Abstract
A new genus, Zlatkocarpus gen. nov., is described from the Peruc Korycany Formation (Cenomanian) of the Bohemian Cretaceous Basin in the Czech Republic based on inflorescence axis, fruits and pollen. Two species are assigned to the new genus, Zlatkocarpus brnikianus and Z. pragensis. Zlatkocarpus has a compound inflorescence consisting of primary axes bearing semi-decussately arranged spikes. Each spike has helically arranged unicarpellate and unilocular fruits. Each fruit apparently contains a single, orthotropous seed. The stigma is indistinct and sessile at the apex. The fruit wall has distinct globular protrusions (probable resin bodies). The fruits are supported at the base by a small floral cup and a bract. Pollen grains adhering to stigmatic areas and also on other surfaces of the fossil are monocolpate with a long colpus and an open reticulum. The pollen is similar to dispersed pollen broadly referred to the extinct pollen genus Retimonocolpites, but none of dispersed pollen genera are suitable for accommodating the fossils described here.
The Peruc-Korycany Formation from the Bohemian Cretaceous is rich in angiosperm remains and the flora, sometimes referred to as the Peruc Flora, is one of the classical leaf floras that has greatly influenced early ideas on the Cretaceous radiation of angiosperms. The first major studies of the flora were those of Velenovský (e.g., Citation1882, Citation1883, Citation1884, Citation1885 a, Citation1885 b, 1889). In more recent years, the flora has been studied by Zlatko and Jiří Kvaček (e.g., Kvaček, Z., Citation1992; Kvaček, J., Citation1995, 1998, Citation1999; Kvaček & Knobloch, Citation1997; Kvaček & Eklund, Citation2003) with the description of many new taxa and revisions of previously described taxa. Currently, more than 120 species of angiosperms have been identified. The fossils are mainly preserved as impressions and compressions in consolidated sediments and in rare cases show reproductive organs attached to leafy stems (Velenovský, Citation1889; Velenovský & Viniklář, Citation1926, Citation1927, Citation1929, Citation1931; Kvaček, Z., Citation1992). Recently, three-dimensionally preserved mesofossils were discovered in the Peruc-Korycany Formation. Combined studies of mesofossils and macrofossils provide an excellent opportunity to assemble more complete reconstructions of inflorescence structure in mid-Cretaceous angiosperms and the plants that produced the reproductive organs (Eklund & Kvaček, Citation1998; Kvaček, J., Citation2000; Kvaček & Eklund, Citation2003; Kvaček et al., Citation2005). These reproductive structures are small and borne in elongated inflorescences/infructescences and include species such as Mauldinia bohemica Eklund and J. Kvaček (Lauraceae), Pragocladus lauroides J. Kvaček and Eklund (Lauraceae) and several species of Myricanthium. Myricanthium was first compared to extant members of Myricaceae (Velenovský, 1889). New studies show, however, that it is probably not related to Myricaceae or other eudicots, and that fossils assigned to the genus are heterogeneous with some species perhaps related to Chloranthaceae (Kvaček & Eklund, Citation2003). The heterogeneity of Myricanthium is also supported by the present study. Unfortunately, the type material for Myricanthium is poorly preserved, but reinvestigation of M. pragense J. Kvaček & Eklund shows that it is distinct from the type material of Myricanthium (type species M. amentaceum Velenovský) and we have therefore included the species in a new genus, Zlatkocarpus (Z. pragensis). In addition to this species, we have also recognised another similar species that is here described as a new species, i.e. Z. brnikensis.
Materials and methods
The fossil plants described here were isolated from sandy mudstone collected in the clay pit near the village Brník, 60 km east of Prague (49° 58′ 59″ N, 14° 54′ 56″ E – Zlatkocarpus brnikensis) and Hloubětín-Hutě, northeast suburb of Prague (50° 06′ 34″ N, 14° 33′ 20″ E – Z. pragensis) within the Peruc-Korycany Formation of the Bohemian Cretaceous Basin (sensu Čech et al., Citation1980). Palynological data show a late mid-Cenomanian age for the formation (Pacltová, Citation1977, Citation1978).
The Brník section is interpreted as a deep palaeo-valley filled with fluvial sandstones and mudstones. It consists of two major parts. The basal part consists of fine granulated sands and claystones and represents fluvial sediments of a meandering river and floodplains. The upper part consists of sandstones interpreted as sediments of incised valley fill of a braided river (Uličný in Nguyen Tu et al., Citation2002). Fossil plants are recorded in the lower part, which consists of claystone bodies of various thicknesses. The fossil bearing claystone is also a resource for mining. Currently, a number of fern, conifer and angiosperm leaves have been recorded including Gleichenia spp., cf. Anemia fremontii Knowlton, Pagiophyllum sp., lauralean foliage “Eucalyptus” angusta Velenovský, “Aralia” formosa Velenovský, Dicotylophyllum velenovskyi Knobloch, Liriodendropsis simplex (Newberry) Newberry, and others. Sandstones of the upper part are usually barren or contain only poorly preserved leaf impressions.
