above and below the pores, separating the poral from the abporal lacunae, echinolophate. The colpi are long, with apices visible in polar view. Pollen size: P = 47.6 (53.7) 63.9 μm, E = 47.6 (53.7) 65.2 μm. Exine thickness, excluding the spines ranging from 5.4 to 8.1 μm. Tectum discontinuous, outline of lacunae more or less regular. Total number of lacunae 27: three poral, six abporal, 12 paraporal and six interporal; polar and equatorial lacunae absent. Tectum surface densely microperforate, spinose. Spines 2.0–5.4 μm long, 1.2–1.61 μm in diameter, acute at the apices.
Species included: Lessingianthus brevifolius, L. carvalhoi, L. durus, L. regis and L. soderstroemii.
Type “B-2” (–I and , B)
Pollen grains oblate-spheroidal (P/E = 0.99), 3-porate, showing lophae across the colpi above and below the pores, separating the poral of the abporal lacunae, echinolophate. Colpi long, with the apices visible in polar view. Pollen size: P = 46.2 (48.5) 50.3 μm, E = 46.2 (48.7) 50.3 μm. Exine thickness, excluding the spines ranging from 5.4 to 6.8 μm. Tectum discontinuous, comprising lacunae surrounded by lophae, outline of lacunae more or less regular. Total number of lacunae 30: three poral, six abporal, three equatorial (central mesocolpium), 12 paraporal and six interporal, polar lacunae absent. Tectum surface densely microperforate, spinose. Spines: 2.0–3.4 μm long, 1.15–1.30 μm in diameter, acute at the apex.
Species included: Lessingianthus santosii.
Type “B-3” (–L and , D)
Pollen grains spheroidal (P/E = 1.00), 3-colporate and psilolophate. Colpi are long with the apices visible in polar view. Pollen size: P = 63.9 (66.9) 69.3 μm, E = 63.9 (66.7) 68.0 μm. Exine thickness, excluding the spines ranging from 9.5 to 13.6 μm. Tectum discontinuous. Total number of lacunae 30: three poral, six abporal, three equatorial (central mesocolpium), 12 paraporal and six interporal, polar lacunae absent. Tectum surface densely microperforate, lacking spines.
Species included: Lessingianthus chamaepeuces.
Type “D” (–O and E, F)
Pollen grains spheroidal (P/E = 1.00), 3-colporate and echinolophate. Colpi are long with the apices visible in polar view; the colpi interrupt the lophae that separate the poral lacunae from the abporal lacunae. Pollen size: P = 45.7 μm, E = 45.6 μm. Exine thickness, excluding spines, 5.4–8.1 μm. Tectum discontinuous, comprising lacunae surrounded by lophae, outline of lacunae more or less regular. Total number of lacunae 27: three poral, six abporal, 12 paraporal and six interporal; polar and equatorial lacunae absent. Tectum surface densely microperforate and spinose; spines, 1.3–2.7 μm long, 0.64–0.71 μm in diameter, acute at the apex.
Figure 1. Pollen types of Lessingianthus (LM). A– C. Pollen type “B”, L. argyrophyllus: A. Polar view; B. Equatorial view, mesocolpium; C. Equatorial view showing aperture. D– F. Pollen type “B-1”, L. soderstroemii: D. Polar view; E. Equatorial view, mesocolpium; F. Equatorial view, aperture, high focus. G– I. Pollen type “B-2”, L. santosii: G. Polar view; H. Equatorial view; I. Equatorial view with aperture, high focus. J– L. Pollen type “B-3”, L. chamaepeuces: J. Polar view; K. Equatorial view; L. Equatorial view, aperture. M– O. Pollen type “D”, L. cristalinae: M. Polar view; N. Equatorial view; O. Equatorial view, aperture. Scale bars – 10 μm.
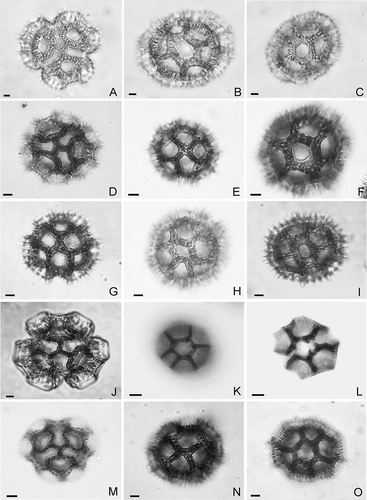
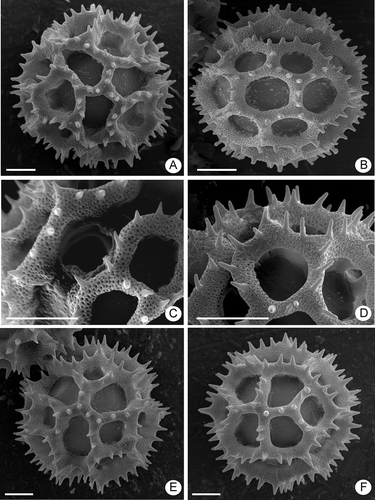
Figure 2. Pollen grains of Lessingianthus (SEM). A– D. Pollen type “B”: A. L. vepretorum, polar view showing the colpi and the absence of polar lacuna; B. L. ibitipocensis, equatorial view, mesocolpium showing the equatorial lacunae; C. L. sellowii, close up of the colpus area with pore; D. L. farinosus, detail of spinae. E–F. Pollen type “B-1”, L. durus: E. Polar view showing the colpi apices; F. Mesocolpium showing the absence of equatorial lacunae. Scale bars – 10 μm.
