Abstract
Melissopalynological analysis of 39 honey samples from Oaxaca, Mexico, enabled us to establish the important plant sources exploited by bees during the principal harvest in four districts of the State of Oaxaca, Mexico. A total of 64 taxa belonging to 29 families were recorded. These subtropical honeys were characterised by their botanical origin as follows: (a) monofloral honeys of Bursera simaruba, Clethra mexicana, Cordia alliodora, Lonchocarpus sp., Mangifera indica, Miconia argentea, Orbignya cohune and Quercus sp.; (b) bifloral honeys with an association of Heliocarpus donnell-smithii and Ceiba sp., Lonchocarpus sp. and Mimosa pudica, H. donnell-smithii and Mangifera indica, Miconia argentea and Miconia tenuiflora; (c) oligofloral honeys of Asteraceae; and (d) multifloral honeys with three or four species ≥10%. Monofloral honeys were placed in classes I, II, III, IV and V. Oligofloral were class II, bifloral were classes I and II, and polyfloral honeys were assigned to classes I, II and III. Honey samples of Apis mellifera had a diversity index range of 0.3 to 2.7. It is well known that this bee is polylectic and has a heterogeneous foraging behaviour. In the State of Oaxaca, it prefers resources of secondary vegetation from low deciduous forest, although taxa of economic importance were also utilised, for instance, Mangifera indica and Citrus sinensis. Oaxaca has important and diverse native resources, and beekeeping activity needs to be promoted because of its potential to develop new types of honey.
Mexican beekeeping has a high social and economic value; there are about 40 000 beekeepers that produce on average of 26 000 honey tons per annum. The State of Oaxaca has a rich biodiversity with 8431 vascular plants (García-Mendoza et al., Citation2004), and contributes to 5.2% of the national honey production. This percentage might increase over the next few years. Mexico exports honey principally to Germany, England and the United States, but the problem is that beekeepers are selling their honey without a characterisation. The botanical origin of honey could be determined by melissopalynology, the pollen analysis might give honey at additional value and enable an increase in beekeeping at Oaxaca. However, natural ecosystems have been affected for many years because man has destroyed original vegetation in order to use land for agriculture or cattle in different regions of Mexico. Thus, melissopalynological research could aid beekeepers, because the knowledge of nectariferous plants might help them preserve and recover some affected vegetation types. This may be achieved by reforesting some of them with native plants of melliferous importance.
Although there are some Mexican melissopalynological studies of Apis mellifera L. (Souza-Novelo, Citation1940; Alvarado & Delgado, Citation1985; Martínez-Hernández et al., Citation1993; Piedras & Quiroz, Citation2007; Ramírez-Arriaga & Martínez-Hernández, Citation2007), only one has been carried out in Oaxaca (Acosta-Castellanos & Palacios-Chávez, Citation2001). As a result of the lack of melissopalynological research in this state, beekeepers do not know all the important apiflora that contributes to honey production. The present study provides information on the botanical characterisation of Mexican honey from southern Oaxaca, in order to determine the most important resources that are being exploited by Apis mellifera. Besides, by using ecological parameters, the foraging behaviour is analysed as well as the pollen diversity in honey samples.
Materials and methods
A total of 39 honey samples were collected during the principal harvest from four districts in the State of Oaxaca (). Seven honeys sampled from January to March 2008 and one sample 2007–2008 were collected at Jamiltepec; five honey samples were collected from February to March 2008 at Juquila; five samples were collected from November 2007 to March 2008 at Pochutla and 21 samples were analysed for the periods 2006–2007, 2007–2008, November 2007–March 2008 and May 2008 at Juchitán (, ). The predominant climate is subtropical with average annual temperatures of 26.2 °C in Jamiltepec, 16 °C in Juquila, 20 °C in Pochutla and 26 °C in Juchitán. The principal types of vegetation are savannah, low deciduous forest and sub-deciduous forest as well as crop zones of Mangifera indica L.
Figure 1. Map with the sampling localities in the Jamiltepec, Juquila, Pochutla and Juchitán districts in Oaxaca, Mexico, including municipalities. Abbreviations see text.
