Abstract
This study is the first contribution to knowledge of the relationships between Geotrigona argentina and the plants of the Argentine Dry Chaco forest. A total of 1260 g of honey (corresponding to 146 pots) and 763 g of pollen (63 pots) stored in four underground nests was studied. The honey pots from each nest were homogenised and the four honey samples were analysed by melissopalynological methods, whereas the pollen pots were studied individually. Both classical counts and counts affected by the volume of the pollen types were carried out. Pollen data were statistically analysed. Additional data on both protein and lipid content is also provided. A total of 39 pollen taxa were identified. Pollen collection was focused on a few pollen taxa: Prosopis, Castela coccinea, Maytenus and Capparis; these taxa, together with Ziziphus mistol and Pisonia zapallo, were also important nectar sources. The preliminary results show that pollen collection varied seasonally, being most diverse in the summer when G. argentina incorporates herbaceous plants into its diet. The pollen collection spectrum of G. argentina is similar to that of other Trigonina bees in that the main plant species collected are a few large shrubs or trees, whose flowering consists of small and clustered flowers. Pots with large amounts of monofloral loads with pollen from only a few species suggests an organised foraging behaviour that includes the recruitment of foragers, such as that observed in other eusocial bees.
Stingless bees or meliponines are important eusocial pollinators in tropical and subtropical regions over the world. They live in communal and perennial nests with a population ranging from a few dozens to 100 000 or more workers (Michener, Citation2000). Both nectar and pollen are required for colony maintenance and growth. Nectar, which becomes honey, is the main energy source for the colony, whereas pollen is the main protein and lipid source essential for brood development (e.g., Roubik, Citation1989). Most studies dealing with plants exploited by stingless bees examined species that build internal nests in tree trunk holes (Ramalho et al., Citation1990; Biesmeijer & Slaa, Citation2006). However, little is known about the pollen and nectar collected by ground-nesting stingless bees. It is unknown if environmental conditions, such as the seasonal floods common in the Dry Chaco forest, influence the collecting behaviour of ground-nesting stingless bees.
Historically, stingless bee foraging has been examined by looking at the corbicular pollen loads from returning foragers (Ramalho et al., Citation1989, Citation1994; Nagamitsu & Inoue, Citation2002), using garbage traps placed at nest entrances (Eltz et al., Citation2001) or examining the pollen and honey from colony storage pots (Cortopassi-Laurino & Ramalho, Citation1988; Ramalho et al., Citation1989). Some species of ground-nesting Meliponini, such as Geotrigona argentina Camargo et Moure, are “timid”, and their guards retreat into the entrance when the nest is disturbed by humans (Couvillon et al., Citation2008). The safest way to examine the floral resources over a long period of time for “timid” species is to examine the food stored in the nests. However, it is difficult to maintain their underground nests in hives for study (Cortopassi-Laurino et al., Citation2006), making examination of the storage pots of G. argentina more difficult than other stingless bees.
Geotrigona (subtribe Trigonina) is a Neotropical genus of stingless bees found from southern Mexico to Central America and throughout most of South America (Camargo & Pedro, Citation1992). The bees build underground nests made of cerumen (bee's wax mixed with resin; Baumgartner & Roubik, Citation1989; Camargo & Moure, Citation1996). The nesting cavities are not constructed by the bees, but by ants, which failed to inhabit them (Nogueira-Neto & Sakagami, Citation1966; Roubik, Citation2006). Although there are some scattered reports from field observations of G. mombuca Smith (Neves et al., Citation2006), G. acapulconis Strand (Castañeda-Vildózola et al., Citation1999) and G. subterranea Friese (Jacobi & Laboissière, Citation2007), little is known about the flowers visited by Geotrigona. There are only some scattered reports from field observations on G. argentina (“alpamiski”), a common stingless bee in the Dry Chaco forest (Chaco region), Argentina (Morello & Adámoli, Citation1968). An ethnobiological study conducted in the region reported that during October the honey of G. argentina is harvested by members of the Wichi ethnia (Arenas, Citation2003). The only data of this stingless bee was collected by Chacoff (Citation2006), who observed them foraging on Citrus paradisi Macfad. flowers (grapefruit).
In order to contribute to the knowledge of the foraging behaviour of Geotrigona argentina in the Dry Chaco forest, the goals of this paper were to: (1) identify the pollen and nectar resources used by stingless bees from the analysis of pollen grains present in both pollen and honey storage pots; (2) detect the contribution of the different pollen types to the bees' diet; and (3) provide additional information about the protein and lipid content of the main pollen types collected.