Species included: Lessingianthus cristalinae.
Statistical analysis
The results of PCA indicate that the first two components could explain 62.5% of the available variation (). The bidimensional projection of the axes of the two first components (39.0% and 23.5% of the total of variation, respectively) is observed in . The variables that contribute more to the first principal component are the polar/equatorial diameter and length of lacunae, whereas those that contribute more with the second principal component are the aperture type and the number of lacunae, but the latter operate negatively.
Table IV. Contribution of the variables to Axes 1 and 2 (see )
Figure 4. Diagram of principal component analysis ordination according to the Axes 1 and 2 of the studies species of Lessingianthus.
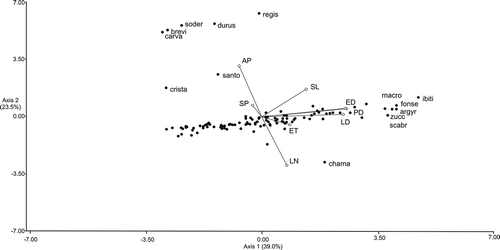
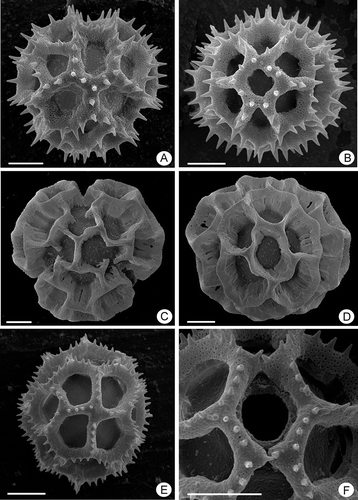
Figure 3. Pollen grains of Lessingianthus (SEM). A–B. Type “B-2” grains, L. santosii: A. Polar view showing the colpus absence; B. Equatorial view, mesocolpium. C–D. Pollen type “B-3”, L. chamaepeuces, grains lacking spinae: C. Polar view; D. Equatorial view, mesocolpium with the equatorial lacuna in the centre. E–F. Pollen type “D”, L. cristalinae: E. Equatorial view, mesocolpium; F. Detail with pore and lophae across the colpus. Scale bars – 10 μm.
The results of the multivariate variance analysis (MANOVA) indicate that the observed differences among species are significant (WILKS' π = 0.0011; F = 1141475.74, p = <0.0001).
Discussion
The genus Lessingianthus was established within the segregation of different Vernonia sections and subsections to new genera (Robinson, Citation1988a ). This new classification was based on different features including vegetative morphology, chromosome number and chemical composition. However, one of the most important features considered was the variation in pollen morphology of the different groups. The pollen type of the genus Lessingianthus was initially described by Stix (Citation1960) as Vernonia argyrophylla-type, and later called type “B” by Keeley & Jones (Citation1979).
In the present study, a total of 111 entities of Lessingianthus have been analysed, including the majority of species of the genus. Most of these entities have not been previously considered in taxonomic treatments of the Vernonieae. Almost all species of Lessingianthus have pollen of type “B”, including the type species of the genus, L. rubricaulis (Homb. & Bonpl.) H. Rob. Only one taxon had type “D” grains. Within the species with type “B” grains some variations in aperture shape, number of lacunae and presence or absence of spines were also found. These variations allowed the acceptance of modified “B-types” of pollen grains.
Five species had triporate pollen grains with a total of 27 lacunae, lacking equatorial lacuna (type “B-1”). A previous analysis carried out by Jones (Citation1979) determined that L. brevifolius and L. durus present type “D” pollen. On the other hand, Robinson (Citation1988a ) described the grains in equatorial view of both taxa as modified “B”-type pollen that has lophae across the colpi above and below the pores. Our results are clearly in agreement with those obtained by Robinson (Citation1988a ).
Lessingianthus santosii was the single species with triporate pollen that also had equatorial lacuna, having a total number of 30 lacunae, in concordance with the species that present the typical “B” pollen grains.
The pollen morphology described for Lessingianthus chamaepeuces differs from a previous study that recorded type “A” pollen for this species (Jones, Citation1979). The psilolophate grains have never been observed before in Lessingianthus or other taxonomical groups having type “B” pollen grains. In general, the psilolophate pollen grains are not frequent in the tribe Vernonieae. The remaining psilolophate palynomorph, called type “E”, has been found in some few species of the New World such as Pacourina edulis Aubl. (Robinson, Citation1999; Wortley et al., Citation2007), Acilepidopsis echitifolia (Mart. ex DC.) H. Rob. (Dematteis & Robinson, Citation1997), Mesanthophora rojasii (Cabrera) H. Rob. (Dematteis & Salgado, Citation2001), M. brunneri H. Rob. (Robinson, Citation1992b ) and Telmatophila scolymastrum Mart. ex Baker (Keeley & Robinson, Citation2009). This pollen, in comparison to the one found in Lessingianthus (type “B-3”), is triporate with lacunae of similar size, without a perforated tectum and spines (Dematteis & Robinson, Citation1997; Dematteis & Pire, Citation2008). The echinate pollen seems to be the primitive condition in the family, while the lophate pollen would be derived from it (Wodehouse, Citation1928).
