Abstract
For the detection of pollen wall layers, the use of different staining methods for one and the same species is highly recommended. The usage of standard transmission electron microscopy (TEM) staining methods showed that the ektexine-layers have always the same contrast behaviour, while the endexine changes its electron opaqueness depending on the method used. However, the endexine can often not be discriminated from the other wall layers. A simple method to detect the endexine is the use of potassium permanganate, which stains the layer electron dense, producing a distinct contrast.
The knowledge on the endexine and its function is rather meagre. In the pollen wall, the endexine is a distinct exine layer between ektexine and intine (Hesse et al., Citation2009). It mainly consists of sporopollenin, lipids and proteins (Heslop-Harrison, Citation1968 a, Citationb; Heslop-Harrison et al., Citation1973). A morphological characteristic is its increasing thickness close to the aperture. Irregular channels within the endexine function as pumping systems (Rowley, Citation1995). The endexine thus plays a role in transport of nutrients and solvents. Furthermore the endexine, as well as other exine layers, are involved in harmomegathy (Wodehouse, Citation1935). This exine flexibility is an adaptation to volume changes (Mohl, Citation1834).
If an endexine is present, it can form a continuous or a discontinuous layer, or it is restricted to the apertural region. Many species are lacking an endexine, but where present, several types can be distinguished: compact, spongy and lamellar (Fægri & Iverson, Citation1989; Weber et al., Citation1998). The lamellar type is typical for all gymnosperm pollen (Guédès, Citation1982; Crane, Citation1985), whereas in angiosperms the endexine is generally compact except close to the apertures (Doyle et al., Citation1975). According to Rowley (Citation1995), the lamellar type often appears early in development in aperture regions. In Orobanche hederae L., microspores have a lamellar endexine (Ulrich, Citation2006), whereas the endexine is compact in mature pollen grains. In mature pollen grains of many angiosperms, the lamellar type is found in aperture regions only (Hesse, Citation2002; Doyle, Citation2005). But as demonstrated for Ambrosia artemisiifolia L., Brasenia schreberi J.F. Gmel. and Utricularia alpina Jacq., there are angiosperm species with continuously lamellar endexines (Jérémie et al., Citation1990; Taylor & Osborn, Citation2006; Diethart et al., Citation2007).
Many species of Araceae and other monocots were reported to lack an endexine (Zavada, Citation1983). Later, Grayum (Citation1992) detected an endexine in five genera of Araceae, while Weber et al. (Citation1998) used different techniques and histochemical staining methods to demonstrate that a distinct endexine is present in many species of Araceae. Especially when using thiocarbohydrazide-silver proteinate (TCH-SP), the endexine could be clearly differentiated from the other wall layers. However, even after this staining technique, the endexine is not always detectable. Also within the same plant family or the same genus, the staining properties can be very different, especially when the layer is thin and less compact or discontinuous. The present paper focuses on the different staining behaviours of various endexine types and shows a simple method how to detect them.
Materials and methods
For transmission electron microscopy (TEM) investigations, anthers of the investigated species were fixed in 3% glutaraldehyde (GA) in 0.1M phosphate buffer (pH 7.4) for six hours at room temperature. After washing in phosphate buffer and distilled water, the material was postfixed with 1% osmiumtetroxide (OsO4) and 0.8% potassium hexacyanoferrate (K4Fe(CN)6 · 3H2O) in a ratio of 2:1, for 16 hours at 4 °C. After washing in distilled water, the fixed material was dehydrated in 2,2-dimethoxypropane. The material was embedded in Spurr's low-viscosity epoxy resin (Spurr, Citation1969) and polymerised at 70 °C for about eight hours. Sections (60–90 nm thick) were cut with a diamond knife on a Reichert Ultracut microtome and collected on grids coated with Formvar. For common contrast, sections were stained for 40 minutes with uranyl acetate (U: prefabricated solution Ultrostain 1 by Leica) followed by five minutes lead citrate (Pb: prefabricated solution Ultrostain 2 by Leica). Sections on gold grids were stained with a modified Thiéry-test with 1% periodic acid (PA) for ten minutes, 0.2% thiocarbohydrazide (TCH) for 15 minutes and with 1% silver proteinate (SP) for ten minutes (see Weber & Frosch, Citation1995). The Thiéry-test was carried out with 1% PA for 30 minutes, 0.2% TCH for 8–16 hours and with 1% SP for 30 minutes (Thiéry, Citation1967). For the lipid-test, sections on gold grids were stained with 0.2% TCH for 5–16 hours, followed by 1% SP for 30 minutes (see Rowley & Dahl, Citation1977). For potassium permanganate staining, sections on copper grids were treated with 1% aqueous potassium permanganate solution (KMnO4) for seven minutes (see Hayat, Citation2000; Ulrich, Citation2006). Sections were examined with Zeiss EM109 and Zeiss EM900 transmission electron microscopes. Images were taken with a digital camera or scanned from negative photographs. Plant taxonomy and nomenclature are in accordance with Adler et al. (Citation1994) and pollen terminology is based on Hesse et al. (Citation2009). Voucher specimens are deposited in the Vienna Herbarium (WU; see ‘Specimens investigated’).