Materials and methods
Study site and nest sampling
The term Chaco is applied to the vegetation covering the vast plains of northern Argentina, western Paraguay, south-eastern Bolivia and the extreme western edge of Mato Grosso do Sul state in Brazil. It extends an area of over 800 000 km2 (Prado, Citation1993, Citation2000). The Chaco is one of the few areas in the world where the transition between the tropics and the temperate belt does not occur in the form of a desert but rather as semiarid forest and woodlands. The most distinctive feature of the Chaco region consists of the dominance of species of the arboreal genus Schinopsis, together with Aspidosperma quebracho-blanco Schldtl., Tabebuia nodosa Griseb., and several species of Acacia and Bulnesia (Pennington et al., Citation2000). The environmental gradients of this extensive region can be divided into three distinct sectors from eastern to western. Each sector is characterised by one species of Schinopsis: S. balansae Engl. in the Humid Chaco, S. lorentzii Engl. in the Dry Chaco and S. marginata Engl. in the Sierra Chaco (Cabrera, Citation1971). A number of woody communities can be found in each sector, such as the “Palosantal” in the Dry Chaco, where the nests of Geotrigona argentina were obtained. In the Dry Chaco, where the vegetation shows the most pronounced xeromorphy, the climate is distinguished by its strong seasonality with summer temperatures up to 49ºC and “severe winter frost”; the mean annual rainfall is c. 500 mm with the heaviest rains experienced during summer (December–March; Pennington et al., Citation2000). In the “Palosantal”, a semiarid thorn woodland can be distinguished, characterised by the dominance of B. sarmientoi Lorentz ex Griseb. (“palo santo”), which is an endemic tree up to 20 m high. This zygophyllacean is accompanied by the apocynacean A. quebracho-blanco, followed by a discontinuous layer of low woody trees and shrubs (), the most important of which belong to the following families: Fabaceae (Prosopis spp., Acacia spp.), Rhamnaceae (Ziziphus mistol Griseb.), Sapotaceae (Sideroxylon obtusifolium (Roem. & Schult.) T. D. Penn.), Capparidadeae (Capparis spp.), Celastraceae (Maytenus vitis-idaea Griseb., Moya spinosa Griseb.), Bignoniaceae (Tabebuia nodosa), Apocynaceae (Aspidosperma triternatum Rojas Acosta), Polygonaceae (Ruprechtia triflora Griseb.), Anacardiaceae (Schinus fasciculatus (Griseb.) I. M. Johnst.), Simaroubaceae (Castela coccinea Griseb.), Achatocarpaceae (Achatocarpus praecox Griseb.), Cactaceae (Stetsonia coryne Britton & Rose, Cereus forbesii Salm-Dyck) and Solanaceae (Grabowskia spp., Lycium spp.). Aquatic plants may be also found in rivers and seasonal swamps, e.g., Menyanthaceae (Nymphoides indica (L.) Kuntze), Limnocharitaceae (Hydrocleys nymphoides (Willd.) Buchenau), Cannaceae (Canna glauca L.), Maranthaceae (Thalia geniculata L.) and Onagraceae (Ludwigia spp.).
Figure 1. The “Palosantal” Dry Chaco forest of Argentina. Site with shrubs and bare soil with sparse bromeliad herbs (photography by F. G. Vossler, 15 January 2008).

Four Geotrigona argentina nests were examined. One nest (Nest I) was found in El Espinillo (25º 23' S; 60º 27' W), and three (Nests II, III and IV) in El Sauzalito (24º 24' S; 61º 40' W) (). Both localities are in the Province of Chaco, Argentina. Nests I and II were collected during summer (February) and Nests III and IV during winter and spring (August and October, ). Nests were built in abandoned ant nests, at a depth varying between 1.0 and 1.8 m. Nests I and II were located on a dirt path lacking vegetation, whereas Nests III and IV were located on paths covered by vegetation.
Figure 2. Map of Argentina indicating the Chaco region in Argentina and the location of the sites with the sampled nests.
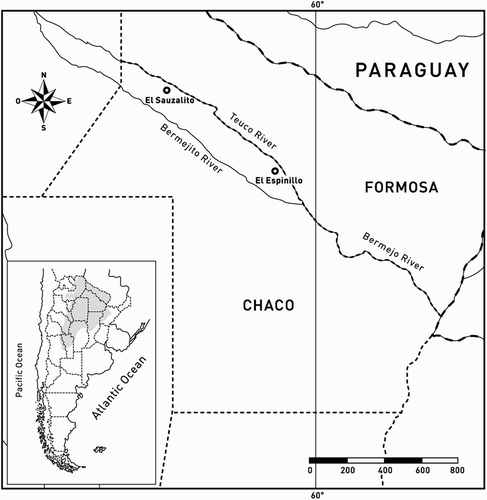
Table I. Data of sampled nests of Geotrigona argentina. Nest I was collected in El Espinillo, the remaining in El Sauzalito, in the Chaco province, Argentina
Pollen analysis
Geotrigona argentina bees store honey and pollen in “pots” that surround the brood chamber; similar to G. matogrossensis Ducke (Camargo & Moure, Citation1996). Pollen loads are stored and pressed into pots (pollen storage pots); nectar turned into honey is kept in other pots (honey storage pots). A total of 146 honey pots and 63 pollen pots were obtained (). Both pollen and honey storage pots were about 6 cm high, but the diameter varied according to the content. The honey storage pots were about 1.3 cm in diameter and the pollen storage pots as large as about 1.7 cm. After extraction, the pots were frozen until their analysis. Pollen pots were numbered. Plastic cylinders (straws, 0.5 cm in diameter and 1.0 cm long) were used to extract the pollen. The cylinders were pushed into different depths of pollen mass depending on the size or appearance (either with or without a uniform colour) of the pollen.