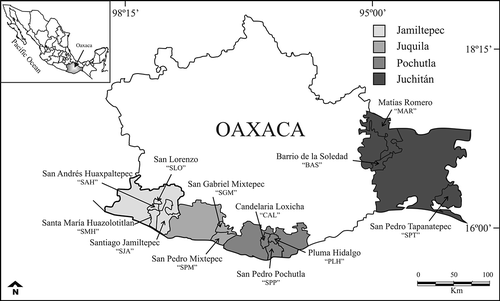
Table I. Pollen types found in ≥1% of the honey samples from Santiago Jamiltepec (SJA), San Andrés Huaxpaltepec (SAH), San Lorenzo (SLO), Santa María Huazolotitlan (SMH), San Pedro Mixtepec (SPM), San Gabriel Mixtepec (SGM), Candelaria Loxicha (CAL), Pluma Hidalgo (PLH) and San Pedro Pochutla (SPP)
Table II. Pollen types with ≥1% found in the honey samples from San Pedro Tapanatepec (SPT), Matías Romero (MAR) and Barrio de la Soledad (BAS)
All honey samples were obtained by centrifugation of combs, and every sample was labelled with date, locality, municipality and district. Following Louveaux et al. (Citation1978) and preserving the sample proportions, 50 g of honey were dissolved in 100 ml of distilled water. Sediments were acetolysed according to Erdtman's (Citation1960) method. Permanent glycerine jelly slides were made and analysed with a Zeiss Axiolab upright microscope. Pollen grains were identified by comparison with the pollen reference collection housed at the Institute of Geology, Universidad Nacional Autónoma de México, and by using additional pollen atlases (Tseng-Chieng, Citation1972; Palacios-Chávez et al., Citation1991; Roubik & Moreno, Citation1991; Martínez-Hernández et al., Citation1993). In order to obtain the percentage and frequency classes of taxa, 1200 pollen grains were counted per sample. Pollen taxa were classified (Louveaux et al., Citation1978) as predominant (P ≥ 45%), secondary (S = 16–45%), important minor (I = 3–15%) or minor pollen (M ≤ 3%). Honeys were characterised as monofloral when a species was predominant. Multifloral honey samples were divided into ‘oligofloral’ when two or more secondary taxa belonged to one botanical family, ‘bifloral’ when two pollen types had secondary percentages, and polyfloral when three or more pollen types were registered with secondary percentages. Also, ecological parameters were calculated using a diversity index (Snannon & Weaver, Citation1949) and the Pielou Index (Citation1977).
The Diversity Index of Shannon and Weaver (Citation1949) is calculated according to the following equation:
The Pielou Index (Citation1977) indicates when heterogeneous utilisation of resources occurred and values approach zero; if the resources were exploited homogeneously, then values approach one. It is calculated as follows:
The absolute quantity of pollen grains in 10 g of honey was calculated using one Lycopodium clavatum L. spore tablet per sample (Stockmarr, Citation1971; Maher, Citation1981). Pollen concentrations were classified following the Maurizio's (Citation1939) scheme.
Results
A total of 64 plant species with percentages ≥1% were found from 29 plant families (, ). Families that occurred in more than 50% of samples include: Anacardiaceae (found in 46.1% of the honey samples), Boraginaceae (53.8%), Asteraceae (76.9%), Fabaceae (92.3%) and Malvaceae (97.4%). The most diverse family in the honey samples analysed was Fabaceae with 14 taxa followed by Malvaceae (six taxa) and Euphorbiaceae (four taxa).
Monofloral honeys are placed in classes I, II, III, IV and V. Oligofloral, bifloral and polyfloral honeys are assigned to classes I, II and III (, ). Based on the absolute quantity of pollen grains, 21% (n = 8) of the samples belong to class I (6757–17 406), 46% (n = 18) to class II (22 497–98 076), 28% (n = 11) to class III (108 757–443 987), 3% (n = 1) to class IV (795 163) and 3% (n = 1) to class V (1 049 609). Individual results are provided as follows.
Figure 2. Percentages of predominant taxa registered in honey samples from Oaxaca, Mexico. Most of the samples are ‘nectar honeys’, with exception of one ‘honeydew honey’ of Quercus. Classes according to pollen concentrations are named with Roman numerals as in the text.