Abbreviations
KMnO4 – potassium permanganate; PA – periodic acid; SP – silver proteinate; TCH – thiocarbohydrazid; PA + TCH + SP – Thiéry-test; PA + TCH + SP (modified) – modified Thiéry-test; Pb – lead citrate; TCH + SP – lipid-test; U – uranyl acetate; U + Pb – uranyl + lead.
Results
The pollen wall of the investigated plants shows the typical organisation with ektexine, endexine and intine. An endexine may be compact (; ), spongy (; ), or lamellar (; ). Each type may be continuous (–C; ) or discontinuous (; ). An endexine that is restricted to the aperture region only, is characteristic for some plants (, C; ), while in other plants, such as Pseudolysimachion spicatum L., the endexine is completely absent (; ). Depending on the staining methods (), the endexine may appear electron-dense or electron-lucent, whereas the ektexine always stains electron-dense. The intine always stains electron-lucent (–D), except with the Thiéry-test ().
Figure 1. Cross-section of pollen walls showing three types of continuous endexines. A. Compact: Galium odoratum, the compact endexine (arrowhead) forms a continuous layer, the endexine is electron-dense with the Thiéry-test. B. Spongy: Thymus odoratissimus, the spongy endexine (arrowhead) forms a continuous layer, the endexine stains electron-dense with KMnO4. C, D. Lamellar: Ambrosia artemisiifolia, the lamellar endexine forms a continuous layer, the endexine is electron-dense with KMnO4 (arrowhead), only the lamellae are electron-lucent (D). Scale bars – 1 μm (A–C), 0.5 μm (D).
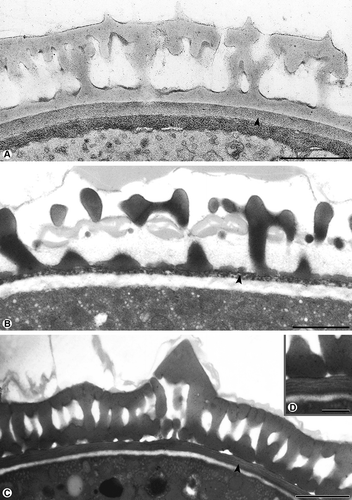
Figure 2. Cross-section of pollen walls. A. Plantago maritime, the compact endexine (arrowhead) forms a discontinuous layer, the endexine stains electron-dense with modified Thiéry-test. B. Corylus avellana, the spongy endexine is present in the aperture only (arrowhead), the endexine is electron-dense with KMnO4. C. Corylus avellana, the pollen wall at the transition zone of the aperture and interapertural area, a thick endexine in the aperture, very thin at the transition zone (arrowhead), the endexine is absent in the interapertural area, stained with KMnO4. D. Pseudolysimachion spicatum, the endexine is absent, stained with TCH+SP (lipid test). Scale bars – 1 μm.
Table 1. Endexine types and examples
Table 2. Staining behaviour of the endexine
Pollen of Galium odoratum L. has a compact endexine forming a continuous layer. The endexine stains electron-dense with the Thiéry-test (). Thymus odoratissimus Willd. pollen is characterised by a spongy endexine forming a continuous layer. The endexine appears electron-dense with the modified Thiéry-test (). The continuous lamellar endexine of Ambrosia artemisiifolia was not apparent by staining with the modified Thiéry-test. However, when staining with potassium permanganate, the lamellar endexine is clearly visible and stains electron-dense while the lamellae remain electron-lucent (). A compact endexine forming a discontinuous layer is present in Plantago maritima L. staining electron-dense with the modified Thiéry-test (). In Corylus avellana L. pollen, the spongy endexine appears in the aperture only and stains electron-dense after treatment with potassium permanganate. In this case, the endexine is enlarging into the apertural area (, C), is getting very thin at the transition to the interapertural area (), and is absent in the interapertural area. Pseudolysimachion spicatum L. pollen are a good example for a pollen wall, where the endexine is absent ().