The material from Prague, Hloubětín-Hutě (Kvaček, J., Citation1992 a, 1992b), was described for the first time by Kvaček and Eklund (Citation2003). It was collected from a temporary excavation for the basement of Hotel Pramen in Prague. Unfortunately, the section was not documented sedimentologically. Currently, 27 taxa have been recorded including various ferns assigned to Gleichenia spp., conifers assigned to “Sequoia” heterophylla Velenovský, Quasisequoia crispa (Velenovský) J. Kvaček, Geinitzia sp., Brachyphyllum sp., several angiosperm leaf taxa including Grevilleophyllum constans (Velenovský) Velenovský, “Eucalyptus” angusta Velenovský and Cocculophyllum cinnamomeum (Velenovský) Velenovský, Proteophyllum araliopsis Velenovský & Viniklář, P. productum Velenovský & Viniklář, P. minutum Velenovský & Viniklář, angiosperm reproductive structures (Mauldinia bohemica, Pragocladus lauroides J. Kvaček & Eklund) and several unnamed reproductive structures (see Kvaček & Eklund, Citation2003).
Specimens were extracted from the mudstone by bulk maceration and treated through standard techniques as described in Eklund and Kvaček (Citation1998). The material was preliminary examined using Wild and Olympus binocular microscopes. More detailed examinations were done using a scanning electron microscope (Phillips 515 and Hitachi S-4300). All specimens and preparations are deposited in the palaeobotanical collections of the National Museum, Prague (NMP).
Systematic palaeontology
Genus Zlatkocarpus gen. nov.
Derivation of generic name
After Zlatko Kvaček in recognition of his contribution to our understanding of the Cretaceous and Tertiary vegetation of the Bohemian Massif.
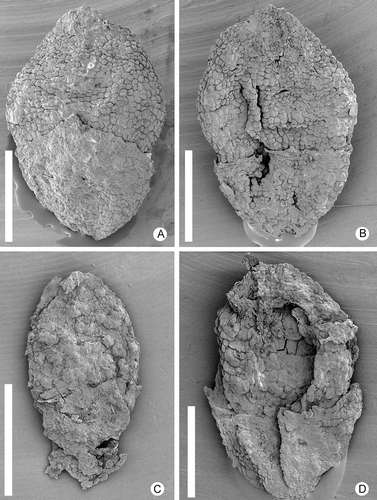
Figure 1. Zlatkocarpus brnikensis sp. nov. A. Larger fragment of secondary axis with helically arranged fruits, holotype, F 3143. B–E. Details of the holotype: B. Fruit with well pronounced perianth; C. Stigmatic area with adhering pollen; D. Attachments of fruits to secondary axis, note perianth and small bracts; E. Detail of fruits with well pronounced resin bodies. Scale bars – 1.5 mm (A); 600 μm (B, D, E); 30 μm (C).
Generic diagnosis
Flowers sessile, aggregated in spikes; each flower supported by a persistent bract arranged helically along an elongated axis. Flowers apparently unisexual. Pistillate flowers consisting of a single carpel surrounded by a floral cup. Stigmatic area sessile, indistinct. Fruit: a one-seeded berry. Fruit wall parenchymatous with resin bodies embedded in the tissue under the epidermis. Pollen adhering to the surface of fruits of Retimonocolpites-type, monocolpate, reticulate-columellate with smooth muri.
Type species
Zlatkocarpus brnikensis sp. nov.
Other species
Zlatkocarpus pragensis (J. Kvaček & Eklund) comb. nov.
Zlatkocarpus brnikensis sp. nov. ( )
Derivation of specific epithet
From the Brník locality, where the fossil was discovered.
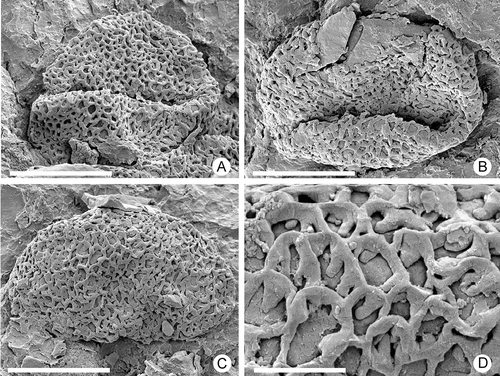
Figure 3. Zlatkocarpus brnikensis sp. nov. A–F. Pollen grains of Retimonocolpites type: A. Group of pollen grains, holotype, F 3143; B. Pollen with colpus, F 3147; C. Group of pollen grains, two of them with partly detached reticulum, F 3146; D. Pollen with colpus and partly detached reticulum, F 3147; E. Detail of exine with heterogenous columellae and smooth muri, holotype, F 3143; F. Pollen adhering on stigma, holotype, F 3143. Scale bars – 30 μm (A); 12 μm (C); 6 μm (B, D, F); 1.2 μm (E).