Only in L. cristalinae was the type “D” pollen observed. It is tricolporate and echinolophate, but differs from the “B” pollen, because the abporal and poral lacunae are not completely separated by lophae. This pollen type is restricted to New World species and it is specially represented in some taxa of Lepidaploa, a genus closely related to Lessingianthus (Robinson, Citation1988a ; Bolick & Keeley, Citation1994). Although L. cristalinae shows almost all the typical morphological features of Lessingianthus, a possible relationship with some species of Lepidaploa with pollen type “D” should be analysed.
The genus Lessingianthus tends to have larger pollen grains than the other members of the tribe. The cytological information indicates that the genus includes the greatest proportion of polyploid species and the highest ploidy levels in the Vernonieae tribe (Dematteis, Citation2002). However, in most cases the pollen size is not particularly correlated with the ploidy level of the species. For example, L. plantaginoides that is tetraploid with 2n=64 chromosomes (Dematteis & Fernández, Citation2000) has a polar diameter of 48.9–53.0 μm, while L. brevifolius is diploid, having 2n=32 chromosomes (Angulo & Dematteis, Citation2009), and presents a polar axis of 50.3–53.0 μm.
The principal component analysis (PCA) ordination of the Lessingianthus species studied () demonstrates a grouping by correspondence to pollen type. The greater cluster consists of taxa with type “B” pollen. The well defined subgroup containing six species (L. argyrophyllus, L. fonsecae, L. ibitipocensis, L. macrocephalus, L. scabrifoliatus and L. zuccarinianus) with the highest polar axis and equatorial diameter values is distinguished within. The five entities having type “B-1” pollen, that have triporate grains without equatorial lacunae, compose a solid group clearly separated from the remaining taxa. Due to presence of triporate pollen with equatorial lacunae, L. santosii (type “B-2”) is isolated from other entities. Lessingianthus chamaepeuces, with type “B-3” pollen, is separated from the species with echinolophate pollen. Likewise, L. cristalinae with pollen type “D” results separated from all the species having type “B” or modified type “B” grains.
Conclusions
The pollen type “B” that characterises the genus Lessingianthus is present in almost all of the analysed species. However, there have been observed some variations of the pollen morphology in different species, particularly in the total number of lacunae and the presence or absence of spines. From the statistical analysis can we conclude that the species of the genus may form definite groups based on characteristics of their pollen grains. The results obtained support the idea that pollen morphology is significant in the delimitation at specific and generic level into the tribe Vernonieae.
Specimens investigated
-
Lessingianthus adenophyllus (Mart. ex DC.) H. Rob. BRAZIL, Minas Gerais: Diamantina, road to Conselheiro Mata, Km 192. Menezes et al. 11878 (CTES).
-
L. ammophyllus (Gardner) H. Rob. BRAZIL, Minas Gerais: Jaboticatubas, Tres Barras, 50 km N of Lagoa Santa. Smith et al. 103262 (CTES).
-
L. arachniolepis (Ekman & Dusen) H. Rob. BRAZIL, Paraná: Município Curitiba, National Park Iguaçu. Barbosa & Costa 1025 (CTES).
-
L. arctatus Dematt. BRAZIL, Goiás: route GO-118 km 174, N of Alto Paraíso, Fazenda Agua Fria. Magenta et al. 266 (CTES).
-
L. argenteus (Less.) H. Rob. BRAZIL, Paraná: Município Jaguariaíva, Rios das Mortes. Barbosa & Silva 2112 (CTES).
-
L. argyrophyllus (Less.) H. Rob. BRAZIL, Distrito Federal: Brasília, National Park. Krapovickas et al. 31158 (CTES).
-
L. asteriflorus (Mart ex DC.) H. Rob. BRAZIL, Paraná: Município Ponta Grossa, Itaia Coca. Silva et al. 6099 (CTES).
-
L. bardanoides (Less.) H. Rob. BRAZIL, Mato Grosso do Sul: Ponta Porá, 22 km W by road to Antonio João. Hatschbach et al. 58736 (CTES).
-
L. barrosoanus Dematt. BRAZIL, Bahía: Municipio Chapada Diamantina, Andaraí, road to Patí. Guedes et al. s. n. (SPF).
-
L. bishopii (H. Rob.) H. Rob. BRAZIL, Goiás: s. l. Saint Hilaire 487 (P).
-
L. brevifolius (Less.) H. Rob. ARGENTINA, Entre Ríos: Dept. Colon, National Park El Palmar, Ayo. El Palmar. Cocucci et al. 2982 (CTES). Corrientes: Dept. San Cosme, Paso de la Patria. Tressens 113 (CTES). PARAGUAY, Dept. Alto Paraná: Est. Santa Elena, 12 km NE of Hernandarias. Schinini et al. 28196 (CTES).