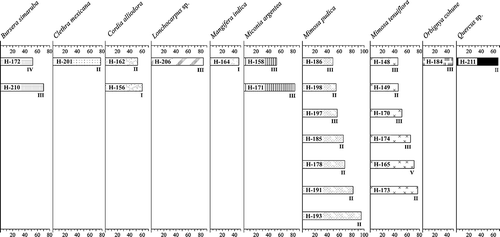
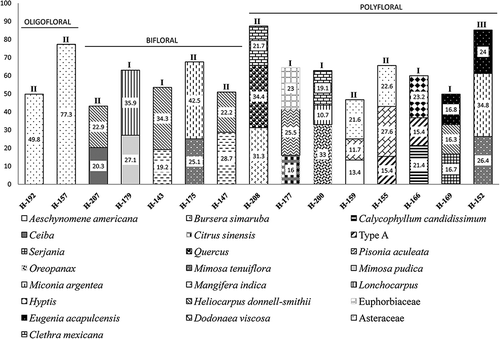
Figure 3. Honey samples characterised as oligofloral, bifloral and polyfloral samples from Oaxaca, Mexico. Numbers in the bars correspond to percentages. The classes according to pollen concentrations were included with Roman numerals: classes I, II, III, IV and V.
Jamiltepec
A total of 55 pollen types were identified, belonging to 21 families (). The most important resources (≥10%) were Asteraceae, Mimosa pudica L. (Fabaceae), Trema micrantha (L.) Blume ()
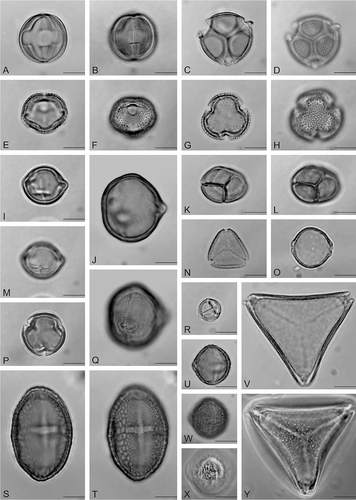
Figure 4. Light microscope photomicrographs of nectariferous resources exploited by Apis mellifera in Oaxaca. A–D. Aeschynomene americana (Fabaceae). E–H. Calycophyllum candidissimum (Rubiaceae). I. Clethra mexicana (Clethraceae). J. Dodonaea viscosa (Sapindaceae). K, L. Mimosa tenuiflora (Fabaceae). M. Clethra mexicana (Clethraceae). N. Eugenia acapulcensis (Myrtaceae). O. Trema micrantha (Ulmaceae). P. Clethra mexicana (Clethraceae). Q. Dodonaea viscosa (Sapindaceae). R. Mimosa pudica (Fabaceae). S, T. Heliocarpus donell-smithii (Malvaceae). U. Mangifera indica (Anacardiaceae). V. Serjania sp. (Sapindaceae). W, X. Mangifera indica (Anacardiaceae). Y. Serjania sp. (Sapindaceae). Scale bars – 10 μm.
and Pisonia aculeata L. (Nyctaginaceae) (, ; ). The honey characterisation revealed seven honeys with predominant pollen of M. pudica () and one oligofloral honey consisting of Asteraceae (, ). Diversity values vary from 0.3 in February 2008 to 1.7 in March 2008; the Pielou's Index revealing heterogeneity in resource exploitation with a mean of 0.4 (, ).
Juquila
A total of 79 pollen types were recorded from 22 families. Plants with percentages ≥10% were Clethra mexicana DC. (, M, P), Euphorbiaceae, Lonchocarpus sp., Mimosa pudica (), Pisonia aculeata, Orbignya cohune (Mart.) Dahlgren ex Standl. and Citrus sinensis Pers. (, ; ). Two samples were considered monofloral honeys from O. cohune and C. mexicana (), one sample was bifloral with secondary pollen of Lonchocarpus sp. and M. pudica and two samples were classified as polyfloral (). The honey diversity ranged from 1 to 2.3 in March 2008 and J ′ values varied from 0.3 to 0.6 showing a heterogeneous honey recollection (, ).
Table III. Shannon–Weaver Diversity Index (H′) and Pielou Index (J′) of pollen found in the honey samples from the Jamiltepec, Juquila, Pochutla and Juchitán districts
Pochutla
A total of 23 families and 66 taxa were recorded. Taxa ≥10% of the sample included Bursera simaruba (L.) Sarg., Ceiba sp., Clethra mexicana (), Heliocarpus donnell-smithii Rose (, T), Lonchocarpus sp. and Quercus sp. (). Three honey samples were characterised as monofloral of Lonchocarpus sp., Quercus sp. and B. simaruba, two samples with secondary pollen grains were bifloral of H. donnell-smithii and Ceiba sp. and Lonchocarpus sp. and Mimosa pudica, respectively. Finally, one honey sample was polyfloral (, ). Shannon–Weaver Diversity Index values ranged from 0.7 to 2 in both samples from November 2007. The lowest J′ value was 0.3 and the highest was 0.7, nevertheless, the mean value (0.5) evidenced also heterogeneous forage behaviour (, ).