Pollen samples were first dissolved by stirring them with a glass rod in 200 ml distilled water at 80–90ºC. Subsequently, the samples were stirred for 10−15 minutes with a magnetic stirrer. Five millilitres of the mixture was centrifuged at 472 × g for 10 minutes. Since most pollen pots shared the same dominant pollen type, general observations on unacetolysed sediments were first made in order to select different sediments for acetolysis. The acetolysis procedure followed the technique described by Erdtman (Citation1960). For scanning electron microscopy (SEM), unacetolysed pollen grains were suspended in 90% ethanol and mounted on stubs and examined in a JEOL JSM-T-100 SEM.
To determine the volume of different pollen types, unacetolysed pollen grains were measured. Mean values with their standard deviation were calculated, and then modelled as sphere (P/E = 0.99−1.01), ellipsoid (prolate P/E > 1.01 or oblate P/E < 0.99) or prism (O'Rourke & Buchmann, Citation1991; Biesmeijer et al., Citation1992). Because Eleocharis produces rather polymorphic pollen, the volume was calculated from the mean between the sphere and ellipsoid volumes. Pollen grain volumes were used to calculate the percentage of different pollen types contained in the pollen storage pots. As honey contained in the honey storage pots contaminate one another during nest handling, all the honey pots extracted from each nest were combined, and then 10−20 g were processed. A preliminary examination of honey revealed a high concentration of pollen grains; for this reason we used distilled water to dissolve the samples following Louveaux et al. (Citation1978). Counts were continued up to percentage stabilisation (Vergeron, Citation1964). In this study, five hundred pollen grains were “the minimal number” that contains the pollen species representatives of the samples. The identification of pollen types was based on reference pollen slides from flowers collected in the “Palosantal” forest. Each reference slide was supported by the corresponding specimens deposited in the herbarium of the Museo de Ciencias Naturales of La Plata (LP), Buenos Aires, Argentina. Specimens of Geotrigona argentina were deposited in the entomological collection of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
Determination of protein and lipid content
The protein and lipid content of the pollen pots were determined from the main pollen types of the monofloral pots and mixtures of pollen types from selected mixed pots. For nitrogen content determination, 507nbsp;mg of pollen (AOAC, Citation1980) from pots selected by botanical origin was analysed by the micro-Kjeldahl method (Bremner & Mulvaney, Citation1982) and crude protein was estimated using the factor 6.25 (Roulston & Cane, Citation1999). Pollen nitrogen content was analysed in LANAIS N15 (National Laboratory of Research and Services UNS-CONICET), Departamento de Agronomía, Universidad Nacional del Sur, Bahía Blanca.
Lipids were determined following Folch et al. (Citation1957). Total lipids of pollen surface were obtained by chloroform-methanol (2:1 v/v) extraction of the dried pollen, and the lipid fraction was estimated from difference in weight.
Data analysis
In order to group pollen storage pots with a similar content, cluster analysis was applied using the Euclidean distance and Ward's method. From this analysis, experimental units (i.e., pots) were grouped at different levels of association. The program PAST, version 1.81 (Hammer et al., Citation2008), was used as statistical package for the analysis. To compare the results of pollen counts with relative volume estimates, chi-square test (p < 0.05) was used leaving out the data of pollen plants present in less than 5% (i.e., those with less than 25 pollen grains). Pollen niche diversity was calculated from diversity index (H') (Hutcheson, Citation1970) expressed as H' = – Σ pi ln (pi), where pi is the proportion of the pollen type i. To compare the pollen collected in spring with that collected in summer, the pollen niche diversity (H'), the richness (S) and the evenness (J') of the sources used were calculated. The t-test was used to compare H' using ln. The richness is expressed as S = ln Pi, where P is the number of different pollen types. The evenness index J' was calculated according to Pielou (Citation1977): J' = H'/S.
Results
Pollen and nectar collected by Geotrigona argentina
Although part of food reserves stored inside the nests of Geotrigona argentina were lost during its extraction, considerable amounts of honey and pollen were extracted. A total of 1260 g of honey (corresponding to 146 honey pots), and 763 g of pollen (63 pollen pots) were analysed (). Most of the stored pollen was dark brownish but in a few pots, pollen loads with different colours could be clearly distinguished (pots 14 and 16). According to Cortopassi-Laurino and Ramalho (Citation1988), stored pollen grains acquire a brownish dark colour as they age.