Specific diagnosis
Floral cup large, encircling the basal part of fruit for about one third of the total length of the fruit. Fruits elliptical in outline with slightly pointed apex and base. Pollen with heterogeneous reticulum and distinct colpus margin.
Holotype designated here
NMP F 3143; illustrated here in .
Paratypes designated here
NMP F 3144–F 3147.
Type locality
Clay pit near the village of Brník, 60 km east of Prague, Czech Republic (49° 58′ 59″ N, 14° 54′ 56″ E).
Type horizon and age
Peruc Member, Peruc-Kory-cany Formation, Cenomanian, Late Cretaceous.
Description
All specimens are pistillate, preserved in the fruiting stage. The most informative specimen (the holotype, F 3143) is a fragment of a spike, about 6.5 mm long and 2.5 mm wide. It has about 50 fruits borne in a helical arrangement (). The inflorescence axis is about 0.6 mm wide and characterised by transversely aligned wrinkles. Fruits are densely spaced and born in the axils of small triangular bracts, spaced at a distance of 0.5–1 mm, each with a long free tip about 0.12–0.15 mm long. The other specimens (F 3144–F 3147) are isolated fruits.
Each fruit is encircled by a floral cup, about 0.3–0.6 mm long (, B, 2A–D). The floral cup is broadly triangular in abaxial view. In adaxial view it has an irregular outline with one or two additional tips (, D). The floral cup is closely adhering to the fruit and apparently fused to the fruit wall at least at the base. Epidermal cells are isodiametric, about 16–25 μm in diameter, and irregularly arranged. No stomata or trichomes were observed. The nature of the floral cup is unclear, although its position suggests that it is a perianth.
Fruits are elliptical in outline with slightly pointed apex and base, about 0.8–1.2 mm long and 0.45–0.7 mm wide (, B, D, E, 2A–D). The stigmatic area is sessile and non-protruding, seen as a circular area of indistinct cells about 0.8–0.1 mm in diameter (, C, E). The surface of the fruit is irregularly bulging from densely spaced resin bodies embedded in the fruit wall under the epidermis (). The epidermis consists of distinct, almost isodiametric, irregularly arranged epidermal cells, about 10–25 μm × 20–50 μm, with convex outer periclinal walls (, B). The fruits are interpreted as unicarpellate. The single seed is apparently orthotropous.
Pollen occurs abundantly on the surface of the fruits, typically close to the stigmatic area and near the margin of the floral cup (). Grains are monocolpate and coarsely reticulate, circular to broadly elliptical in equatorial outline, with an equatorial diameter of about 12–18 μm (–F). The colpus is straight and does not reach the equator. Typically, the grains are folded in the colpus area and the margin is usually hidden (, B), but a single specimen shows a distinct colpus margin (). The reticulum is heterogeneous; smaller lumina are about 0.05–0.2 μm in diameter and larger lumina are up to about 0.4–0.8 μm in diameter (). Muri are narrow and low with a flattened to rounded and smooth profile, about 0.2–3 μm wide; columellae are irregularly spaced, about 0.3 μm long. Columellae and reticulum easily detach from the foot layer (, D).
Zlatkocarpus pragensis (J. Kvaček & Eklund) comb. nov
()
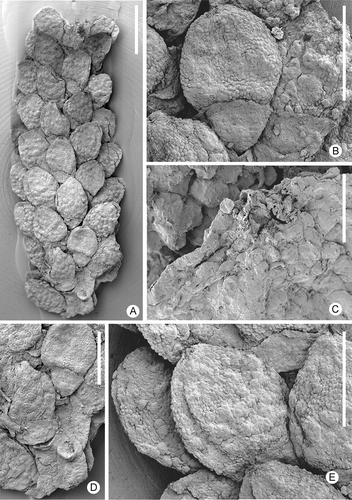
Figure 4. Zlatkocarpus pragensis (J. Kvaček & Eklund) comb. nov. A. Basal and medial parts of secondary axis with helically arranged fruits, holotype, F 2892. B. Apical part of empty secondary axis with bracts, F 3155. C–E. Fragments of secondary axis showing arrangement of fruits and supporting bracts, F 3154: C. Overview; D. Fruit with its bract and perianth; E. Bract and perianth. F. Details of empty bracts, detail of (B). G. Isolated fruit with well preserved perianth bearing conspicuous epidermal structure, F 3150. H. Basal part of primary axis with one secondary axis still attached, F 2894. Scale bars – 5 mm (H); 500 μm (A); 400 μm (B); 300 μm (C, F); 200 μm (E, G); 150 μm (D).