How difficult the detection of the endexine can be, is demonstrated by the pollen walls occurring in three representative genera of the Lamiaceae, i. e. Melissa, Mentha, and Thymus (). In all three examples, a spongy to compact endexine is forming a thin continuous layer. The endexine of Melissa officinalis L. is almost not detectable with the modified Thiéry-test (). After U + Pb staining, the endexine appears electron-dense (), but as the layer is very thin, the results are again not very distinct. This wall layer is not visible after the lipid-test (). After staining with potassium permanganate, the results are positive as the endexine appears electron-dense (). In Mentha aquatica L., the endexine is not visible after staining with the modified Thiéry-test (). It appears slightly electron-dense both after staining with U + Pb () and the lipid-test (), but the results are not very distinct. Staining with potassium permanganate makes the endexine finally clearly visible in that it appears electron-dense ().
Figure 3. Cross-sections of pollen walls of three Lamiaceae species stained with different methods. Endexine spongy, continuous. A–D. Melissa officinalis: A. Endexine (arrowhead) not clearly visible with modified Thiéry-test; B. Endexine (arrowhead) electron-dense with U + Pb; C. Endexine not visible with TCH + SP (lipid test); D. Endexine (arrowhead) electron-dense with KMnO4. E–H. Mentha aquatica: E. Endexine not visible with modified Thiéry-test; F. Endexine electron-dense with U + Pb; G. Endexine (arrowhead) electron-dense with TCH + SP (lipid test); H. Endexine clearly visible, electron-dense with KMnO4 staining (arrowhead). I–L. Thymus odoratissimus: I. Endexine not visible with modified Thiéry-test; J. Endexine (arrowhead) not clearly visible with U + Pb; K. Endexine (arrowhead) only partially visible with TCH + SP (lipid test); L. Endexine (arrowhead) electron-dense with KMnO4. Scale bars – 1 μm.
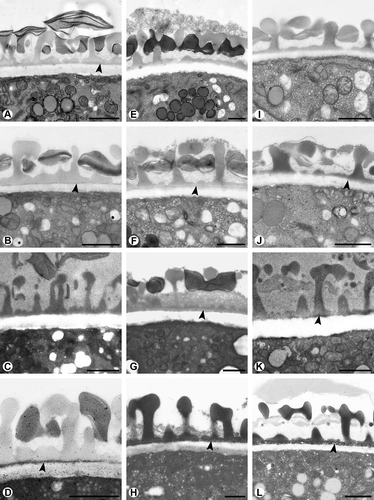
Similar to Mentha aquatica, the endexine of Thymus odoratissimus is not visible with the modified Thiéry-test () and also is not clearly visible after U + Pb staining (). After staining with the lipid-test, the endexine appears discontinuous (). As in Mentha and Melissa, potassium permanganate staining clearly differentiates the continuous endexinic layer ().
Discussion
The pollen walls are highly diverse in morphology and variations from the standard type of a pollen wall with ektexine (tectum, infratectum, and foot layer), endexine and intine. This has been observed in different lineages of angiosperms. All wall layers may be partly or totally reduced. The sporopolleninous ektexine is lacking in many species of Araceae (Weber et al., Citation1999; Anger & Weber, Citation2006), in some genera of Monimiaceae and Lauraceae (Walker, Citation1976) and in the aquatic Ceratophyllum demersum L., Ceratophyllaceae (Takahashi, Citation1995). The lack of foot layer is for instance reported from some genera of Haemodoraceae (Simpson, Citation1983).
The endexine in angiosperm pollen shows also a wide range of structural diversity. A morphological feature of the endexine is its increasing thickness closer to the aperture. According to Hesse et al. (Citation2009), the endexine may be compact, as in Oenothera (Takahashi & Skvarla, Citation1990), spongy, as in several Araceae (Weber et al., Citation1999) or lamellar as seen in Ambrosia (Diethart et al., Citation2007), Brasenia (Taylor & Osborn, Citation2006) and in many gymnosperm taxa (Kurmann, Citation1990, Citation1994). The endexine has a distinct substructure including laterally oriented white line-centred lamellations (Rowley, Citation1988, 1995). Each type may be continuous, discontinuous, restricted to the aperture only, or absent, as for example in Geranium (Weber, Citation1996).