Pollen from 39 different taxa was found in the samples. Fourteen were identified at species level, 16 at generic level, six at family level, and three assigned as ‘types’ (, ). Types include common taxa of the region that are indistinguishable. The Astereae-type includes species of Baccharis and Eupatorium; the Hydrocleys-type includes Hydrocleys nymphoides and Sagittaria montevidensis Cham. & Schltdl., and the Maytenus-type includes Maytenus vitis-idaea and Moya spinosa. The 39 identified taxa belong to 30 families: Achatocarpaceae (Achatocarpus praecox), Alismataceae (Sagittaria montevidensis), Amaranthaceae (Alternanthera spp.), Apocynaceae, Asteraceae (Astereae-type, Holocheilus hieracioides (D. Don) Cabrera, Parthenium hysterophorus L., Tessaria spp.), Basellaceae (Anredera cordifolia (Ten.) Steenis), Boraginaceae (Heliotropium spp.), Cactaceae, Capparidaceae (Capparis spp.), Celastraceae (Maytenus-type), Celtidiaceae (Celtis spp.), Cyperaceae (Eleocharis spp.), Euphorbiaceae (Croton spp.), Fabaceae (Acacia spp., Parkinsonia aculeata L., Prosopis spp.), Limnocharitaceae (Hydrocleys-type), Loranthaceae (Struthanthus spp., Tripodanthus acutifolius Tiegh.), Malpighiaceae, Malvaceae, Menyanthaceae (Nymphoides indica), Nyctaginaceae (Boerharvia diffusa L., Pisonia zapallo Griseb.), Onagraceae (Ludwigia spp.), Polygonaceae (Ruprechtia triflora), Portulacaceae (Portulaca spp.), Rhamnaceae (Ziziphus mistol), Sapotaceae (Sideroxylon obtusifolium (Roem. & Schult.) T. D. Penn.), Scrophulariaceae (Scoparia spp.), Simaroubaceae (Castela coccinea), Typhaceae (Typha spp.), Verbenaceae (Phyla spp.), and Zygophyllaceae (Bulnesia sarmientoi). Measurements, means, standard deviations, and the volume of each pollen taxon with a frequency higher than 5% of total pollen are given ().
Figure 3. Dendrogram showing the four groups and 11 subgroups of 63 monofloral pollen pots from the four nests of Geotrigona argentina using the Euclidean distance and Ward's method (generated with the PAST statistical package).
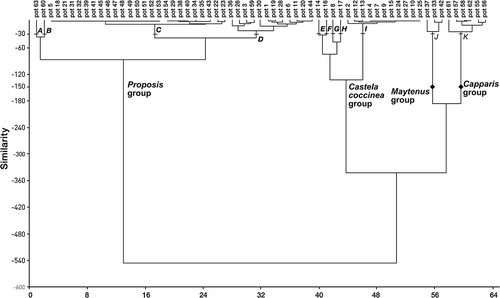
Table II. Pollen taxa present in mixed pollen storage pots of Castela coccinea, the Maytenus and Capparis groups. Monofloral pollen pots (more than 95% of single pollen type) were excluded. Order of pot numbers follows the order in . The percentage derived from the pollen counts are mathematically rounded. Percentages are listed first, followed by the volume estimate of each taxon. Percentages and volume are divided by a slash. A + indicates pollen taxa present in less than 5% (they were also excluded from volume estimates). Pot numbers 8, 14, 16, 37, and 55 demonstrate the Major Differences Between The Two Counting methods
Table III. Pollen taxa present in mixed pollen storage pots of the Prosopis group. Order of pot numbers follows the order in . Monofloral pollen pots of Prosopis spp. were excluded. Percentages and volume are divided by a slash. A + indicates pollen taxa present in less than 5%. Pots 30 and 63 demonstrate the major differences between the two counting methods
Table IV. Pollen grain volume calculated from means of polar (P) and equatorial (E) diameters; values obtained from measurements of 20 pollen grains. The asterisk indicates a measure belonging to the widest pollen region in equatorial view. Pollen shape includes: S, sphere; E, ellipsoid; P, prism. For Struthanthus spp., P and E coincide with the height and the side of a prism. The volumes were calculated using the following formulas: sphere = 1/6 π P3; ellipsoid = 1/6 π P E2; prism = 1/4 E2 P √3
In order to classify the pollen storage pots, we established two categories of pollen contents: ‘monofloral’ (with a single pollen species exceeding 95% of the total) and ‘mixed’ (where no pollen species exceeded 95% of the total). For monofloral pots, we consider that the remainder 5% of pollen grains (25 pollen grains of a total of 500 counted grains) may be due to contamination that occurred during the handling of nests.
Four groups and 11 subgroups with a similarity level of 150 and 30, respectively, were obtained from the cluster analysis (). In each group, a variable number of pots share a dominant pollen type whereas in each subgroup a variable number of pots additionally share a similar contribution of pollen types. The Prosopis group contained four subgroups A–D. The Castela coccinea group contained five subgroups E–I, and the Maytenus and Capparis groups each contained one subgroup (J and K, respectively). The Prosopis group was not only monofloral but also co-dominant with Ziziphus mistol in subgroup A and with Capparis spp. in subgroup B. It included only monofloral pots of Prosopis in subgroup C; and occurred from 85–95% of total pollen in subgroup D. In the Castela coccinea group, subgroups E–F comprised mixed pollen pots: in E, Castela coccinea was together with Croton spp. and Struthanthus spp.; in F with Croton spp., Hydrocleys-type, Eleocharis spp., Verbena spp. and Scoparia spp.; and in G and H with Prosopis spp. and Capparis spp., respectively. Subgroup I included only monofloral pollen pots of Castela coccinea. The Maytenus group comprised both monofloral and mixed pots where this taxon was together with Prosopis spp. The Capparis group included both monofloral and mixed pots that also contained pollen from Prosopis spp. and Ruprechtia triflora.
