Abstract
Mature pollen usually has a conspicuously thick intine in the aperture regions, whereas in the interapertural area(s) the intine is thin. In Carex (and in other Cyperaceae with pseudomonads), usually five to six circular or elongated apertures (poroids) are present, one situated distally and the others equatorially. In the species studied here the situation is opposite: the apertures (poroids) are characterised by a very thin intine, whereas in the interapertural area(s) the intine is conspicuously thickened. A similar condition is only known for Vinca (Apocynaceae). A second unusual sporoderm feature in Carex relates to the foot layer. In the interapertural area(s) the foot layer is thin and discontinuous. The equatorial poroids exhibit a compact, thick and homogeneous foot layer, whereas the distal poroid is distinguished by an elaborated foot layer, structurally similar to a transfer-cell wall labyrinth. This unique elaborated foot layer in the distal poroid probably functions as a nutrimental passage between the tapetum and the maturing pollen. The pseudomonads are usually pear-shaped, sometimes spherical. Ornamenting elements are microechini, granules, and perforations, all sometimes placed on verrucae.
In Cyperaceae, only a single row of meiotic tetrads border the tapetal tissue (Carniel, Citation1962; Kirpes et al., Citation1996: “…a single, uniseriate cylinder of pollen grains…”). In species with pseudomonads, the meiotic products do not separate and form an almost unique permanent tetrad (Selling, Citation1947), sometimes called cryptotetrad (Erdtman, Citation1952). Only one microspore of the pear-shaped tetrad is functional and the other three abort: an example of programmed cell death (Furness, Citation2009). The degenerative microspores are packed tightly in the apex of the pyriform pseudomonad, i.e., the pointed portion, and their remnants are sealed by an extraordinarily thick intine (Furness & Rudall, Citation1999; Brown & Lemmon, Citation2000).
In order to describe the location of features (e.g., apertures), the pseudomonad (in fact, the hidden tetrad) with its single functional pollen grain is treated as one “pollen grain” (Van Wichelen et al., Citation1999). This pollen grain usually has c. five to six apertures (poroids), one at the distal pole (i.e., the basis of the pyriform pseudomonad), and four to five in the equatorial region. During development only the distal aperture (poroid) of the surviving functional monad is in contact with the tapetum cells, but not the equatorial ones. A poroid is defined as a circular or elliptic aperture with indistinct margin (Hesse et al., Citation2009).
The uncommon absence of a thick intine within the aperture of Carex pollen was already shown by light micrographs (Furness & Rudall, Citation1999, Citation2003). Pollen walls of Carex and other Cyperaceae have been the subject of only a few transmission electron microscopy (TEM) investigations (e.g., Nilsson et al., Citation1977; Furness & Rudall, Citation1999; Simpson et al., Citation2003). The absence of a thick apertural intine was also reported for Vinca rosea L. (Apocynaceae) by Cousin (Citation1979). Details of the aperture stratification, however, are poorly known.
In this paper, we present a detailed study on features of the distal and lateral poroids in combination with developmental aspects, and relate these findings to the current view of apertural functions.
Materials and methods
For TEM-investigations, mature anthers were fixed for 20 hours in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and after washing in buffer and distilled water, postfixed overnight in 2% osmium tetroxide (OsO4) and 0.8% potassium ferrocyanide (K4Fe(CN)6.3H2O) 2:1 at 4 °C (Weber, Citation1992). After fixation and washing in distilled water, the material was dehydrated in 2.2-dimethoxypropane (DMP) and acetone and embedded in Spurr's low-viscosity epoxy resin. Sections of about 70–90 nm were made on a Reichert-Jung Ultracut S microtome with a diamond knife and transferred to copper and gold grids. Ultrathin sections were post-stained either in 2% alcoholic UA-PB, or in KMnO4 for 30 minutes, or treated for detection of unsaturated lipids in osmium-fixed material according to a modified Thiéry reaction (Weber et al., Citation1998). Specimens were examined with a ZEISS EM-900 transmission electron microscope.
For light microscopy (LM) investigations, fresh, mature, partially germinating pollen was examined in water, and for scanning electron microscopy (SEM) investigations, mature pollen samples were dehydrated in 2.2-dimethoxypropane (DMP) and critical-point dried in acetone (Halbritter, Citation1998). All samples were sputter-coated with gold. Specimens were examined with a JEOL JSM 6390 scanning electron microscope.