-
L. brevipetiolatus (Sch. Bip. ex Baker) H. Rob. BRAZIL, Minas Gerais: Betim. Roth 1615 (CESJ).
-
L. buddleiifolius (Mart. ex DC.) H. Rob. BRAZIL, Goiás: 6 km E de Morrinhos, road to Caldas Novas. Krapovickas et al. 33270 (CTES).
-
L. bupleurifolius (DC.) H. Rob. BRAZIL, Mato Grosso: Santa Anna da Chapada. Malme 3361 (S).
-
L. carduoides (Baker) H. Rob. BRAZIL, Goiás: Bushy uplands between Arrayas and São Domingos. Gardner 4192 (P).
-
L. carvalhoi (H. Rob.) H. Rob. BRAZIL, Bahía: Guiné. Conceição785 (ALCB).
-
L. cataractarum (Hieron.) H. Rob. ARGENTINA, Misiones: Dept. Iguazú, Cataratas, Superior Circuit. Gatti 28 (CTES).
-
L. cephalotes (DC.) H. Rob. BRAZIL, Goiás: between Chapadinha and Guariraba. Glaziou 21577 (G).
-
L. chamaepeuces (Sch. Bip. ex Baker) H. Rob. BRAZIL, Mato Grosso: Serra da Chapada. Malme 3442 (S).
-
L. clavatus (Gardner) Dematt. BRAZIL, Minas Gerais: Corinto, Fazenda do Diamante. Mexia 5536 (P).
-
L. compactiflorus (Mart. ex Baker) H. Rob. BRAZIL, Goiás: Municipio Cristalina, Cubículo. Hatschbach 43705 (CTES).
-
L. constrictus (Matzenb. & Mafioleti) Dematt. BRAZIL, Río Grande do Sul: Torres, 4 km S of the access to Torres. Krapovickas & Cristóbal 44772 (CTES).
-
L. cordiger (Mart. ex DC.) H. Rob. BRAZIL, Minas Gerais: Município Joaquin Felício, Serra do Cabral. Hatschbach et al. 64821 (CTES).
-
L. coriaceus (Less.) H. Rob. BRAZIL, Minas Gerais: 53 km E de Araxá, River Quebra Anzol. Krapovickas et al. 33375 (CTES).
-
L. correntinus (Cabrera & Cristób.) Dematt. ARGENTINA, Corrientes: Dept. Curuzú Cuatiá. 8 km N of Curuzu Cuatia. Schinini & Ahumada 13895 (CTES).
-
L. cristalinae (H. Rob.) H. Rob. Brazil, Goiás: Chapada dos Veadeiros, Barroso 522 (UB).
-
L. declivium (Malme) Dematt. BRAZIL, Mato Grosso: Santa Anna da Chapada. Malme 2104 (R).
-
L. durus (Mart. ex DC.) H. Rob. BRAZIL, Goiás: Mun. Planaltina, 8–10 km N of border with Distrito Federal. Hatschbach et al. 59292 (CTES). Goiás: Mun. Água Fria, access to the Torre Repetidora de Roncador. Hatschbach et al. 70856 (CTES). BOLIVIA, Santa Cruz: 61 km E of San Javier on the road to Concepción. Dematteis et al. 2102 (CTES).
-
L. eitenii (H. Rob.) H. Rob. BRAZIL, Distrito Federal: Reserva Ecológica IBGE, river Taquara area. Fonseca & Alvarenga 2220 (RB).
-
L. elegans (Gardner) H. Rob. BRAZIL, Minas Gerais: Serra do Cipó, road Belo Horizonte- Mato Dentro. Souza et al. 10107 B (CTES).
-
L. exiguus (Cabrera) H. Rob. BRAZIL, Paraná: Vila Velha. Krapovickas & Cristóbal 40842 (CTES).
-
L. farinosus (Baker) H. Rob. BRAZIL, Bahía: Road Itaçu-Barra da Estiva, 8 km from Barra da Estiva. Giulietti et al. 1293 (CTES).
-
L. floccosus (Gardner) H. Rob. BRAZIL, Minas Gerais: Upland campos Serra das Araras. Gardner 4786 (G).
-
L. fonsecae (H. Rob.) H. Rob. BRAZIL, Goiás: Chapada dos Veadeiros, road GO-118, next to the Rio das Almas. Pirani 1835 (K).
-
L. glabratus (Less.) H. Rob. ARGENTINA, Corrientes: 12 km NE of San Miguel, Estancia Curupayty. Vanni et al. 1476 (CTES).
-
L. graminifolius (Gardner) Dematt. BRAZIL, Goiás: Dry grassy campos Villa de Arrayas. Gardner 3799 (G).
-
L. grandiflorus (Less.) H. Rob. PARAGUAY, Amambay: Chirigüelo, ca. 550 m.s.m. Dematteis & Schinini 859 (CTES).
-
L. grearii (H. Rob.) H. Rob. BRAZIL, Goiás: National Park Chapada dos Veadeiros, road to Corredeiras. Paula-Souza et al. 4563 (CTES).