The honey samples had the same pollen composition (). Six taxa appeared as the main nectar sources. Prosopis spp., Ziziphus mistol, Capparis spp. and Maytenus-type represented over 50% of total pollen, whereas Castela coccinea and Pisonia zapallo varied between 10% and 14%. Conversely, some pollen taxa, such as Alternanthera spp., Bulnesia sarmientoi, Celtis spp., Malvaceae, Ruprechtia triflora, and Schinopsis lorentzii (“Minor pollen” in ), occurred infrequently (less than 1% of the total pollen).
Table V. Percentages of pollen identified in honey of Geotrigona argentina. “Minor pollen” includes pollen types that occurred less than 1% of total pollen
Contribution of pollen types in the diet
The collected pollen types were remarkably different in shape and size (). Results from two different counting methods (, ) showed significant differences (p < 0.05) in pollen storage pots belonging to the Castela coccinea group (pots 14, 16 and 8), the Maytenus group (pot 37) and the Capparis group (pot 55), as well as in pots belonging to the Prosopis group (only pots 30 and 63). For these pollen pots, chi-square test ranged between 4.48 and 54.09 and thus H0 was rejected; this means that differences of pollen counts and volume estimate percentages are significant. When diversity indices (H') were compared, the t-test revealed that the differences observed in the pollen collected during winter/spring and summer nests were significant (t = −2.77, df = 15, p = 0.014; ) and thus H0 was rejected. This means that the pollen niche diversity varied seasonally and was larger during the summer.
Figure 4. SEM micrographs of some pollen types harvested by Geotrigona argentina in the Dry Chaco forest. A. Croton spp. B. Struthanthus spp. C. Castela coccinea. D. Ziziphus mistol. E. Hydrocleys-type. F. Astereae-type. Scale bars – 15 μm (A), 10 μm (B), 5 μm (C–E), and 2 μm (F).
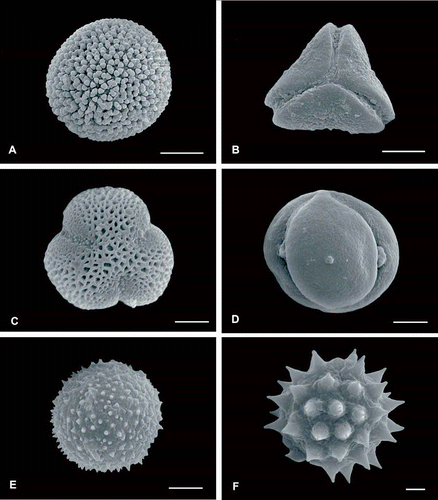
Table VI. Compared values of H', S and J' calculated from the volume percentages of pollen stored in winter-spring, summer and all nests
Protein and lipid content of collected pollen
The protein content generally exceeded 20% (). Prosopis spp. had the highest protein content (30.41%) and Capparis spp. the lowest (9.78%). The lipid content differed strongly among pollen from different taxa. The highest value was found in a mixture of pollen where Prosopis spp. was dominant followed by Ziziphus mistol (23.62%), whereas the lowest value was found in Castela coccinea (3.10%).
Table VII. Nitrogen, protein, and lipid content of the main pollen stored by Geotrigona argentina
Discussion
Pollen study of food reserves stored in nests of Geotrigona argentina allowed us to detect the sources of pollen and nectar, calculate the niche diversity and speculate about the foraging behaviour of this stingless bee. The analysis of protein and lipid content of stored pollen provided us preliminary information about its nutritional quality.
Plants foraged by Geotrigona argentina in the Dry Chaco forest
Within the great diversity of the floral resources available in the Dry Chaco forest, Geotrigona argentina bees visited a wide range of plant species, but only a small group of trees and woody shrubs constantly occurred in both pollen and honey reserves. Pollen analysis revealed that these plants are intensely collected because they either characterised most monofloral pots or were co-dominant in mixed pots (, ). However, in the mixed pots, the relevance of the main pollen taxa was significantly affected by their volume. In the Castela coccinea and Maytenus groups, pollen counts overestimated the contribution of Castela coccinea and Maytenus in the diet and underestimated that of Croton spp. and Prosopis spp. (). Conversely, there were no significant differences among counts within the Prosopis group. In the latter, differences in the volume of pollen grains were underestimated mainly by the high number of pollen grains of Prosopis spp. (). Honey analysis indicated that Prosopis spp., Capparis spp. and Maytenus spp. together with Ziziphus mistol and Pisonia zapallo are important nectar sources (), however, the latter two have less significance as pollen sources. Flowering of main food plants for stingless bees increased during spring (September–October) and declined in summer, as it occurs in tropical environments (Wilms et al., Citation1996). Almost all the main plants visited by G. argentina offer large amounts of food in their small and clustered flowers. The selective exploitation of rich food sources by stingless bees has been reported by many authors (e.g., Ramalho, Citation2004).