Vouchers were deposited in the herbarium at the Faculty Centre of Biodiversity of Vienna University (WU). The pollen reference database is available at the Department of Palynology and Structural Botany, University of Vienna, Vienna, Austria.
Figure 1. A–F. (SEM). A, B. Carex remota: A. Pseudomonad, equatorial view; B. Sexine ornamentation microechinate and perforate. C, D. Carex flacca: C. Pseudomonads in hydrated state with different dimensions and profiles, note especially the variable shape of the narrow (proximal) apices; D. Poroid with circular outline. E, F. Carex montana: E. Pseudomonad, equatorial view, note the oval outline of the large equatorial poroid, whereas the inconspicuous poroid (arrow) is much smaller and circular; F. Pseudomonad, proximal polar view. Scale bars – 10 μm (A, D–F); 1 μm (B); 100 μm (C).
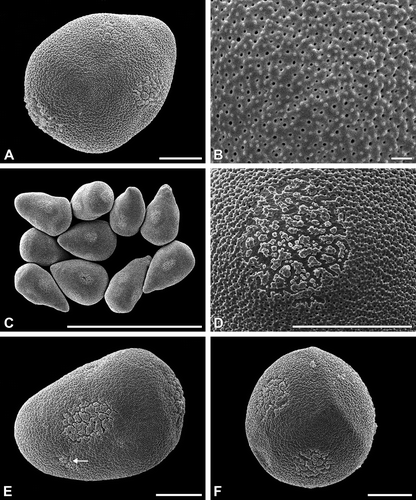
Figure 2. A–F. (SEM). A, B. Carex atrata: A. More or less spherical, verrucate pseudomonads; B. Sexine ornamentation verrucate and perforate. C, D. Carex alba: C. Poroid with elongated outline; D. Pseudomonads in dry state, note the sunken poroids. E, F. Carex nigra: E. Pseudomonads with six to seven poroids; F. Ubisch bodies adhering on the locular wall. Scale bars – 10 μm (A, C–E); 1 μm (B, F).
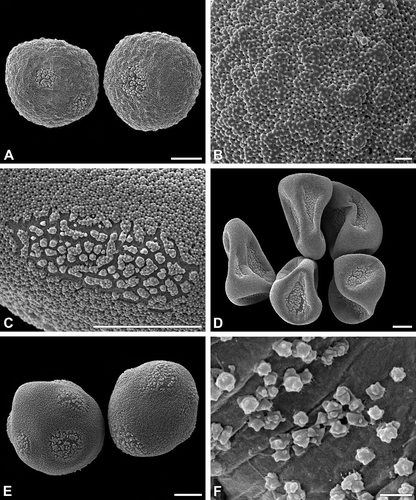
Figure 3. A–F. (SEM). A, B. Cyperus longus: A. Pseudomonad, equatorial view, note the poroids of different shape and size; B. Poroid with irregular outline. C, D. Schoenoplectus lacustris: C. Pseudomonads in dry condition, the distal poles are directed to the locular wall; D. Pseudomonads, equatorial view. E. Scirpus sylvaticus: Pseudomonads, equatorial view. F. Eleocharis palustris: Pseudomonad, equatorial view. Scale bars – 10 μm.
Results
Carex and other Cyperaceae with pseudomonads ( )
External morphology
The pseudomonads (in fact hidden tetrads) are in the hydrated/turgescent state usually more or less pear-shaped (, C, E, F, 2D, E, 3A, D–F). Some species show almost spherical pseudomonads (). The apex ( = proximal pole) of the pyriform pseudomonad includes the remnants of the three aborted microspores. Within one species the shape of the pseudomonads is usually more or less elongated or nearly spherical. Individual pseudomonads, however, may have ±flexuous or straight, pointed (elongated) or rounded apices (). The pseudomonads border the tapetum tissue only in a single row (, ).