The staining behaviour of the endexine is very heterogeneous and often leads to misinterpretations, especially when the layer is very thin and/or discontinuous. There are a number of studies reporting difficulties in discerning endexines (Praglowski, Citation1974; Zavada Citation1983; Grayum Citation1992). Particularly, in early-diverging angiosperms, the existence of an endexine was and still is a matter of discussion (Doyle et al., Citation1975; Doyle & Walker, Citation1975; Walker, Citation1976; Gabarayeva, Citation1995; Xu & Kirchoff, Citation2008). Even within the same plant family, as shown for the three Lamiaceae species Melissa officinalis, Mentha aquatica, and Thymus odoratissimus, which all have a thin, spongy to compact endexine, the staining properties are very different. The application of different methods to the pollen wall reveals that the ektexine layers have the same staining behaviour, while the endexine changes its electron-opaqueness depending on the method used. In the three species mentioned earlier, the endexine is not or is only barely detectable by staining with U + Pb, the standard TEM staining technique, just as staining with the modified Thiéry-test, which is used for general contrast enhancement in osmium-ferrocyanide-fixed material (Weber & Frosch, Citation1995). The endexine usually stains electron-dense in osmium-fixed material with the lipid-test, indicating lipidic compounds and electron-lucent with the Thiéry-test in osmium-free material (Weber, Citation1992; Weber et al. Citation1998). Nevertheless, the endexine can remain hidden even after the lipid-test, as shown in Melissa and Mentha. In such cases, staining with potassium permanganate can contribute to clarification. Potassium permanganate was used in the past as fixative and stain, with rather limited success. Potassium permanganate preserves and stains phospholipid-protein complexes in tissues; it is used as a stain for localising lignin in cell walls after GA-Os-fixation (Hayat, Citation2000). It was applied only recently as a stain for pollen walls. Used in a 1% aqueous solution of potassium permanganate, it particularly stains the endexine producing a distinct contrast. This has been confirmed for many species (Ulrich, Citation2006; Diethart et al., Citation2007). The application of different TEM staining techniques for one and the same sample is very important and highly recommended in order to avoid misinterpretations in the pollen wall architecture.
Specimens investigated
References
- Adler , W. , Oswald , K. and Fischer , R. 1994 . Exkursionsflora von Österreich , Stuttgart, , Wien : Eugen Ulmer .
- Anger , E. M. and Weber , M. 2006 . Pollen-wall formation in Arum alpinum . Annals of Botany , 97 : 239 – 244 .
- Crane , P. R. 1985 . Phylogenetic analysis of seed plants and the origin of angiosperms . Annals of the Missouri Botanical Garden , 72 : 716 – 793 .
- Diethart , B. , Sam , S. and Weber , M. 2007 . Walls of allergenic pollen: special reference to the endexine . Grana , 46 : 1 – 13 .
- Doyle , J. A. 2005 . Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses . Grana , 44 : 227 – 251 .
- Doyle , J. A. , Van Campo , M. and Lugardon , B. 1975 . Observations on exine structure of Eucommiidites and Lower Cretaceous angiosperm pollen . Pollen et Spores , 17 : 429 – 484 .
- Doyle , J. A. and Walker , J. W. 1975 . The bases of angiosperm phylogeny: palynology . Annals of the Missouri Botanical Garden , 62 : 664 – 723 .
- Fægri , K. and Iverson , J. 1989 . Textbook of pollen analysis , Chichester : John Wiley & Sons .
- Gabarayeva , N. 1995 . Pollen wall and tapetum development in Anaxagorea brevipes (Annonaceae): sporoderm substructure, cytoskeleton, sporopollenin precursor particles, and the endexine problem . Review of Palaeobotany and Palynology , 85 : 123 – 152 .
- Grayum , M. H. 1992 . Comparative external pollen ultrastructure of the Araceae and putatively related taxa . Monographs in Systematic Botany from the Missouri Botanical Garden , 43 : 1 – 167 .
- Guédès , M. 1982 . Exine stratification, ektexine structure and angiosperm evolution . Grana , 21 : 161 – 170 .
- Hayat , M. A. 2000 . Principles and techniques of electron microscopy: Biological applications , Cambridge : Cambridge University Press .
- Heslop-Harrison , J. 1968a . Pollen wall development . Science , 161 : 230 – 237 .
- Heslop-Harrison , J. 1968b . Tapetal origin of pollen coat substances in Lilium . New Phytologist , 67 : 779 – 786 .
- Heslop-Harrison , J. , Heslop-Harrison , Y. , Knox , R. B. and Howlett , B. 1973 . Pollen-wall proteins: ‘gametophytic’ and ‘sporophytic’ fractions in the pollen walls of the Malvaceae . Annals of Botany , 37 : 403 – 412 .
- Hesse , M. 2002 . The uniquely designed pollen aperture in Lasioideae (Araceae) . Aroideana , 25 : 51 – 59 .