Although in our research pollen collection was monopolised on some woody plants, few pots evidenced that Geotrigona argentina may exploit herbaceous plants as well, either from the lowest stratum of the forest, such as plants from aquatic communities like Alternanthera spp., Holocheilus hieracioides, Ludwigia spp., Sagittaria montevidensis, Eleocharis spp., or from open areas, such as Croton spp., Verbena spp., and Scoparia spp. These plants proliferate in summer, when the flowering of the main woody food sources declines. The incorporation of herbaceous plants into the diet accounted for the higher resource diversity during the summer. However, major sampling of both the regional flowering and the plants visited is necessary to confirm this foraging behaviour. The foraging spectrum of G. argentina seems to be narrow and similar to those of medium-sized non-aggressive Trigonina bees such as Scaptotrigona and Partamona (Wilms et al., Citation1996; Biesmeijer & Slaa, Citation2006). Some authors have explained the foraging niche size in stingless bees from different points of view, e.g., in relation with food source communication (e.g., Lindauer & Kerr, Citation1960; Biesmeijer & Slaa, Citation2004), colony size (Sommeijer et al., Citation1983), head width (Van Nieuwstadt & Ruano Iraheta, Citation1996), body size (Araújo et al., Citation2004), and aggressiveness (Biesmeijer & Slaa, Citation2006). There is not enough published data on the biology of G. argentina to make comparisons with our results. However, we speculate that these stingless bees are able to communicate the location of sources by means of some recruitment system (group foraging), as proposed from studies on pollen foraging of Meliponula in Uganda (Kajobe, Citation2007). If foragers were not able to communicate efficiently, pollen diversity of the pots should be higher than that found in this work and they would have represented a random collection (dispersal foraging). Some plant families visited by G. argentina, such as Fabaceae (Mimosoideae), Euphorbiaceae, Loranthaceae and Rhamnaceae, are also foraged by other stingless bees (Imperatriz-Fonseca et al., Citation1988; Ramalho et al., Citation1990).
Protein and lipids of pollen stored by Geotrigona argentina
The nutritional requirements of some social Apidae are well-known and it has been determined that the quantity and quality of collected pollen affects the productivity of the colony, i.e., reproduction, brood rearing and longevity (Tasei & Aupinel, Citation2008). However, there is not enough data on the nutritional value of the pollen collected by stingless bees so as to make comparisons with our results. The protein content of pollen collected by Geotrigona argentina commonly ranged between 20% and 27%. These values fall into the broad range of protein content of pollen collected by honeybees, which is between 12% and 61% (Roulston & Cane, Citation1999). The pollen mixes of Prosopis spp. and Ziziphus mistol (pot number 11) had the highest protein and lipid content. However, further experiments are necessary to reach clearer conclusions about the nutritional requirements of G. argentina and pollen collection.
Conclusions
By comparing the pollen collection spectrum of Geotrigona argentina with those of other members of the tribe Meliponini, we concluded that G. argentina has a pollen spectrum similar to that of other medium-sized non-aggressive Trigonina. Main plant species collected by medium-sized non-aggressive Trigonina bees and G. argentina are a few large shrubs or trees, whose inflorescences consist of small and clustered flowers. The individual analysis of the pots demonstrated that most of them presented large amounts of monofloral pollen loads from a few plant species only. This behaviour might evidence that G. argentina is efficient in group foraging. These stingless bees accumulate large loads of pollen from the flowerings of the end of the winter and early spring (August–October). This food storage persisted in the nests for several months after being collected (February and October). The nutritional requirements and the climatic conditions probably contributed to shape the foraging behaviour of G. argentina. The Chaco plain is subject to low soil moisture and freezing in the dry season (winter), to water logging and extremely high air temperatures during part of the rainy season (summer) (Pennington et al., Citation2000). During seasonal inundations, ground nesting stingless bees cannot supply the nest with pollen and nectar. Under such conditions, the efficient use of floral resources might play an essential role for the survival of the colony. These results may represent only a very general picture about the relationships between G. argentina and the vegetation of the Dry Chaco forest. However, these results could be the first step to develop further studies on particular aspects of the foraging behaviour of these bees.
Acknowledgements
The authors are grateful to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for financial support, Dr Arturo Roig Alsina for identifying specimens of stingless bees, Laura Cavaleiro for helping us with lipid analysis, Dr Arturo Kehr for helping us with statistical analysis, César Albornoz and Rogelio Burgardt for logistical support and two anonymous reviewers for making useful suggestions and linguistic improvements concerning the manuscript.
References
- AOAC (Association of Official Analytical Chemists) . 1980 . Official methods of analysis , Washington, DC : AOAC .
- Araújo , E. D. , Costa , M. , Chaud-Netto , J. and Fowler , H. G. 2004 . Body size and flight distance in stingless bees (Hymenoptera, Meliponini): Inference of flight range and possible ecological implications . Brazilian Journal of Biology , 64 : 563 – 568 .