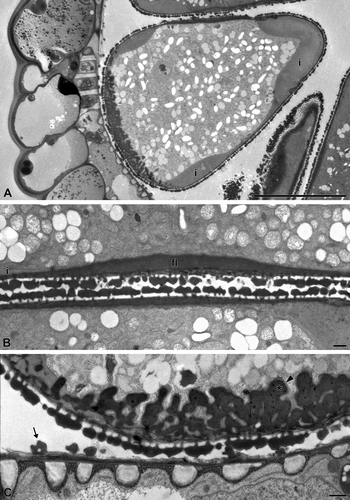
Figure 4. Carex acutiformis (TEM). Pollen sac in cross section. A single row of young pseudomonads (m) border with their distal face the tapetal cells (t). Scale bar – 10 μm.
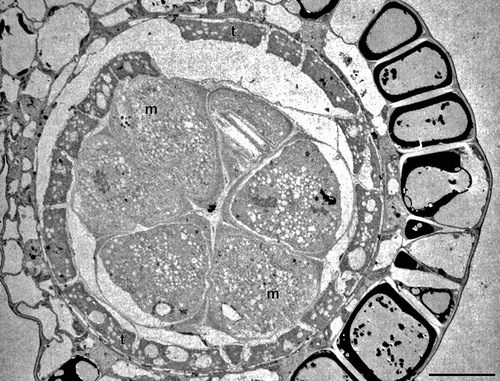
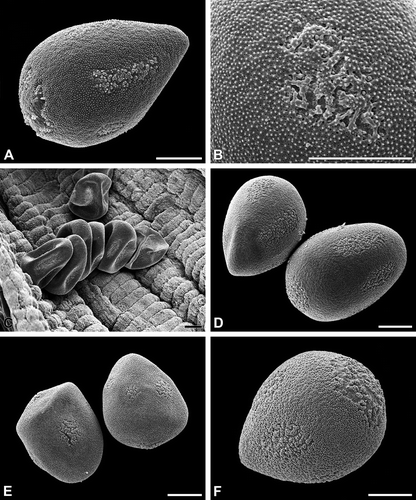
Figure 5. A–D. Carex flacca (A–C. LM; D. SEM): A. Pseudomonad in optical cross section. The apertural regions lack a thick intine. Note structured dense layer (arrow) at the distal pole; B. Pollen tube formed from the distal poroid; C. Pollen tube formed from an equatorial poroid; D. Pollen tube formed from the distal poroid. Note widely expanded poroid region. Scale bars – 10 μm.
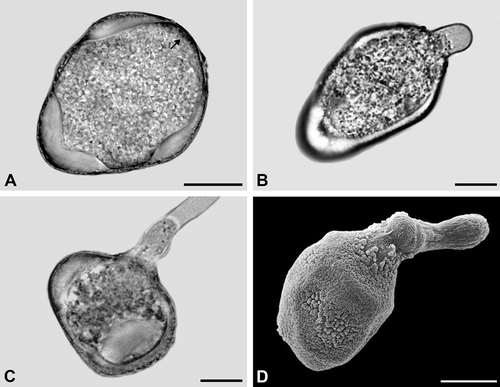
Ornamenting elements are small granules or microechini, and perforations are placed upon more or less indistinct verrucae (). Sometimes verrucae are more prominent (). Such an exine ornamentation may be called, e.g., verrucate, granulate and perforate, or verrucate, microechinate and perforate.
Pseudomonads have mostly five to six circular or elongated apertures (poroids) with indistinct margin: one aperture is situated at the distal pole; all others (usually about four) are situated equatorially. The species-specific ornamentation is found also in the apertures (poroids), where the exine is divided into distinctly separate elements (, 2C–E, 3A, B). The poroids may be nearly circular (, 2A, E) or more or less elongated (, 3A, D). Their size varies with much smaller poroids occuring in the vicinity of the conspicuous poroids (). Under dry condition, the pseudomonads collapse and the poroids are characteristically infolded (, 3C) following the specific pollen wall construction in apertural (thin intine) and interapertural areas (very thick intine). Ubisch bodies line the locular wall. Their ornamentation resembles more or less that of the exine elements in the poroids ().