- Hesse , M. , Halbritter , H. , Zetter , R. , Weber , M. , Buchner , R. , Frosch-Radivo , A. and Ulrich , S. 2009 . Pollen terminology. An illustrated handbook , New York, , Wien : Springer .
- Jérémie , J. , Lobreau-Callen , D. and Suarez-Cervera , M. 1990 . Un pollen à endexine lamellaire chez une angiosperme . Utricularia alpina Jacq. (Lentibulariaceae). Comptes Rendus de l'Académie des Sciences, Paris , 310 ( Série II ) : 1005 – 1010 .
- Kurmann , M. H. 1990 . Development of the pollen wall in Tsuga canadensis (Pinaceae) . Nordic Journal of Botany , 10 : 63 – 78 .
- Kurmann , M. H. 1994 . Pollen morphology and ultrastructure in the Cupressaceae . Acta Botanica Gallica , 141 : 141 – 147 .
- Mohl , H. 1834 . Über den Bau und die Formen der Pollenkörner. Beiträge zur Anatomie und Physiologie der Gewächse. Erstes Heft , Bern : Chr. Fischer und Comp .
- Praglowski , J. 1974 . Magnoliaceae Juss . World Pollen Spore Flora , 3 : 1 – 45 .
- Rowley , J. R. 1988 . Substructure within the endexine, an interpretation . Journal of Palynology , 24 : 29 – 42 .
- Rowley , J. R. 1995 . Are the endexines of pteridophytes, gymnosperms and angiosperms structurally equivalent? . Review of Palaeobotany and Palynology , 85 : 13 – 34 .
- Rowley , J. R. and Dahl , A. O. 1977 . Pollen development in Artemisia vulgaris with special reference to glycocalyx material . Pollen et Spores , 19 : 169 – 284 .
- Simpson , M. G. 1983 . Pollen ultrastructure of the Haemodoraceae and its taxonomic significance . Grana , 22 : 79 – 103 .
- Spurr , A. R. 1969 . A low-viscosity epoxy resin embedding medium for electron microscopy . Journal of Ultrastructure Research , 26 : 31 – 43 .
- Takahashi , M. 1995 . Development of structure-less pollen wall in Ceratophyllum demersum L. (Ceratophyllaceae) . Journal of Plant Research , 108 : 205 – 208 .
- Takahashi , M. and Skvarla , J. J. 1990 . Pollen development in Oenothera biennis (Onagraceae) . American Journal of Botany , 77 : 1142 – 1148 .
- Taylor , M. L. and Osborn , J. M. 2006 . Pollen ontogeny in Brasenia (Cabombaceae, Nympheales) . American Journal of Botany , 93 : 344 – 356 .
- Thiéry , J. P. 1967 . Mise en évidence des polysaccharides sur coupes fines en microscope électronique . Journal of Microscopy , 6 : 987 – 1018 .
- Ulrich , S. 2006 . Pollenmorphologie und Ultrastruktur ausgewählter Lamianae , Vienna : University Vienna. Unpublished diploma (‘master’) thesis .
- Walker , J. W. 1976 . “ Evolutionary significance of the exine in the pollen of primitive angiosperms ” . In The evolutionary significance of the exine , Edited by: Ferguson , I. K. and Muller , J. 251 – 308 . London : Academic Press .
- Weber , M. 1992 . Nature and distribution of the exine-held material in mature pollen grains of Apium nodiflorum L. (Apiaceae) . Grana , 31 : 17 – 24 .
- Weber , M. 1996 . The existence of a special exine coating in Geranium robertianum pollen . International Journal of Plant Sciences , 157 : 195 – 202 .
- Weber , M. and Frosch , A. 1995 . The development of the transmitting tract in the pistil of Haquetia epipactis (Apiaceae) . International Journal of Plant Sciences , 156 : 615 – 621 .
- Weber , M. , Halbritter , H. and Hesse , M. 1998 . The spiny pollen wall in Sauromatum (Araceae) – with special reference to the endexine . International Journal of Plant Sciences , 159 : 744 – 749 .
- Weber , M. , Halbritter , H. and Hesse , M. 1999 . The basic pollen wall types in Araceae . International Journal of Plant Sciences , 160 : 415 – 423 .
- Wodehouse , R. 1935 . Pollen grains. Their structure, identification and significance in science and medicine , New York, , London : McGraw-Hill .
- Xu , F.-X. and Kirchoff , B. K. 2008 . Pollen morphology and ultrastructure of selected species of Magnoliaceae . Review of Palaeobotany and Palynology , 150 : 140 – 153 .
- Zavada , M. S. 1983 . Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structures . Botanical Review , 49 : 331 – 379 .