-
L. hasslerianus (Chodat) Dematt. PARAGUAY, Itapúa: Capitan Meza. Montes 7161 (CTES).
-
L. hovaefolius (Gardn.) H. Rob. BRAZIL, Goiás: Municipio Catalão, 233 km, border Catalão-Campo Alegre. Hatschbach et al. 70576 (CTES).
-
L. hypochaeris (DC) H. Rob. BRAZIL, Paraná: Municipio Porto Amazonas, Recanto dos Papagaios. Silva & Ribas 3491 (CTES).
-
L. hystricosus (Cabrera & Dematt.) Dematt. PARAGUAY, Amambay: 34 km S of Bella Vista, River Negla and Route 3. Schinini et al. 30446 (CTES).
-
L. ibitipocensis Borges & Dematt. BRAZIL. Minas Gerais: Serra do Ibitipoca, Saint Hilaire 440 (P).
-
L. intermedius (DC.) Dematt. URUGUAY, Montevideo. Sellow s. n. (BR).
-
L. irwinii (G.M. Barroso) H. Rob. BRAZIL, Goiás: Santa Luzia. Glaziou s. n. (G).
-
L. ixiamensis (Rusby) H. Rob. BOLIVIA, Beni: Prov. Ballivián, San Borja. Krapovickas & Schinini 34882 (CTES).
-
L. kuntzei (Hieron.) Dematt. BOLIVIA, Santa Cruz: Vallegrande, between El Zapallar and Pujio, 15 km S of Pucará. Vargas 880 (CTES).
-
L. lacunosus (Mar. ex DC.) H. Rob. BRAZIL, Minas Gerais: s. l. Riedel 81 (G).
-
L. laevigatus (Mar. ex DC.) H. Rob. BRAZIL, Minas Gerais: Municipio Joaquin Felício, Serra do Cabral. Hatschbach et al. 64730 (CTES).
-
L. lanatus (Cabrera) Dematt. PARAGUAY, Dept. Caaguazú: river Yhú, 12 km S of Yhú. Schinini et al. 36145 (CTES).
-
L. laniferus (Cristobal & Dematt.) Angulo ARGENTINA, Misiones: Dept. General Manuel Belgrano, Campina de Américo. Keller 3663 (CTES).
-
L. lanuginosus Dematt. BRAZIL, Goiás: Alto Paraíso, route to the water treatment station. Paula-Souza et al. 4455 (ESA).
-
L. lapinhensis Dematt. BRAZIL, Minas Gerais: Serra do Cipó, próximo da localidade da Lapinha. Pirani et al. 12129 (CTES).
-
L. laurifolius (DC.) H. Rob. BOLIVIA, La Paz: Prov. Sud Yungas, Huancané, 9.5 km to San Isidro. Beck 19805 (CTES).
-
L. liguliifolius (Mart. ex DC.) H. Rob. BRAZIL, Goiás: Route GO-118, 16 km of São Gabriel. Hatschbach et al. 59996 (CTES).
-
L. linearifolius (Less.) H. Rob. BRAZIL. Minas Gerais: Serra de Ibitipoca. Sucre & Krieger 6850 (CTES).
-
L. linearis (Spreng.) H. Rob. BRAZIL, Minas Gerais: Municipio Santana do Riacho, Serra do Cipó. Simão-Bianchini 11488 (CTES).
-
L. longicuspis Dematt. BOLIVIA, Santa Cruz: San Ignacio. Dematteis et al. 2186 (CTES).
-
L. lorentzii (Hieron.) H. Rob. ARGENTINA, Entre Ríos: Dept. Concordia. Federal. Martinez Crovetto 4818 (CTES).
-
L. macrocephalus (Less.) H. Rob. URUGUAY, Rivera: Cuñapirú. Pedersen 11648 (CTES).
-
L. mansoanus (Baker) H. Rob. BRAZIL, Mato Grosso: Fazenda Nueva Era. Souza 16307 (ESA).
-
L. mollissimus (D. Don ex Hook & Arn.) H. Rob. ARGENTINA, Misiones: 23 km SE de San Javier. Krapovickas & Cristóbal 28841 (CTES).
-
L. morii (H. Rob.) H. Rob. BRAZIL, Bahia: Municipio Brotas de Macaúbas, 5–10 km of the BR-242. Hatschbach et al. 67666 (CTES).
-
L. myrsinites H. Rob. BRAZIL, Goiás: Municipio de Água Fria, Estação Repetidora de Roncador. Hatschbach et al. 60128 (CTES).
-
L. niederleinii (Hieron.) H. Rob. BRAZIL, Mato Grosso do Sul: Municipio Jardín, Boqueirão. Hatschbach et al. 77097 (CTES).
-
L. obscurus (Less.) H. Rob. BRAZIL, Goiás: Villa de Arrayas. Gardner 3791 (G).
-
L. obtusatus (Less.) H. Rob. BRAZIL, Goiás: Municipio de Água Fría, Estação Repetidora de Roncador. Hatschbach et al. 60116 (CTES).