Juchitán
Eighty pollen types were identified belonging to 30 plant families. Many families were recorded in low numbers and only those taxa that occurred in more than 1% of the samples are listed (). The most common plant resources were: Aeschynomene americana L. (–D), Bursera simaruba, Calycophyllum candidissimum DC. (–H), Cordia alliodora Cham., Asteraceae, Dodonaea viscosa Jacq. (, Q), Eugenia acapulcensis Steud. (), Heliocarpus donnell-smithii (, T), Hyptis sp., Mangifera indica (, W, X), Miconia argentea DC., Mimosa tenuiflora L. (, L), Serjania sp. (, Y) and Type A. Twelve honey samples had predominant pollen from Mimosa tenuiflora, Miconia argentea, Mangifera indica, Cordia alliodora and B. simaruba. Three honey samples were bifloral with secondary pollen of H. donnell-smithii and Mangifera indica, or Miconia argentea and Mimosa tenuiflora, respectively. One sample was oligofloral from Asteraceae, and five were considered as polyfloral (, ). The Shannon–Weaver Diversity Index varies from 0.4 in November 2007 to 2.7 in January 2008. Pielou Index values ranged from 0.1 in November 2007 to 0.7 in January 2008. The mean value showed a heterogeneous honey recollection (, ).
Discussion
Nectariferous flora
Even though a high diversity of pollen types in honey samples was encountered, only 23 taxa were the most common resources for nectar in Oaxaca's coast and San Pedro Tapanatepec (district abbreviations as follows: Jamiltepec JAM; Juquila JUQ; Pochutla POCH; Juchitán JUCH): Aeschynomene americana, Bursera simaruba, Calycophyllum candidissimum, Ceiba sp., Citrus sinensis, Clethra mexicana, Asteraceae, Cordia alliodora, Diphysa sp., Dodonaea viscosa, Eugenia acapulcensis, Euphorbiacae, Heliocarpus donnell-smithii, Hyptis sp., Lochocarpus sp., Mangifera indica, Miconia argentea, Mimosa pudica, Mimosa tenuiflora, Orbignya cohune, Oreopanax sp., Pisonia aculeata and Type A. In addition, Quercus was a honeydew resource (Ruoff et al., Citation2006).
The high diversity of nectar resources found in honey samples from Oaxaca is not surprising since it is due to flora richness and because Apis mellifera has a polylectic behaviour, which characterises social bees (Michener, Citation1979). Most of the important taxa belonged to native plants from low deciduous forest, but there were also registered crop plants such as Citrus sinensis and Mangifera indica ().
Most of the honeys were monofloral (61.6%). Monofloral honey samples were mostly from trees such as Bursera simaruba (POCH, JUCH), Cordia alliodora (JUCH), Lonchocarpus sp. (POCH), Mangifera indica (JUCH), Orbignya cohune (JUQ), Quercus sp. (POCH) and Trema micrantha. There were also predominant pollen types of shrubs and herbs, for example, Clethra mexicana (JUQ), Mimosa pudica (JAM), Miconia argentea (JUCH) and Mimosa tenuiflora (JUCH).
Two samples were classified as oligofloral (5.1%) because of the foraging behaviour of Apis mellifera, which reflected the preference of different species of Asteraceae (JAM, JUCH).
Bifloral honeys had a representation of 12.8% in the samples. Most of the nectariferous resources registered in bifloral honey were trees (Ceiba sp., Heliocarpus donnell-smithii, Lonchocarpus sp., Mangifera indica), some others were shrubs (Miconia argentea and Mimosa tenuiflora) and there were also herbs (Mimosa pudica) that belong to secondary vegetation, with exception of Mangifera indica, a plant of economic importance.
Besides, eight samples (20.5%) were classified as polyfloral honeys. In this kind of honey, three or four taxa were registered as important minor pollen or secondary pollen types, including Aeschynomene americana, Calycophyllum candidissimum, Ceiba sp., Citrus sinensis, Compositae, Dodonaea viscosa, Eugenia acapulcensis, Euphorbiaceae, Gramineae, H. donnell-smithii, Hyptis sp., Miconia argentea, Mimosa tenuiflora, Pisonia aculeata and Serjania sp.