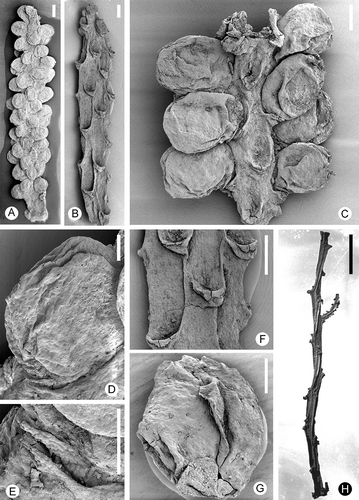
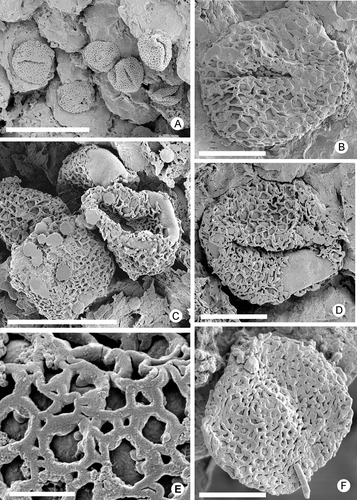
Figure 5. Zlatkocarpus pragensis (J. Kvaček & Eklund) comb. nov. A–E. Details of the holotype, F 2892 (see ): A. Detail of stigma with adhering pollen; B. Fruit with resin bodies; C. Basal part of secondary axis with attached fruits; D. Retimonocolpites pollen adhering on stigma; E. Retimonocolpites pollen adhering near to stigma, with elongate outline. Scale bars – 1 mm (C); 400 μm (B); 30 μm (A); 6 μm (D, E).
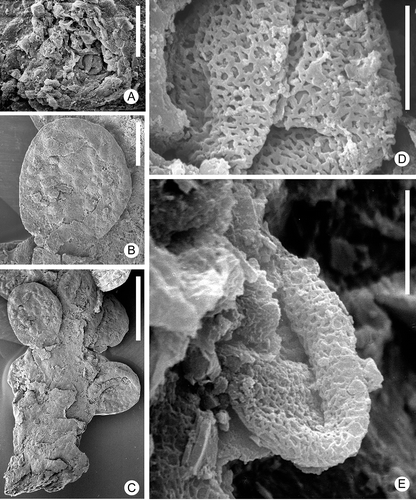
Figure 6. Zlatkocarpus pragensis (J. Kvaček & Eklund) comb. nov. A–D. Retimonocolpites pollen, details of F 3155 (): A. Pollen with colpus, adhering on empty secondary axis; B. Pollen with colpus, adhering on empty secondary axis; C. Pollen with nearly homogenous reticulum, adhering on empty secondary axis; D. Detail of exine with columellae and smooth muri. Scale bars – 6 μm (A–C); 1.2 μm (D).
Basionym
Myricantheum pragense (Kvaček & Eklund, Citation2003, p. 1025, , ).
Emended diagnosis
As in Kvaček and Eklund (Citation2003) with the following addition: Floral cup short, about one fourth of the total length of the fruit, most pronounced adaxially. Pollen with almost homogeneous reticulum.
Holotype
NMP F 2892 (see Kvaček & Eklund, Citation2003).
Material
NMP F 2893, F 2895–F 2898, F 3148–F 3155, F 3461–F 3565.
Type locality
Hloubětín-Hutě, north-eastern suburb of Prague, Czech Republic (50° 06′ 34″ N, 14° 33′ 20″ E).
Type horizon and age
Peruc Member, Peruc-Kory-cany Formation, Cenomanian, Late Cretaceous.
Description and comments
The type material (holotype and paratypes) originally ascribed to Myricantheum pragense by Kvaček and Eklund (Citation2003) includes remnants of compound inflorescence/infructescence axes () as well as isolated secondary axes (spikelets) with or without attached floral organs (–C). The spikelets with the attached floral units and the empty inflorescences/infructescence fragments are assigned to the same species based on the characteristic secondary bracts and rare remnants of fruits in the empty inflorescences/infructescence. Additional material includes axes with a various number of fruitlets attached (F 2547, F 3148, F 3149, F 3151, F 3152, F 3461).
Pollen attached to the stigma surface (, D, E) is monocolpate and coarsely reticulate, circular to ellipsoidal in equatorial outline, with an equatorial diameter of about 8–10 μm. The reticulum is almost homogeneous with only a few smaller lumina (–D). Larger lumina are about 0.1–0.3 μm in diameter. Muri are narrow and low with slightly triangular and smooth profile, about 1 μm wide (). Columellae are about 0.3 μm long and widely spaced.