-
L. onopordioides (Baker) H. Rob. BRAZIL, Minas Gerais: Lagoa Santa. Warming 2595 (G).
-
L. parvifolius (Chodat) H. Rob. PARAGUAY, Paraguari: Colonia Achoteí, Estancia Ypoá. Mereles et al. 8453 (CTES).
-
L. paulensis Dematt. BRAZIL, São Paulo: campos da Bocaina. Glaziou 14985 (P).
-
L. plantaginoides (Kuntze) H. Rob. ARGENTINA, Entre Rios: Dept. Concordia. Park Rivadavia. Krapovickas & Cristóbal 46566 (CTES).
-
L. platyphyllus (Chodat) H. Rob. PARAGUAY, Amambay: 40 km N of P. J. Caballero, on the road to Estrella. Dematteis & Schinini 865 (CTES).
-
L. polyphyllus (Sch. Bip. ex Baker) H. Rob. ARGENTINA, Misiones: Dept. San Ignacio, road to Provincial Park Teyú Cuaré. Dematteis et al. 2752 (CTES).
-
L. profusus (Dematt. & Cabrera) Angulo. BRAZIL, Mato Grosso do Sul: Ponte do Grego, 48 km N of Terenos. Krapovickas & Cristóbal 34490 (CTES).
-
L. pseudoincanus (Hieron.) Dematt. ARGENTINA, Corrientes: Dept. Bella Vista, 10 km S of Bella Vista, Arroyo Toropí. Angulo 9 (CTES).
-
L. pseudopiptocarphus (H. Rob.) H. Rob. BRAZIL, Goiás: estrada Niquelandia-Colinas, 32.5 km de Niquelandia. Machado Teles et al. 1906 (RB).
-
L. psilophyllus (Sch. Bip. ex Baker) H. Rob. BRAZIL, Minas Gerais: Municipio Joaquin Felicio, road to Serra do Cabral. Hatschbach et al. 64716 (CTES).
-
L. pumillus (Vell.) H. Rob. BRAZIL, Paraná: Mun. Ipiranga, Río Capivari. Hatschbach 25932 (CTES).
-
L. pusillus (Dematt.) Angulo ARGENTINA, Corrientes: Dept. Capital, Perichón, on the road to the river. Dematteis et al. 2769 (CTES).
-
L. pycnostachyus (DC.) H. Rob. BRAZIL, Minas Gerais: Jequetinhonha, 47 km de Pedra Azul, Paula-Souza et al. 5616 (CTES).
-
L. regis (H. Rob.) H. Rob. BRAZIL, Minas Gerais: Serra do Cipó, ca.3–4 km da ponte sobre o Rio Capibara. Zappi et al. 10301 (CTES).
-
L. reitzianus (Cabrera) H. Rob. BRAZIL, Paraná: Serra do São Luis, route BR-277. Ferrucci et al. 236 (CTES).
-
L. rigescens (Malme) Dematt. BRAZIL, Mato Grosso: Santa Anna da Chapada, Malme 1462 (S).
-
L. robustus (Rusby) H. Rob. BRAZIL, Mato Grosso: Cuyabá. Riedel 1474 (P).
-
L. roseus (Mart. ex DC.) H. Rob. BRAZIL, Minas Gerais: Cachoeira do Campo. Damazio 1465 (RB).
-
L. rosmarinifolius (Less.) H. Rob. BRAZIL, Minas Gerais: 10–20 km NE of C. Mota road to Conceição do Mato Dentro. Arbo et al. 4170 (CTES).
-
L. rubricaulis (Humb. & Bonpl.) H. Rob. ARGENTINA, Misiones: Dept. Capital, Posadas, Arroyo Zaimán. Dematteis & Surenciski 2452 (CTES).
-
L. saltensis (Hieron.) H. Rob. ARGENTINA, Salta: Dept. Guachipas, National Route 68, 1 km S of La Viña. Pozner & Belgrano 454 (CTES).
-
L. sancti-pauli (Hieron.) Dematt. BRAZIL, Paraná: Mun. Jaguariaíva. Parque Estadual do Cerrado. Von Lisingen & Sonehara 115 (CTES).
-
L. santosii (H. Rob.) H. Rob. BRAZIL, Minas Gerais: 15 km of Diamantina, on the road to Milho Verde. Arbo et al. 5133 (CTES). Bahía: Route Itaçu-Barra da Estiva, 84 km of Barra da Estiva. Morro do Ouro. Giulietti 1275 (CTES).
-
L. scabrifoliatus (Hieron.) H. Rob. BOLIVIA, Santa Cruz: 5.4 km de Concepción on road to San Antonio de Lomería. Seijo 3215 (CTES).
-
L. secundus (Sch. Bip. ex Baker) H. Rob. BRAZIL, Goiás: campiment du Córrego do Brejo. Glaziou 21634 (R).
-
L. sellowii (Less.) H. Rob. ARGENTINA, Misiones: on the road to Profundidad, 5 km of Route 12. Dematteis & Solis Neffa 508 (CTES).