According to the Codex STAN, 12–1981 (CAC, Citation2001), honey can be produced from the nectar of plants (nectar honey), from secretions of living parts of plant parts, or from the excretion of plant sucking insects (honeydew honey). Most of the honeys analysed were nectar honeys, but there was also one honeydew honey of Quercus and a natural mixture honey (nectar and honeydew honey) of Bursera simaruba, Clethra mexicana and Quercus. There were also two honey samples rich in pollen resources from non-nectariferous plants, for instance, Cecropia obtucifolia (14%) and Poaceae (11.2%). The main resources in monofloral, oligofloral, bifloral and polyfloral honeys will be analysed later.
Main resources in monofloral, oligofloral, bifloral and polyfloral honeys
Secondary pollen grains of Aeschynomene americana (13.4%) were registered in polyfloral honey. They have also been reported in honey samples of Colombia (Girón-Vanderhuck, Citation1996). Aeschynomene americana colonises disturbed lands, it is an invasive weed species (Tejero-Díez et al., Citation2008) and is often found in low deciduous forests (Salas-Morales et al., Citation2007).
Oligofloral honeys have predominant pollen of Asteraceae (ranging from 49.8 to 77.3%). This family was also considered as a secondary resource in polyfloral samples (21.6–22.6%). Asteraceae have been considered as a nectar resource (Crane et al., Citation1984; Villegas-Durán et al., Citation1998, Citation1999, Citation2003, Citation2004; Martínez-Hernández et al., Citation1993). There are more than 140 Asteraceae species reported along the Oaxaca coastal margin (Salas-Morales et al., Citation2003).
Bursera simaruba was registered with a range of 52.7 to 70.1% in monofloral honeys. It was also recorded with 31.3% in a polyfloral honey. The genus Bursera is a native plant that has been reported in honey samples of Apis mellifera and Scaptotrigona mexicana Guérin-Méneville in Puebla (Ramírez-Arriaga & Martínez-Hernández, Citation2007). It was expected in A. mellifera ligustica Spinola honey samples from Pluma Hidalgo, Oaxaca (Acosta-Castellanos & Palacios-Chávez, Citation2001) and it is cited as a nectar-pollen resource in the Yucatán Peninsula (Villanueva et al., Citation2009). Bursera simaruba grows in a variety of habitats from low sub-deciduous forests to low and high evergreen forest (personal observation). It produces nectar and pollen and is used by man to construct live fences, houses, in carpentry, and medicinally (Arellano-Rodríguez et al., Citation2003; Villegas-Durán et al., Citation2003).
The pollen type of Calycophyllum candidissimum was secondary (21.4%) in polyfloral honeys; Calycophyllum candidissimum has been reported as a melliferous plant by Villegas-Durán et al. (Citation2003), but not in melissopalynological studies. This species grows in low deciduous forest (Salas-Morales et al., Citation2007).
Ceiba was a secondary resource (20.3%) in bifloral honey. The Ceiba tree is a resource that produces nectar but honeybees also collect pollen (Villanueva, Citation1984). By means of direct observation, it was reported as a polleniferous plant (Espina & Ordetx, Citation1983). This tree occurs in low deciduous forests and has medical importance to treat diarrhoea, fever, gonorrhoea, parasitic infections, and as a diuretic and an emollient (Salas-Morales et al., Citation2007).
Clethra mexicana was recorded with 77.6% in a monofloral honey and as secondary pollen in polyfloral samples (ranging from 19.1% to 21.7%). It is reported in the honeys of Apis mellifera and stingless bees in Chiapas, Puebla and Oaxaca (Ramírez-Arriaga, Citation1989; Martínez-Hernández et al., Citation1993). Clethra mexicana grows in pure pine and pine-oak forests (Verduzco & Rodríquez, Citation1995).
Citrus sinensis was a secondary resource (16%) in polyfloral honeys. It has been well documented as a nectar resource for Apis mellifera and stingless bees in Oaxaca, Puebla and Chiapas (Ramírez-Arriaga, Citation1989; Martínez-Hernández et al., Citation1993; Acosta-Castellanos & Palacios-Chávez, Citation2001). In South America, Citrus sinensis has been reported in honeys from Chile (Ramírez & Montenegro, Citation2004), Argentina (Valle et al., Citation2001) and Brazil (Bastos et al., Citation2003). It has been cited in the melliferous flora of Mexico from Michoacán, Chiapas, Veracruz and Tabasco (Espina & Ordetx, Citation1983; Villegas-Durán et al., Citation1999, Citation2002, Citation2003, Citation2004).