Zlatkocarpus pragensis is in general structure very similar to Z. brnikensis, but the inflorescence/infructescences are more completely preserved showing a compound organisation with lateral spikelets arranged in a semi-decussate to helical arrangement along the main axis (). Zlatkocarpus pragensis differs mainly by its much smaller floral cup that is also much more pronounced at the abaxial side than on the adaxial side (). Fruits are broadly elliptical to almost circular in outline with an almost smooth surface and with a thick cuticle (, G). The stigmatic area is sessile and circular similar to that in Z. brnikensis.
Discussion
Recognition of the new genus
Zlatkocarpus is based on structurally preserved inflorescence/infructescence axes with attached floral/fruiting units and adhering monocolpate pollen with a semitectate-reticulate pollen wall. Muri of the reticulum are smooth without supratectal ornamentation. Similar dispersed pollen grains are typically assigned to the pollen genus Retimonocolpites. The current use of this dispersed pollen genus is very broad and taxa included in Retimonocolpites clearly represent a heterogeneous assemblages of lineages with some species probably related to monocototyledons and others perhaps related to various basal lineages of angiosperms (Friis et al., Citation2010).
Comparison of Zlatkocarpus with extant plants indicates that this fossil may represent an extinct lineage of basal angiosperms. It shows some similarity with Chloranthaceae, but the fossil inflorescences have distinct helical phyllotaxis in contrast to the prevailing decussate phyllotaxis of Chloranthaceae. However, pollen very similar to that of Zlatkocarpus has been found in unequivocal Araceae (Friis et al., Citation2010) and affinity of Zlatkocarpus with early diverging monocots cannot be excluded.
Fossil inflorescences/infructescences similar in gross morphology to the fossils described here are known from several other Early and mid-Cretaceous floras. They are particularly similar to a variety of inflorescences/infructescences from the Peruc flora of the Bohemian Basin generally assigned to the extinct genus Myricanthium. The type species, M. amentaceum, was established by Velenovský (Citation1889) based on compression/impression material that lacks details on supporting organs or flowers. Later studies have identified structurally preserved material together with the compression/impression fossils that clearly demonstrates that Myricanthium-like fossils from the Bohemian Cretaceous include a diverse and heterogeneous complex of plants (Kvaček, Z., 1992; Eklund & Kvaček, Citation1998; Kvaček & Eklund, Citation2003). Currently, three species have been distinguished. Mauldinia bohemica and Pragocladus lauroides both have bisexual and trimerous flowers and were assigned to the Laurales (Eklund & Kvaček, Citation1998; Kvaček & Eklund, Citation2003). Myricanthium pragense was described for fossils with unisexual and simple flowers and was tentatively compared with extant Chloranthaceae (Kvaček & Eklund, Citation2003).
Because of the poor preservation of the type material of Myricanthium amentaceum it is unknown whether this species had unisexual or bisexual flowers and detailed comparison with the structurally preserved fossils is not possible. We therefore suggest that the genus Myricanthium is reserved for inflorescences/infructescences for which details of floral units and supporting organs are uncertain and exclude M. pragense from the genus. Instead a new genus, Zlatkocarpus, is established for organically preserved inflorescences/infructescences bearing densely packed, simple floral units supported by small bracts borne helically along an elongated axis as described here, including Z. brnikensis as a new species and Z. pragensis transferred from Myricanthium. In Z. pragensis, the spikes are clearly secondary axes from a compound spike. The secondary axes are borne in a semi-decussate to helical arrangement. Although the inflorescences of Z. brnikensis are more fragmentary preserved, the similarity to Z. pragensis suggests that Z. brnikensis also had compound inflorescences. Each floral unit consists of a single ovary/fruit encircled by a floral cup. In Z. brnikensis, the floral cup completely encircles the ovary/fruit and is fused to the ovary wall for most of its length. The most straightforward interpretation of this structure is that it represents a perianth of several fused parts that are marked on the cupule margin as extending tips. A complication in this interpretation is, however, that the floral cup is more bract-like in Z. pragensis and does not completely encircle the ovary/fruit as it does in Z. brnikensis. An interpretation of the floral cup as a supporting bract rather than a perianth would imply the floral unit represents a condensed tertiary axis of a compound inflorescence with small naked flowers. The spherical bodies, probably resin bodies, preserved in the ovary wall and other tissues may be remains of ethereal oil cells.
We have interpreted the fossil flowers and inflorescences as unisexual and pistillate. Most of the specimens appear to be preserved in a post-anthetic stage and the possibility that stamens have been shed from an originally bisexual flower without leaving any traces cannot be excluded. However, this alternative is less likely. None of the specimens has any remains of filaments or scars from detached stamens. In addition, the floral cup is fused to the ovary wall for most of its length in Zlatkocarpus brnikensis and shows no vascularisation or other evidence for the presence of stamens.