Internal structure
In young developmental stages, the pseudomonads of Carex acutiformis border the tapetal cells with their distal face (). In mature Carex acutiformis pollen, the pollen wall is formed by an ektexine with tectum, columellae and foot layer, and an intine, whereas an endexine is lacking throughout. In interapertural areas, columellae are distinct and the tectum is covered with small granules or microechini. The wall elements covering the poroids consist of few small columellae and a discontinuous tectum, covered with granules or microechini. In the interapertural areas, the foot layer is perforated and rather inconspicuous, whereas in the apertures (poroids) it is peculiarly thickened (, 6A, C). In the equatorial apertures, the foot layer is remarkably thickened and continuous (). In the distal aperture, the foot layer forms a complex wall ingrowth structure resembling a wall labyrinth in a transfer cell (, 6A, C). This wall ingrowth is composed of two layers: an electron-opaque inner layer and an electron-translucent, thin outer layer ().
In the interapertural areas, the intine is conspicuously thickened and 3–4 μm thick (, ). In the apertural areas (poroids), the intine is very thin (sometimes less than 0.1 μm thick). The ektexine in the poroids consists of a discontinuous tectum, a few and low columellae, while the foot layer is peculiarly thickened (, C). Germination experiments demonstrate that all poroids can form a pollen tube (–D).
Vinca major and V. minor ( )
External structure
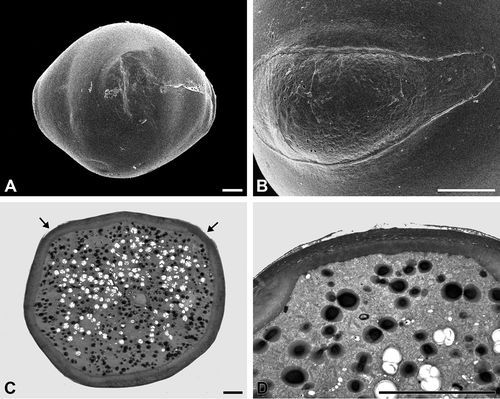
The oblate, tricolporate pollen grains are psilate throughout (, B). Additionally, the pollen grains exhibit indistinct pseudocolpi between the colpori ().
Internal structure
The exine consists of a continuous, thin tectum and a low, granular to columellar infratectum. The infratectum is situated upon a poorly defined basal layer, if foot layer and/or endexine cannot be differentiated (, D). The intine is conspicuously thick, except in the apertural areas. The apertures (the colpori) are covered by a rather fragile exine and are characterised by a thin intine (). However, the pseudocolpi neither have a fragile exine nor a thin intine, and so their non-apertural character is demonstrated.
Discussion
External morphology
Cyperaceae pseudomonads are predominantly pear-shaped, but in some taxa, they are more or less spheroidal. The ornamentation is usually characteristic and easy to recognise. However, using current palynological terms, it is somewhat complicated to describe the ornamentation sufficiently. Ornamenting elements are small granules, or microechini, and perforations. Sometimes these elements are placed upon distinct or indistinct verrucae. Such exine ornamentation may be called verrucate, granulate and perforate (e.g., Nagels et al., Citation2009), or verrucate, microechinate and perforate. Other authors call it, imprecisely, baculate (Moar & Wilmshurst, Citation2003) or scabrate (Pignotti & Mariotti, Citation2004). However, a highly variable ornamentation is always species-specific. Sometimes, one of the ornamenting categories may dominate.
The species-specific ornamentation is also found in the apertures (poroids), where the exine is divided into separate elements. A poroid is defined as a circular or elliptic aperture, with an indistinct margin (Hesse et al., Citation2009).
In Carex, five to six or seven apertures (poroids) are arranged in a typical manner, which have been variously interpreted. One aperture is placed distally and was termed as ulcus by Simpson et al. (Citation2003) and Nagels et al. (Citation2009); in contrast, Furness and Rudall (Citation1999) describe it as a pore. All other poroids are situated equatorially, being variously termed as lacunae (Moar & Wilmshurst, Citation2003), pseudoapertures (Meltsov et al., Citation2008) or pores/colpi (Pignotti & Mariotti, Citation2004). Ubisch bodies are common; their ornamentation may resemble that of the pseudomonads (Nagels et al., Citation2009, for review).