Cordia alliodora was recorded with 52.7% and 60.2% in monofloral honeys; it is a nectariferous resource (Villegas-Durán et al., Citation2002) and has been reported in honey and pollen loads of Apis mellifera and stingless bees in Chiapas (Martínez-Hernández et al., Citation1993). Cordia alliodora is abundant in secondary vegetation and it is distributed along the Pacific and Gulf of Mexico coasts (Pennington & Sarukhán, Citation1998).
Secondary pollen grains of Eugenia acapulcensis (ranging from 16.4 to 24%) were observed in polyfloral honeys. The genus Eugenia has been cited as a nectar-polliniferous resource in Yucatán's Peninsula (Villanueva et al., Citation2009) and it has been found in honey samples of Apis mellifera from Formosa, Argentina (Cabrera, Citation2006). Nevertheless, the species Eugenia acapulcensis is reported as nectariferous for the first time.
Heliocarpus donnell-smithii was important in bifloral (ranging 22.9 to 34.3%) and polyfloral honeys (16.3%). It is exploited by stingless bees and honeybees (Ramírez-Arriaga, Citation1989; Martínez-Hernández et al., Citation1993; Acosta-Castellanos & Palacios-Chávez, Citation2001).
Lonchocarpus sp. was a significant resource in monofloral (83.9%), bifloral (35.6%) and polyfloral (10.7%) honeys. Lonchocarpus is an important nectar source for common bees and stingless bees in Chiapas and Jalisco (Martínez-Hernández et al., Citation1993; Quiroz-García & Palacios-Chávez, Citation1999). It has also been reported in melissopalynological studies (Espina & Ordetx, Citation1983; Villegas-Durán et al., Citation2003, Citation2004). There are at least 15 Lonchocarpus species along the Oaxaca coast (Salas-Morales et al., Citation2003), some occur in middle and high evergreen forest, while others grow in low sub-deciduous forests (Pennington & Sarukhán, Citation1998).
A species that has economic importance in the region is Mangifera indica. It was recorded in monofloral (46.6%) and bifloral honeys (ranging from 19.2 to 28.7%). This plant has been previously reported in honey samples of stingless bees and Apis mellifera in Chiapas and Puebla (Martínez-Hernández et al., Citation1993; Ramírez-Arriaga & Martínez-Hernández, Citation2007).
Miconia has been reported in Scaptotrigona mexicana and Apis mellifera honeys from Chiapas and Puebla (Martínez-Hernández et al., Citation1993; Ramírez-Arriaga & Martínez-Hernández, Citation2007). Ribeiro dos Santos et al. (Citation2006) cite different species of Miconia as important sources for common bees in a semi-arid ecosystem of Brazil. Mexico has about 100 Miconia species and they are found in cloud forests, pine and pine-oak forests and exceptionally in the low sub-deciduous forests of the western and southern ranges, as well as in the Sierra de Juárez in Oaxaca (de Santiago, Citation2000).