The individual ovaries/fruits of Zlatkocarpus are very similar to those of Couperites mauldinensis Pedersen, Crane, Drinnan & Friis from the Cenomanian Mauldin Mountain flora of Maryland, USA (Pedersen et al., Citation1991) and Couperites sp. from the early or mid-Albian Puddledock flora of Virginia, USA (Friis et al., Citation1997). In both genera, fruits are apparently berries with a single seed and the fruit wall is characterised by abundant resin bodies embedded in the parenchyma tissue below the epidermis. Both also have a sessile stigma and monocolpate reticulate pollen, but ovaries/fruits of Couperites are distinct in having an elongate, slightly decurrent stigmatic ridge. Pollen grains have supratectal ornamentation of minute spines. Seed structure is well-known for Couperites, anatropous and pendant, while it is less clear, but apparently orthotropous in Zlatkocarpus. Fruits of Couperites are always found isolated and there is no information on inflorescences structure for this fossil.
Comparison with dispersed pollen taxa
Pollen grains broadly similar to those observed on the stigmatic areas of Zlatkocarpus pragense and Z. brnikensis are common in dispersed palynological assemblages from the Early and mid-Cretaceous. This kind of dispersed grains is typically assigned to the form genus Retimonocolpites or sometimes to Liliacidites. Both genera have been used in a very broad sense and include a diverse and heterogeneous assemblage of pollen types that are probably systematically widely separated.
The type species for Retimonocolpites, R. dividuus Pierce, was first described from the Cenomanian of Minnesota, USA, by using light microscopy (LM) alone. Thus, no details of micromorphology or ultrastructure were described or illustrated (Pierce, Citation1961). The presence of a distinct aperture almost encircling the grain and dividing it into two halves as well as a reticulum that occasionally separates from the main body of the grain were mentioned as defining characters for the species (Pierce, Citation1961). Other features that are probably of systematic importance such as presence or absence of supratectal ornamentation and ultrastructure of the pollen wall are unknown. A study of pollen assigned to R. dividuus from the Early Cretaceous (Late Albian) of Delaware, USA, using LM, scanning electron microscopy (SEM) and transmission electron microscopy (Walker & Walker, Citation1984), showed distinct striate ornamentation of the muri, but that pollen has a long, extended colpus and may not be identical to the type.
The type species for Liliacidites, L. kaitangataensis Couper, was first described from the Late Cretaceous and Early Tertiary of New Zealand (Couper, Citation1953). It is monocolpate, characterised by a distinctly graded reticulum and muri with beaded supratectal ornamentation. Liliacidites has often been used as a repository for other kinds of reticulate pollen, including grains with a non-graded reticulum and smooth muri. One of these described by Walker and Walker (Citation1984) from the Middle-Late Albian of Maryland, USA as “Liliacidites” minutus (=Clavatipollenites minutus Brenner) is similar to the grains of Zlatkocarpus pragensis, but the reticulum in Z. pragensis is denser and number of small lumen is higher.
The pollen grains observed on the surfaces of Zlatkocarpus have smooth muri and shorter colpi than in Retimonocolpites dividuus and the Bohemian pollen is clearly distinct from the type species of Retimonocolpites. Other pollen grains assigned to Retimonocolpites that have been studied using SEM are distinguished from the pollen of Zlatkocarpus by differences in lumen size and shape, length of columellae, or presence of supratectal ornamentation. Pollen grains closely similar to those of Zlatkocarpus have also been found in situ in stamens borne in distinct aroid inflorescences (Friis et al., Citation2010).
Systematic position of Zlatkocarpus
The character combination in Zlatkocarpus is unusual and based on the data currently available, it is not possible to place the fossils in a modern family or order. Monocolpate, reticulate pollen is restricted to non-eudicot angiosperms. The presence of a heterogeneous reticulum with smaller and larger lumina in monocolpate pollen has been suggested as a defining feature for monocotyledons (Walker & Walker, Citation1984), and very similar pollen has been observed in situ in undisputable Araceae flowers from the Early Cretaceous of Portugal (Friis et al., Citation2010). However, if the resin bodies embedded in the Zlatkocarpus fruit wall represent ethereal oil cells, affinities with monocotyledons are unlikely. In monocots, only Acorus has ethereal oil cells, but Acorus is distinguished from Zlatkocarpus by its bisexual flowers, much denser arrangement of the flowers in the inflorescence, and the tectate-perforate pollen wall. Among other non-eudicot angiosperms several corresponding features occur in members of the Chloranthaceae. Ascarina and Hedyosmum have unisexual flowers borne in compound spikes and typically are supported by bracts. Flowers are either naked or have an epigynous, trimerous perianth that is fused to the ovary for most of its length and only free at the top of the gynoecium like in the pistillate flowers of Hedyosmum.