- Arenas , P. 2003 . Etnografía y alimentación entre los toba-ñachilamoleek y wichi lhuku'tas del Chaco Central (Argentina) , Buenos Aires : Edición del autor [[email protected]] .
- Baumgartner , D. L. and Roubik , D. W. 1989 . Ecology of necrophilous and filth-gathering stingless bees (Apidae: Meliponinae) of Peru . Journal of the Kansas Entomological Society , 62 : 11 – 22 .
- Biesmeijer , J. C. and Slaa , E. J. 2004 . Information flow and organization of stingless bee foraging . Apidologie , 35 : 143 – 157 .
- Biesmeijer , J. C. and Slaa , E. J. 2006 . The structure of eusocial bee assemblages in Brazil . Apidologie , 37 : 240 – 258 .
- Biesmeijer , J. C. , Van Marwijk , B. , Deursen , K. , Punt , W. and Sommeijer , M. J. 1992 . Pollen sources for Apis mellifera L. (Hym., Apidae) in Surinam, based on pollen grain volume estimates . Apidologie , 23 : 245 – 256 .
- Bremner , J. M. and Mulvaney , C. S. 1982 . “ Nitrogen-total ” . In Methods of soil analysis. Part 2. Chemical and microbiological properties , Edited by: Page , A. L. , Miller , R. H. and Keeney , D. R. 595 – 624 . Madison, WI : American Society of Agronomy .
- Cabrera , A. L. 1971 . Fitogeografía de la República Argentina . Boletín de la Sociedad Argentina de Botánica , 1-2 : 1 – 42 .
- Camargo , J. M. F. and Moure , J. S. 1996 . Meliponini neotropicais: O gênero Geotrigona Moure, 1943 (Apinae, Apidae, Hymenoptera) com especial referência à filogenia e biogeografía . Archivo de Zoologia São Paulo , 33 : 95 – 161 .
- Camargo , J. M. F. and Pedro , S. R. M. 1992 . Systematics, phylogeny and biogeography of the Meliponinae (Hymenoptera, Apidae): A mini-review . Apidologie , 23 : 509 – 522 .
- Castañeda-Vildózola , A. , Equihua-Martínez , A. , s- , Valdé , Carrasco , J. , Barrientos-Priego , A. F. , Ish-Am , G. and Gazit , S. 1999 . Insectos polinizadores del aguacatero en los estados de México y Michoacán . Revista Chapingo – Serie Horticultura , 5 : 129 – 136 .
- Chacoff , N. P. 2006 . Los ecosistemas naturales como fuente de polinizadores para Citrus paradisi en el Pedemonte de las Yungas , Tucumán: Universidad Nacional de Tucumán, Facultad de Ciencias Naturales e Instituto Miguel Lillo . PhD Diss.
- Cortopassi-Laurino , M. , Imperatriz-Fonseca , V. L. , Roubik , D. W. , Dollin , A. , Heard , T. , Aguilar , I. , Venturieri , G. C. , Eardley , C. and Nogueira-Neto , P. 2006 . Global meliponiculture: Challenges and opportunities . Apidologie , 37 : 275 – 292 .
- Cortopassi-Laurino , M. and Ramalho , M. 1988 . Pollen harvest by africanized Apis mellifera and Trigona spinipes in Sâo Paulo: Botanical and ecological views . Apidologie , 19 : 1 – 24 .
- Couvillon , M. J. , Wenseleers , T. , Imperatriz-Fonseca , V. L. , Nogueira-Neto , P. and Ratnieks , F. L. W. 2008 . Comparative study in stingless bees (Meliponini) demonstrates that nest entrance size predicts traffic and defensivity . Journal of Evolutionary Biology , 21 : 194 – 201 .
- Eltz , T. , Brühl , C. A. , Van der Kaars , S. and Linsenmair , K. E. 2001 . Assessing stingless bee pollen diet by analysis of garbage pellets: A new method . Apidologie , 32 : 341 – 353 .
- Erdtman , G. 1960 . The acetolysis method. A revised description . Svensk Botanisk Tidskrift , 54 : 561 – 564 .
- Folch , J. M. , Lees , M. and Sloane-Stanley , G. H. 1957 . A simple method for the isolation and purification of lipids from animal tissues . Journal of Biological Chemistry , 226 : 497 – 509 .
- Hammer , Ø , Harper , D. A. T. and Ryan , P. D. 2008 . PAST-Palaeontological Statistics , : 81 ver.1
- Hutcheson , K. 1970 . A test for comparing diversities based on the Shannon formula . Journal of Theoretical Biology , 29 : 151 – 154 .
- Imperatriz-Fonseca , V. L. , Kleinert-Giovannini , A. and Ramalho , M. 1988 . Pollen harvest by eusocial bees in a non-natural community in Brazil . Journal of Tropical Ecology , 5 : 239 – 242 .
- Jacobi , C. M. and Laboissière del Sarto , M. C . 2007 . Pollination of two species of Vellozia (Velloziaceae) from high-altitude quartzitic grasslands . Acta Botanica Brasilica , 2 : 325 – 333 .