Ultrastructural aspects
An angiosperm pollen aperture is most usually characterised by a conspicuously thick, often bi-layered intine. In contrast, the apertural areas of mature Carex pollen show a very thin intine, whereas in the interapertural areas, the intine is remarkably thick. This feature is quite unusual in angiosperms, but most probably a common feature of the pseudomonads in Cyperaceae (Furness & Rudall, Citation1999; Simpson et al., Citation2003; our observations). In other genera of Cyperaceae with monads as pollen units (pseudomonads are absent), apertures show the standard type with a thick intine, e.g., in many Hypolytreae, such as Mapania and Diplasia (Simpson et al., Citation2003, Citation2007).
Figure 6. A–C. Carex acutiformis (TEM): A. The intine (i) is intensely thickened only in the interapertural areas; B. Two adjacent pseudomonads in cross section. The equatorial poroids with homogeneous, thick foot layer (fl). The thickened intine (i) is restricted to the interapertural area; C. Distal poroid with highly elaborated foot layer, similar to a reticulate wall ingrowth. This wall ingrowth consists of an electron-opaque inner region (asterisk) and an electron-transparent outer layer (arrowhead). The thickened intine (i) is restricted to the interapertural area. Ubisch bodies line the locular wall (arrow). Scale bars – 10 μm (A); 1 μm (B, C).
As far as known, the uncommon occurrence of either a thick or a thin intine is, in addition to Cyperaceae, only found in Vinca (Apocynaceae): A thin apertural intine and a thick intine in the interapertural areas were reported by Cousin (Citation1979) for Vinca rosea. We add corresponding data for Vinca major and V. minor.
The foot layer design in Carex apertures is unique. The equatorial poroids show a thickened and unstructured foot layer, whereas the distal poroid displays a conspicuous, elaborated foot layer, structurally similar to a transfer-cell wall labyrinth. These structures have already been documented at the light microscopic level, but have not been interpreted (Furness & Rudall, Citation1999).
The wall ingrowth (i.e. the elaborated foot layer) of the distal poroid can be compared with reticulate-type labyrinthine cell wall ingrowths of transfer cells (Vaughn et al., Citation2007; McCurdy et al., Citation2008). In Carex, this structure was shown at the light microscopic level (Reille, Citation1992, Citation1995; Furness & Rudall, Citation1999; Van Wichelen et al., Citation1999), and TEM level (Furness & Rudall, Citation1999: fig. ). Indeed, it consists of a foot layer material, but in a quite unusual quality. As common for wall ingrowths in transfer cells, the inner region (the foot layer material) is electron-opaque, covered by an outer, thin, electron-translucent layer, which is probably continuous with the intine.
Figure 7. A, B. Vinca minor (SEM): A. Tetracolporate pollen grain in equatorial view; B. Close-up of a colporus. C, D. Vinca major (TEM): C. Pollen grain in cross section. The inconspicuous apertures are equipped with a thin intine (arrows); D. Cross section of an aperture, note the exine with partially ruptured tectum and thin intine. Scale bars – 10 μm.
Functional aspects
The combination of a thin apertural intine and the simultaneous presence of an unusually structured foot layer in the distal aperture of Carex is unique amongst angiosperms. The observation of a transfer cell-like wall labyrinth in the distal poroid area sheds light on the role of the apertures. An aperture is defined as a region of the pollen wall, which differs significantly morphologically and/or anatomically from the rest of the pollen wall, presumably functioning as germination site and playing a role in harmomegathy (Hesse et al., Citation2009). The term ‘harmomegathy’ (harmomegathic effect) is circumscribed as a mechanism permitting changes in shape and size of the pollen grain (i.e., by varying the hydration status: water uptake and release). An unfamiliar function of the aperture is to provide the uptake of nutritional substances (from the tapetum). While the formation of pollen tubes may take place in all Carex apertures, the distal poroid plays a special role in maintaining the interaction between the secretory tapetal cells and the developing and mature pseudomonad [three of the four meiotic tetrad members undergo a programmed cell death (Furness, Citation2009)]. Carniel (Citation1962) referred to a possible nutrimental role of the distal poroid, while Christensen and Horner (Citation1974) and Barnes and Blackmore (Citation1992) pointed towards the analogue pore-tapetum orientation and uptake of nutrients in Poaceae. The elaborated structure of the distal poroid, which is the only poroid with permanent contact to the secretory tapetum, is now interpreted as nutrition passage for the remaining functional monad (, 5A, 6C). The lateral poroids have never contact with the tapetum, and are – as demonstrated by their compact foot layer – not equipped for a nutritional role. A mechanism for the transfer of nutritional substances through labyrinthine cell wall ingrowths of transfer cells, which are likewise typical for syncytia caused by plant-parasitic nematodes (Szakasits et al., Citation2009), needs further investigation.