If our interpretation of the floral cup in Zlatokocarpus as a perigynous perianth is correct, it is comparable to the perianth of Hedyosmum. The gynoecium in Chloranthaceae consists of a single carpel with a single orthotropous ovule. The stigma is sessile. The phyllotaxis of Chloranthaceae is mostly opposite and decussate, while in Zlatkocarpus only the secondary spikes are borne in a semi-decussate arrangement, but floral units are borne in a helically arrangement. Pollen grains in Chloranthaceae are monocolpate-trichotomocolpate in Ascarina, while the other genera of the family have more unusual aperture configurations. All have reticulate pollen grains. Grains with smooth muri are known in Sarcandra and Chloranthus, but none of these have monocolpate pollen, while Hedyosmum and Ascarina have muri with distinct supratectal ornamentation.
Simple, unisexual flowers borne in spikes are also known for some extant members of the Piperales, but pollen in Piperales is tectate with a continuous, non-reticulate tectum. Inflorescences in Piperales are mostly simple spikes, but an exception was described for the perianthless. Peperomia fraseri C.DC. has numerous spikes borne along a raceme (Remizova et al., Citation2005); it is, however, clearly distinct from Zlatkocarpus by having bisexual flowers proximally and pistillate flowers distally in the spikes, a high degree of polymorphism in the structure of bracts in the same inflorescence, and much denser secondary branching (Remizova et al., Citation2005).
Conclusion
The two new species of Zlatkocarpus described here add further diversity to the complex of Myricanthium-like inflorescences. The organisation of the inflorescence and flowers is in line with that of many other Early and mid-Cretaceous angiosperms: flowers are small, often unisexual with few floral parts and arranged in dense inflorescences. Although the radiation of eudicot angiosperms was well under way in the Cenomanian, the diversity of non-eudicot angiosperms was still much higher in the Cenomanian than later in the Cretaceous and today, and includes a considerable extinct component. Zlatkocarpus with its monocolpate pollen clearly belongs to this non-eudicot component of Cretaceous floras. The precise systematic placement of Zlatkocarpus is, however, unclear. Features of the pollen grains may suggest affinity with monocotyledons, but organisation of the flowers and fruit does not allow an unambiguous phylogenetic assignment. In flower and fruit characters, the fossils show particular similarity to some Chloranthaceae, but Zlatkocarpus is distinguished from Chloranthaceae by the spiral phyllotaxis of the spikes, and the pollen grains differ in several respects from those of Chloranthaceae.
Acknowledgements
We thank H. Eklund and Y. Arremo, Stockholm, for help with SEM photographs of Zlatkocarpus pragensis. This work was supported by Synthesys (SE-TAF-1153), the Academy of Sciences of the Czech Republic (grant no. IAA 304070701), the Ministry of Culture of the Czech Republic (grant no. MK 00002327201), and the Swedish Natural Science Research Council.
References
- Čech , S. , Klein , V. , Kříž , J. and Valečka , J. 1980 . Revision of the Upper Cretaceous stratigraphy of the Bohemian Cretaceous Basin . Věstník Ústředního ústavu geologického , 55 : 277 – 296 .
- Couper , R. A. 1953 . Upper Mesozoic and Cainozoic spores and pollen grains from New Zealand . New Zealand Geolological Survey Palaeontological Bulletin , 22 : 1 – 77 .
- Eklund , H. and Kvaček , J. 1998 . Lauraceous inflorescences and flowers from the Cenomanian of Bohemia (Czech Republic, Central Europe) . International Journal of Plant Sciences , 159 : 668 – 686 .
- Friis , E. M. , Crane , P. R. and Pedersen , K. R. 1997 . “ Fossil history of magnoliid angiosperms ” . In Evolution and diversification of land plants , Edited by: Iwatsuki , K. and Raven , P. H. 121 – 156 . Tokyo : Springer .
- Friis , E. M. , Pedersen , K. R. and Crane , P. R. 2010 . Diversity in obscurity: fossil flowers and the early history of angiosperms . Philosophical Transactions of the Royal Society B: Biological Sciences , 65 : 369 – 382 .
- Kvaček , J. 1992a . “ Leaf and fruit compressions from the Bohemian Cenomanian ” . In Palaeovegetational development in Europe and regions relevant to its palaeofloristic evolution, Proceedings of the Pan-European Palaeobotanical Conference, Vienna, 19–23 September 1991 , Edited by: Kovar-Eder , J. 301 – 304 . Vienna : Museum of Natural History .
- Kvaček , J. 1992b . A new Cenomanian floral locality from Praha . Časopis Národního muzea v Praze, Řada přírodovědná , 158 : 42
- Kvaček , J. 1995 . Cycadalean and bennettitalean leaf compressions of the Bohemian Cenomanian, Central Europe . Review of Palaeobotany and Palynology , 84 : 389 – 412 .
- Kvaček , J. 1998 . Cuticle analysis of gymnosperms of the Bohemian Cenomanian , Prague : Charles University, PhD Diss .