- Kajobe , R. 2007 . Pollen foraging by Apis mellifera and stingless bees Meliponula bocandei and Meliponula nebulata in Bwindi Impenetrable National Park, Uganda . African Journal of Ecology , 45 : 265 – 274 .
- Lindauer , M. and Kerr , W. E. 1960 . Communication between the workers of stingless bees . Bee World , 41 : 29 – 71 .
- Louveaux , J. , Maurizio , A. and Vorwhol , G. 1978 . Methods of melissopalynology by International Commission for Bee Botany of IUBS . Bee World , 59 : 139 – 157 .
- Michener , C. D. 2000 . The bees of the world , Baltimore, MD : Johns Hopkins University Press .
- Morello , J. and Adámoli , J. 1968 . Las grandes unidades de vegetación y ambiente del Chaco argentino. Primera parte: Objetivos y metodología. Serie fitogeográfica no. 10 , Buenos Aires : INTA .
- Nagamitsu , T. and Inoue , T. 2002 . Foraging activity and pollen diets of subterranean stingless bee colonies in response to general flowering in Sarawak, Malaysia . Apidologie , 33 : 303 – 314 .
- Neves , E. L. , Taki , H. , Silva , F. O. , Viana , B. F. and Kevan , P. G. 2006 . Flower characteristics and visitors of Merremia macrocalyx (Convolvulaceae) in the Chapada Diamantina, Bahia, Brazil . Lundiana , 2 : 97 – 102 .
- Nogueira-Neto , P. and Sakagami , S. F. 1966 . Nest structure of a subterranean stingless bee Geotrigona mombuca Smith (Meliponinae, Apidae, Hymenoptera) . Anais da Academia Brasileira de Ciências , 38 : 187 – 194 .
- O'Rourke , M. K. and Buchmann , S. L. 1991 . Standardized analytical techniques for bee-collected pollen . Environmental Entomology , 20 : 507 – 513 .
- Pennington , R. T. , Prado , D. E. and Pendry , C. A. 2000 . Neotropical seasonally dry forests and Quaternary vegetation changes . Journal of Biogeography , 27 : 261 – 273 .
- Pielou , E. C. 1977 . Mathematical ecology , New York, NY : John Wiley and Sons .
- Prado , D. 1993 . What is the Gran Chaco vegetation in South America? I. A review. Contribution to the study of flora and vegetation of the Chaco. V . Candollea , 48 : 145 – 172 .
- Prado , D. 2000 . Seasonally dry forests of tropical South America: From forgotten ecosystems to a new phytogeographic unit . Edinburgh Journal of Botany , 57 : 437 – 461 .
- Ramalho , M. 2004 . Stingless bees and mass flowering trees in the canopy of Atlantic Forest: A tight relationship . Acta Botanica Brasilica , 18 : 37 – 47 .
- Ramalho , M. , Giannini , T. C. , Malagodi-Braga , K. S. and Imperatriz-Fonseca , V. L. 1994 . Pollen harvest by stingless bee foragers (Hymenoptera, Apidae, Meliponinae) . Grana , 33 : 239 – 244 .
- Ramalho , M. , Kleinert-Giovannini , A. and Imperatriz-Fonseca , V. L. 1989 . Utilization of floral resources by species of Melipona (Apidae, Meliponinae): Floral preferences . Apidologie , 20 : 185 – 195 .
- Ramalho , M. , Kleinert-Giovannini , A. and Imperatriz-Fonseca , V. L. 1990 . Important bee plants for stingless bees (Melipona and Trigona) and Africanized honeybees (Apis mellifera) in neotropical habitats: A review . Apidologie , 21 : 469 – 488 .
- Roubik , D. W. 1989 . Ecology and natural history of tropical bees , Cambridge : Cambridge University Press .
- Roubik , D. W. 2006 . Stingless bee nesting biology . Apidologie , 37 : 124 – 143 .
- Roulston , T. H. and Cane , J. H. 1999 . Pollen nutritional content and digestibility for animals . Plant Systematics and Evolution , 222 : 187 – 209 .
- Sommeijer , M. J. , de Rooy , G. A. , Punt , W. and de Bruijn , L. L. M. 1983 . A comparative study of foraging behaviour and pollen resources of various stingless bees (Hym., Meliponinae) and honey bees (Hym., Apinae) in Trinidad, West-Indies . Apidologie , 14 : 205 – 224 .
- Tasei , J. N. and Aupinel , P. 2008 . Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae) . Apidologie , 39 : 397 – 409 .
- Van Nieuwstadt , M. G. L. and Ruano Iraheta , C. E . 1996 . Relation between size and foraging range in stingless bees (Apidae, Meliponinae) . Apidologie , 27 : 219 – 228 .
- P , Vergeron . 1964 . Interprétation statistique des resultants en matière d'analyse pollinique des miels . Annales de l'Abeille , 7 : 349 – 364 .
- Wilms , W. , Imperatriz-Fonseca , V. L. and Engels , W. 1996 . Resource partitioning between highly eusocial bees and possible impact of the introduced Africanized honey bee on native stingless bees in the Brazilian Atlantic rainforest . Studies on Neotropical Fauna and Environment , 31 : 137 – 151 .