-
L. soderstroemii (H. Rob.) H. Rob. BRAZIL, Minas Gerais: Municipio Santana do Riacho, Serra do Cipó, Souza et al. 25168 (ESA). Goiás: route GO-118, 10 km S of São João da Aliança. Hatschbach et al. 54521 (CTES). Goiás: Teresina, on the road to Alto Paraíso, Chapada dos Veadeiros. Hatschbach et al. 58357 (CTES).
-
L. souzae (H. Rob.) H. Rob. BRAZIL, Goiás: Cabeceiras do Rio Sant'Anna prés du Pouso Alto. Glaziou 21632 (BR)
-
L. spicatus (Cabrera) Dematt. ARGENTINA, Misiones: Dept. San Ignacio, La Plantadora. Schwartz 5491 (CTES).
-
L. stoechas (Mart. ex DC.) H. Rob. BRAZIL, Goiás: Chapada dos Veadeiros, Vargem Grande. Glaziou 21604 (BR).
-
L. subcarduoides (H. Rob.) H. Rob. BRAZIL, Minas Gerais: Serra do Espinhaço, 3 km N of São João da Chapada. Irwin et al. 28237 (RB).
-
L. subobscurus (Malme) H. Rob. BOLIVIA, Santa Cruz: 80 km E de Concepción, road to San Ignacio. Seijo & Solis Neffa 3248 (CTES).
-
L. syncephalus (Sch. Bip. ex Baker) H. Rob. BRAZIL, Minas Gerais: Serra do Caminho do Tapeiva. Saint Hilaire B1 N° 935 (P).
-
L. teyucuarensis (Cabrera) Dematt. ARGENTINA, Misiones: Dept. San Ignacio, house of Horacio Quiroga. Dematteis et al. 515 (CTES).
-
L. tomentellus (Mart. ex DC.) H. Rob. BRAZIL, Minas Gerais: São Gonçalo do Abaeté, 40 km S of road BR-040. Hatschbach et al. 64579 (CTES).
-
L. ulei (Hieron.) H. Rob. BRAZIL, Paraná: Municipio Ponta Grossa, Fazenda Santana. Ribas et al. 5060 (CTES).
-
L. varroniifolius (DC.) H. Rob. BOLIVIA, Santa Cruz: 10 km E of San Javier, on the road to Concepción. Dematteis et al. 2076 (CTES).
-
L. vepretorum (Mart. ex DC.) H. Rob. BRAZIL, Minas Gerais: s. l. Wedell 1073 (G).
-
L. vestitus (Baker) H. Rob. BRAZIL, Minas Gerais: s. l. Claussen s. n. (P).
-
L. warmingianus (Baker) H. Rob. BRAZIL, Minas Gerais: Santa Luzia do Rio das Velhas. Glaziou 20383 (R).
-
L. westermanii (Ekman & Dusén) H. Rob. BRAZIL, Paraná: Jaguariahyva. Dusén 16400 (G).
-
L. xanthophyllus (Mart. ex DC.) H. Rob. BRAZIL, Tocantins: Taguatinga, road to the district of L. E. Magalhães. Paula-Souza et al. 4763 (CTES).
-
L. zuccarinianus (Mart. ex DC.) H. Rob. BRAZIL, Goiás: s. l. Weddell 2060 (P).
Acknowledgements
This work has been supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretaría General de Ciencia y Técnica of the Universidad Nacional del Nordeste, which are greatly appreciated.
Notes
References
- Angulo , M. B. and Dematteis , M. 2009 . Caryological analysis of South American species of Vernonia (Vernonieae, Asteraceae) . Plant Biosyst. , 142 : 20 – 24 .
- Baker , J. G. 1873 . “ Compositae I. Vernoniaceae ” . In Flora brasiliensis , Edited by: Martius , C. 1 – 179 . Leipzig : Fleischer & Co . Vol. 6 Part 2
- Bolick , M. R. and Keeley , S. C. 1994 . Pollen morphology and classification of the Vernonieae (Compositae) . Acta Bot. Gall. , 141 : 279 – 284 .
- Bremer , K. 1994 . Asteraceae. Cladistics and classification , Portland : Timber Press .
- Dematteis , M. 2002 . Cytotaxonomic analysis of South American species of Vernonia (Vernonieae: Asteraceae) . Bot. J. Linn. Soc. , 139 : 401 – 408 .
- Dematteis , M. 2006 . Two new species of Lessingianthus (Vernonieae, Asteraceae) from the Brazilian highlands . Bot. J. Linn. Soc. , 150 : 487 – 493 .
- Dematteis , M. 2007 . Taxonomic notes on the genus Chrysolaena (Vernonieae, Asteraceae), including a new species endemic to Paraguay . Ann. Bot. Fenn. , 44 : 56 – 64 .
- Dematteis , M. 2009 . Revisión taxonómica del género sudamericano Chrysolaena (Vernonieae, Asteraceae) . Bol. Soc. Argent. Bot. , 44 : 103 – 170 .