Specimens investigated
Cyperaceae
Carex acutiformis Ehrh., Bad Vöslau, Lower Austria, 170902Car
Carex alba Scop., Baden, Helenental, Lower Austria, 050402Car
Carex atrata L., plants cultivated in the Botanical Garden of the University of Vienna (HBV), 090905Car
Carex flacca Schreb., Bad Vöslau, Lower Austria, 110705Car
Carex montana L., Ödlitz, Lower Austria, 071102Car
Carex nigra (L.) Reichard, Krakautal, Überlinger Moor, Styria, 121108Car
Carex remota L., Großau, Lower Austria, 090702Car
Cyperus longus L., Bad Vöslau, Lower Austria, 221004Cyp
Eleocharis palustris (L.) Roem. & Schult., plants cultivated in the Botanical Garden of the University of Vienna (HBV), 060606Ele
Schoenoplectus lacustris (L.) Palla, Bad Vöslau, Lower Austria, 080600Sch
Scirpus sylvaticus L., Großau-Berndorf, Lower Austria, 160701Sci
Apocynaceae
Vinca major L., plants cultivated in the Botanical Garden of the University of Vienna (HBV), 190407Vin
Vinca minor L., plants cultivated in the Botanical Garden of the University of Vienna (HBV), 230402Vin
Acknowledgements
We thank Mrs Andrea Frosch-Radivo, Mrs Ursula Schachner, and Mag. Silvia Ulrich (Department of Structural and Functional Botany, University of Vienna) for assistance in technical matters. Prof. Dr Tod Stuessy and Prof. Dr Rose Samuel (Department of Systematic and Evolutionary Botany, University of Vienna) are acknowledged for linguistic improvements.
References
- Barnes , S. H. and Blackmore , S. 1992 . Ultrastructural organization of two tapetal types in angiosperms . Arch. Histol. Cytol. , 55 ( Suppl ) : 217 – 224 .
- Brown , R. C. and Lemmon , B. E. 2000 . The cytoskeleton and polarization during pollen development in Carex blanda (Cyperaceae) . Am. J. Bot. , 87 : 1 – 11 .
- Carniel , K. 1962 . Beiträge zur Entwicklungsgeschichte des sporogenen Gewebes der Gramineen und Cyperaceen. II. Cyperaceae. Österr . Bot. Z. , 109 : 81 – 95 .
- Christensen , J. E. and Horner , H. T. Jr . 1974 . Pollen pore development and its spatial orientation during microsporogenesis in the grass Sorghum bicolor . Am. J. Bot. , 61 : 604 – 623 .
- Cousin , M.-T. 1979 . Tapetum and pollen grains of Vinca rosea (Apocynaceae) . Grana , 18 : 115 – 128 .
- Erdtman , G. 1952 . Pollen morphology and plant taxonomy. Angiosperms , Stockholm : Almqvist & Wiksell .
- Furness , C. A. 2009 . Pollen evolution and development in Ericaceae, with particular reference to pseudomonads and variable pollen sterility in Styphelioideae . Int. J. Plant Sci. , 170 : 476 – 495 .
- Furness , C. A. and Rudall , P. J. 1999 . Microsporogenesis in monocotyledons . Ann. Bot. , 84 : 475 – 499 .
- Furness , C. A. and Rudall , P. J. 2003 . Apertures with lids: Distribution and significance of operculate pollen in monocotyledons . Int. J. Plant Sci. , 164 : 835 – 854 .
- Halbritter , H. 1998 . Preparing living pollen material for scanning electron microscopy using 2.2-dimethoxypropane (DMP) and critical-point drying . Biotechn. Histochem. , 73 : 137 – 143 .
- Hesse , M. , Halbritter , H. , Zetter , R. , Weber , M. , Buchner , R. , Frosch-Radivo , A. and Ulrich , S. 2009 . Pollen terminology. An illustrated handbook , Vienna : Springer .