The genus Mimosa includes about 510 species, 90% are from America (Martínez-Bernal et al., Citation2008); about 110 species grow in tropical, subtropical, arid and semi-arid Mexican ecosystems (Grether et al., Citation2006; Martínez-Bernal et al., Citation2008). Mexico is a secondary centre of diversity for Mimosa (Martínez-Bernal et al., Citation2008). Some studies of pollen loads and honey analysis consider M. pudica only as a polleniferous resource (Villanueva, Citation2002; Villanueva et al., Citation2009). Nevertheless, in South America, Mimosa species have been reported as a nectar source (Barth, Citation2004; Lima et al., Citation2006; Oliveira et al., Citation2010). In this paper, we suggest that M. pudica and M. tenuiflora also produce nectar. Both species belong to the Piptadenia group, and although this group does not have well-developed nectaries, they have a stemozone. This is a small disc formed by fusion of petals and stamens that produces nectar (Lewis & Elias, Citation1981). Even though, M. pudica and M. tenuiflora pollen grains were recorded as predominant types, honey samples were not considered as monofloral honeys because these species could be over-represented (Barth, Citation1989). Mimosa pudica was predominant with a range of 49.2–82.3% in some honey samples and was a secondary resource in bifloral honeys (27.1%). The M. pudica pollen type has been reported in Mexico as a nectariferous resource for Apis mellifera and stingless bees (Alvarado & Delgado, Citation1985; Ramírez-Arriaga, Citation1989; Martínez-Hernández et al., Citation1993; Ramírez-Arriaga & Martínez-Hernández, Citation2007), as well as polliniferous (Villanueva, Citation2002; Villanueva et al., Citation2009). Field observations made in the region of the Yucatán Peninsula (Arellano-Rodríguez et al., Citation2003) reported M. pudica as a melliferous plant that produces pollen and nectar. This plant occurs in secondary vegetation and it has medicinal uses. In South America, M. pudica has been reported in melissopalynological studies in A. mellifera as nectariferous and nectar-polleniferous in Antioquia (Colombia), Formosa (Argentina), Bahia and Cerrado de Minas, Brazil (Girón-Vanderhuck, Citation1995; Camargo-Ricalde, Citation2000; Bastos et al., Citation2003; Borges et al., Citation2006; Cabrera, Citation2006; Ribeiro dos Santos et al., Citation2006). Mimosatenuiflora, a predominant species, was recorded from 45.2 to 77%; in addition, it was a secondary resource in bifloral (25.1%) and polyfloral samples (26.4%). Mimosa tenuiflora is a melliferous plant that produces pollen and nectar; it has been reported in melissopalynological studies from Brazil (Borges et al., Citation2006; Ribeiro dos Santos et al., Citation2006). In Mexico, it has been reported in field observations by Villegas-Durán et al. (Citation2002). Mimosa tenuifolia ‘tepezcohuite’ has medical uses to treat lesions, it protects the body from infection (Miranda, Citation1976; Barneby, Citation1991) and it is found in low deciduous and sub-deciduous forests, pine and pine-oak forests (Camargo-Ricalde, Citation2000).
Other secondary pollen in polyfloral honeys was Pisonia aculeata. By means of field observations, P. aculeata was reported as a melliferous plant in the Yucatán Peninsula (Villegas-Durán et al., Citation1998). Moreover, this is the first melissopalynological report. Pisonia aculeata is a native species that grows in low deciduous and middle sub-deciduous forests in Oaxaca (Salas-Morales et al., Citation2007).
Quercus pollen grains had a percentage of 67.6% in a honeydew honey and 34.4% in a polyfloral sample. Quercus has been documented in honeys of Apis mellifera and stingless bees in Puebla and Oaxaca (Acosta-Castellanos & Palacios-Chávez, Citation2001; Ramírez-Arriaga & Martínez-Hernández, Citation2007). This genus has been cited in melissopalynological studies from Spain (Gómez-Ferreras & Sáenz de Rivas, Citation1980; Luis-Villota & Gómez-Ferreras, Citation1989; Terrab et al., Citation2004). By means of field observations, Quercus has been considered to be a polleniferous resource (Espina & Ordetx, Citation1983). This tree forms forests and it is an important element of the pine-oak forests on the Oaxaca coast (Salas-Morales et al., Citation2003).
Serjania, a secondary resource in polyfloral honeys (16.7%), has been found in honey samples from Brazil (Bastos et al., Citation2003) and Argentina (Cabrera, Citation2006). It is an indigenous plant that grows in low deciduous forests (Salas-Morales et al., Citation2007).
Two new taxa have been found in honey samples of Apis mellifera: Dodonaea viscosa, a shrub from low deciduous forests and Oreopanax sp., a tree from the native flora.
Beekeeping potentiality
From the present analysis there is huge potential for beekeeping activity in southern Oaxaca. Eight different monofloral honey types were produced as a result of the abundance of native plant resources from low deciduous forests. Although every district within Oaxaca produces specific monofloral honeys, the Juchitán district has the greatest diversity of monofloral honey production.
Oaxaca has high floral diversity that honey bees exploit to produce honey. The information obtained in this research can improve beekeeping performance in that beekeepers need to maintain the native plants and reforest disturbed sites with native plants that now are known to be honeybee plants.