- Dematteis , M. and Fernández , A. 2000 . Chromosome studies on nine South American species of Vernonia (Vernonieae, Asteraceae) . Caryologia , 53 : 55 – 61 .
- Dematteis , M. and Pire , S. M. 2008 . Pollen morphology of some species of Vernonia s. l. (Vernonieae, Asteraceae) from Argentina and Paraguay . Grana , 47 : 117 – 129 .
- Dematteis , M. and Robinson , H. 1997 . Chromosome studies and taxonomic considerations in Acilepidopsis (Vernonieae, Asteraceae) . Phytologia , 83 : 366 – 370 .
- Dematteis , M. and Salgado , C. R. 2001 . Pollen morphology and chromosome number of Vernonia rojasii (Vernonieae, Asteraceae) . Comp. Newsl. , 36 : 69 – 76 .
- Erdtman , G. 1966 . Pollen morphology and plant taxonomy. Angiosperms , New York : Hafner Publ. Co .
- Jones , S. B. 1979 . Synopsis and pollen morphology of Vernonia (Compositae: Vernonieae) in the New World . Rhodora , 81 : 425 – 447 .
- Jones , S. B. 1981 . Synoptic classification and pollen morphology of Vernonia (Compositae: Vernonieae) in the Old World . Rhodora , 83 : 59 – 75 .
- Keeley , S. C. and Jones , S. B. 1977 . Taxonomic implications from of external pollen morphology to Vernonia (Compositae) in the West Indies . Am. J. Bot. , 64 : 576 – 584 .
- Keeley , S. C. and Jones , S. B. 1979 . Distribution of the pollen types in Vernonia (Vernonieae: Asteraceae) . Syst. Bot. , 4 : 195 – 202 .
- Keeley , S. C. and Robinson , H. 2009 . “ Vernonieae ” . In Systematics, evolution and biogeography of Compositae , Edited by: Funk , V. A. , Susanna , A. , Stuessy , T. F. and Bayer , R. J. 439 – 469 . Wien : IAPT .
- Mendonça , C. B. F. , Carrijo , T. T. , Esteves , R. L. and Gonçalves-Esteves , V. in press . Lessingianthus H. Rob. (Vernonieae-Asteraceae): Generic and infrageneric relationships based on pollen morphology . Nord. J. Bot ,
- Punt, W., Hoen, P. P., Blackmore, S., Nilsson, S. & Le Thomas, A. (2007). Glossary of pollen and spore terminology. 2nd rev. ed. Utrecht: LPP Found. Univ. Utrecht. LPP Contrib. 1 (also available: 1' Rev. Palaeobot. Palynol. 143; 2'' http://www3.bio.uu.nl/palaeo/glossary/glos-int.htm
- Robinson , H. 1988a . Studies in the Lepidaploa complex (Vernonieae: Asteraceae). IV. The new genus Lessingianthus . Proc. Biol. Soc. Wash. , 100 : 929 – 951 .
- Robinson , H. 1988b . Studies in the Lepidaploa complex (Vernonieae: Asteraceae). V. The new genus Chrysolaena . Proc. Biol. Soc. Wash. , 101 : 925 – 958 .
- Robinson , H. 1988c . Studies in the Lepidaploa complex (Vernonieae: Asteraceae). VI. A new genus Aynia . Proc. Biol. Soc. Wash. , 101 : 959 – 965 .
- Robinson , H. 1992a . The Asteraceae of the Guianas, III: Vernonieae and restoration of the genus Xiphochaeta . Rhodora , 94 : 348 – 361 .
- Robinson , H. 1992b . Mesanthophora, a new genus of Vernonieae (Asteraceae) from Paraguay . Novon , 2 : 169 – 172 .
- Robinson , H. 1993 . Three new genera of Vernonieae from South America, Dasyandantha, Dasyandanthina and Quechualia (Asteraceae) . Proc. Biol. Soc. Wash. , 106 : 775 – 785 .
- Robinson , H. 1999 . Generic and subtribal classification of American Vernonieae . Smithsonian Contrib. Bot. , 89 : 1 – 116 .
- Robinson , H. 2007 . “ Tribe Vernonieae ” . In The families and genera of vascular plants. Vol. 8. Asterales , Edited by: Kadereit , J. and Jeffrey , C. 165 – 192 . Berlin : Springer .
- Skvarla , J. J. , DeVore , M. L. and Chissoe , W. F. 2005 . Lophate sculpturing of Vernonieae (Compositae) pollen . Rev. Palaeobot. Palynol. , 133 : 51 – 68 .
- Stix , E. 1960 . Pollenmorphologische Untersuchungen an Compositen . Grana Palynol. , 2 : 41 – 104 .
- Wodehouse , R. P. 1928 . Phylogenetic value of pollen grain characters . Ann. Bot. , 168 : 891 – 934 .
- Wortley , A. H. , Funk , V. A. , Robinson , H. , Skvarla , J. J. and Blackmore , S. 2007 . A search for pollen morphological synapomorphies to classify rogue genera in Compositae (Asteraceae) . Rev. Palaeobot. Palynol. , 146 : 169 – 181 .