- Kirpes , C. C. , Clark , L. G. and Lersten , N. R. 1996 . Systematic significance of pollen arrangement in microsporangia of Poaceae and Cyperaceae: Review and observations on representative taxa . Am. J. Bot. , 83 : 1609 – 1622 .
- McCurdy , D. W. , John , W. , Patrick , J. W. and Offler , C. E. 2008 . Wall ingrowth formation in transfer cells: Novel examples of localized wall deposition in plant cells . Curr. Opin. Plant Biol. , 11 : 653 – 661 .
- Meltsov , V. , Poska , A. and Saar , M. 2008 . Pollen size in Carex: The effect of different chemical treatments and mounting media . Grana , 47 : 220 – 233 .
- Moar , N. T. and Wilmshurst , J. M. 2003 . A key to the pollen of New Zealand Cyperaceae . N. Zeal. J. Bot. , 41 : 325 – 334 .
- Nagels , A. , Muthama Muasya , A. , Huysmans , S. , Vrijdaghs , A. , Smets , E. and Vinckier , S. 2009 . Palynological diversity and major evolutionary trends in Cyperaceae . Plant Syst. Evol. , 277 : 117 – 142 .
- Nilsson , S. , Praglowski , J. and Nilsson , L. 1977 . Atlas of airborne pollen grains and spores in Northern Europe , Stockholm : Natur & Kultur .
- Pignotti , L. and Mariotti , L. M. 2004 . Micromorphology of Scirpus (Cyperaceae) and related genera in south-west Europe . Bot. J. Linn. Soc. , 145 : 45 – 58 .
- Reille , M. 1992 . Pollen et spores d'Europe et d'Afrique du Nord , Marseille : Lab. Bot. Hist. Palynol. Fac. St. Jerome Marseille Univ. III/URA CNRS .
- Reille , M. 1995 . “ Pollen et spores d'Europe et d'Afrique du Nord, Suppl. 1 ” . Marseille : Lab. Bot. Hist. Palynol. Fac. St. Jerome Marseille Univ. III/URA CNRS .
- Selling , O. 1947 . Studies in Hawaiian pollen statistics , Gothenburg : Bernice P. Bishop Mus . Sp. Publ. 38
- Simpson , D. A. , Furness , C. A. , Hodkinson , T. R. , Muasya , A. M. and Chase , M. W. 2003 . Phylogenetic relationships in Cyperaceae subfamily Mapanioideae inferred from pollen and plastid DNA sequence data . Am. J. Bot. , 90 : 1071 – 1086 .
- Simpson , D. A. , Muasya , A. M. , Alves , M. , Bruhl , J. J. , Dhooge , S. , Chase , M. W. , Furness , C. A. , Ghamkhar , K. , Goetghebeur , P. , Hodkinson , T. R. , Marchant , A. D. , Nieuborg , R. , Reznicek , A. A. , Roalson , E. H. , Smets , E. , Starr , J. R. , Thomas , W. W. , Wilson , K. L. and Zhang , X. 2007 . Phylogeny of Cyperaceae based on DNA sequence data – a new rbcL analysis . Aliso , 23 : 72 – 83 .
- Szakasits , D. , Heinen , P. , Wieczorek , K. , Hofmann , J. , Wagner , F. , Kreil , D. P. , Sykacek , P. , Grundler , F. M. W. and Bohlmann , H. 2009 . The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots . Plant J. , 57 : 771 – 784 .
- Van Wichelen , J. , Camelbeke , K. , Chaerle , P. , Goetghebeur , P. and Huysmans , S. 1999 . Comparison of different treatments for LM and SEM studies and systematic value of pollen grains in Cyperaceae . Grana , 38 : 50 – 58 .
- Vaughn , K. C. , Talbot , M. J. , Offler , C. E. and McCurdy , D. W. 2007 . Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of Arabinogalactan proteins . Plant Cell Physiol. , 48 : 159 – 168 .
- Weber , M. 1992 . Nature and distribution of the exine-held material in mature pollen grains of Apium nodiflorum L. (Apiaceae) . Grana , 31 : 17 – 24 .
- Weber , M. , Halbritter , H. and Hesse , M. 1998 . The spiny pollen wall in Sauromatum (Araceae) – with special reference to the endexine . Int. J. Plant Sci. , 159 : 744 – 749 .