- Kvaček , J. 1999 . New data and revision of three gymnosperms from the Cenomanian of Bohemia – Sagenopteris variabilis (Velenovský) Velenovský, Mesenea bohemica (Corda) comb. n. and Eretmophyllum obtusum (Velenovský) comb. n . Acta Musei Nationalis Pragae, Series B, Historia Naturalis , 55 : 15 – 24 .
- Kvaček , J. 2000 . Frenelopsis alata and its microsporangiate and ovuliferous reproductive structures from the Cenomanian of Bohemia (Czech Republic, Central Europe) . Review of Palaeobotany and Palynology , 112 : 51 – 78 .
- Kvaček , J. and Eklund , H. 2003 . A report on newly recovered reproductive structures from the Cenomanian of Bohemia (Central Europe) . International Journal of Plant Sciences , 164 : 1021 – 1039 .
- Kvaček , J. , Falcon-Lang , H. and Dašková , J. 2005 . A new Upper Cretaceous ginkgoalean reproductive structure Nehvizdyella from the Czech Republic and its whole-plant reconstruction . American Journal of Botany , 92 : 1958 – 1969 .
- Kvaček , J. and Knobloch , E. 1997 . Representatives of the genus Nilsonia Brogniart from the Cenomanian of the Bohemian Massif (Czech Republic, Central Europe) . Review of Palaeobotany and Palynology , 97 : 41 – 52 .
- Kvaček , Z. 1992 . Lauralean angiosperms in the Cretaceous . Courier Forschungsinstitut Senckenberg , 147 : 345 – 367 .
- Nguyen Tu , T. T. , Kvaček , J. , Uličný , D. , Bocherens , H. , Mariotti , A. and Broutin , J. 2002 . Isotope reconstruction of plant palaeoecology. Case study of Cenomanian floras from Bohemia . Palaeogeography, Palaeoclimatology, Palaeoecology , 183 : 43 – 70 .
- Pacltová , B. 1977 . Cretaceous angiosperms of Bohemia, Central Europe . Botanical Review , 43 : 128 – 142 .
- Pacltová , B. 1978 . “ Significance of palynology for the biostratigraphic division of the Cretaceous of Bohemia ” . In Paleontological Conference, Department of Paleontology, Faculty of Natural Sciences, Charles University, Praha , Edited by: Pokorný , V. 93 – 109 . Prague : Charles University .
- Pedersen , K. R. , Crane , P. R. , Drinnan , A. N. and Friis , E. M. 1991 . Fruits from the mid-Cretaceous of North America with pollen grains of the Clavatipollenites type . Grana , 30 : 577 – 590 .
- Pierce , R. L. 1961 . Lower Upper Cretaceous plant microfossils from Minnesota . Minnesota Geological Survey Bulletin , 42 : 1 – 86 .
- Remizova , M. , Rudall , P. L. and Sokoloff , D. D. 2005 . Evolutionary transitions among flowers of perianthless Piperales: inferences from inflorescence and flower development in the anomalous species Peperomia fraseri (Piperaceae) . International Journal of Plant Sciences , 166 : 925 – 943 .
- Velenovský , J. 1882 . Die Flora der böhmischen Kreideformation . Beiträge zur Paläontologie Österreich-Ungarns und des Orientes , 2 : 8 – 32 .
- Velenovský , J. 1883 . Die Flora der böhmischen Kreideformation . Beiträge zur Paläontologie Österreich-Ungarns und des Orientes , 3 : 1 – 22 .
- Velenovský , J. 1884 . Die Flora der böhmischen Kreideformation . Beiträge zur Paläontologie Österreich-Ungarns und des Orientes , 4 : 1 – 14 .
- Velenovský , J. 1885a . Die Flora der böhmischen Kreideformation . Beiträge zur Paläontologie Österreich-Ungarns und des Orientes , 5 : 62 – 75 .
- Velenovský , J. 1885b . Die Gymnospermen der böhmischen Kreideformation , Prague : E. Greger .
- Velenovský , J. 1889 . Květena Českého Cenomanu . Rozpravy Královské České Společnosti Nauk , 7 : 1 – 75 .
- Velenovský , J. and Viniklář , L. 1926 . Flora Cretaca Bohemiae I , Prague : Státní Geologický ústav Československé Republiky .
- Velenovský , J. and Viniklář , L. 1927 . Flora Cretaca Bohemiae II , Prague : Státní Geologický ústav Československé Republiky .
- Velenovský , J. and Viniklář , L. 1929 . Flora Cretaca Bohemiae III , Prague : Státní Geologický ústav Československé Republiky .
- Velenovský , J. and Viniklář , L. 1931 . Flora Cretaca Bohemiae IV , Prague : Státní Geologický ústav Československé Republiky .
- Walker , J. W. and Walker , A. G. 1984 . Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants . Annals of the Missouri Botanical Garden , 71 : 464 – 521 .