Conclusions
During the principal honey harvest, Apis mellifera prefers resources of secondary vegetation from low deciduous forests, although taxa of economic importance were also exploited. A total of 64 plant species were found to be utilised in Oaxaca with percentages ≥1% belonging to 29 families. The most diverse family in the honey samples analysed was Fabaceae with 14 taxa. Subtropical honeys were characterised by their botanical origin as (a) monofloral honeys of Bursera simaruba (classes IV and III), Clethra mexicana (class II), Cordia alliodora (classes I and II), Lonchocarpus sp. (class III), Mangifera indica (class I), Miconia argentea (class III), Orbignya cohune (class III) and Quercus sp. (class II); (b) bifloral honeys with association of Heliocarpus donnell-smithii and Ceiba sp. (class II), Lonchocarpus sp. and Mimosa pudica (class I), H. donnell-smithii and Mangifera indica (classes I and II), and Miconia argentea and M. tenuiflora (class II); (c) oligofloral honeys of Asteraceae (class II); and (d) polyfloral honeys with three or four species ≥10% (classes I, II and III). Apis mellifera is polylectic and it has heterogeneous forage behaviour. According to our study, beekeeping activity needs to be promoted in Oaxaca because of its huge potential for better development in the state.
Acknowledgements
We would like to express our sincere thanks to David Cantrill and two anonymous reviewers for their valuable suggestions. We acknowledge Enrique Martínez-Hernánez for his useful comments as well as the Oaxacan beekeepers that provided the honey samples and Gisela Fuentes Mascorro for her valuable cooperation with the fieldwork. The authors are grateful to Magdalena Alcayde Orraca and Miguel Angel Calderón Ramírez for reviewing the English version. This work was supported by the Instituto de Geología, Universidad Nacional Autónoma de México (UNAM).
References
- Acosta-Castellanos , S. and Palacios-Chávez , R. 2001 . “ Plants of apicultural interest in the Pluma Hidalgo Zone, Oaxaca, Mexico ” . In Proceedings of the IX International Palynological Congress, Houston, Texas, USA , Edited by: Goodman , O. K. and Clarke , R. T. 459 – 469 . College Station, TX : American Association of Stratigraphic Palynologists .
- Alvarado , J. L and Delgado , R. M. 1985 . Flora apícola de Uxpanapa,Veracruz, México . Biotica , 19 : 257 – 275 .
- Arellano-Rodríguez , A. J. , Flores , G. J. S. , Tun , G. J. and Cruz , B. M. M. 2003 . Etnoflora yucatanense: nomenclatura, forma de vida, uso, manejo y distribución de las especies vegetales de la península de Yucatán , Yucatán : Universidad Autónoma de Yucatán .
- Barneby , R. C. 1991 . Sensitive censitae. A description of the genus Mimosa Linnaeus (Mimosaceae) in the New World . Memoirs of the New York Botanical Garden , 65 : 1 – 835 .
- Barth , O. M. 1989 . O pólem no mel brasileiro , Rio de Janeiro : Gráfica Luxor .
- Barth , O. M. 2004 . Melissopalynology in Brazil: a review of pollen analysis of honeys, propolis and pollen loads of bees . Science Agriculture (Piracicaba, Brazil) , 61 : 342 – 350 .
- Bastos , E. M. A. , Silveira , V. M. and Soares , A. E. E. 2003 . Pollen spectrum of honey produced in Cerrado areas of Minas Gerais State (Brazil) . Brazilian Journal of Biology , 63 : 599 – 615 .
- Borges , R. L. B. , Lima , L. C. L. , Oliveira , P. P. , Silva , F. H. M. , Novais , J. S. , Dórea , M. C. and dos Santos , F. A. R. 2006 . “ O pólen no mel do semi-árido Brasileiro ” . In Apium Plantae , Edited by: Santos , F. A. Ribeiro dos . 103 – 118 . Recife : Instituto do Milênio do Semi-árido and Ministério da Ciência e Tecnologia .
- Cabrera , M. M. 2006 . Caracterización polínica de las mieles de la provincia de Formosa, Argentina . Revista del Museo Argentino de Ciencias Naturales , 8 : 135 – 142 .
- Camargo-Ricalde , S. L. 2000 . Descripción, distribución, anatomía, composición química y usos de Mimosa tenuiflora (Fabaceae-Mimosoideae) en México . Revista de Biología Tropical , 48 : 939 – 954 .
- Codex Alimentarius Commission (CAC) . 2001 . “ CODEX STAN, 12–1981 ” . In Revised codex standard for honey , Rome : CAC .
- Crane , E. , Walker , P. and Day , R. 1984 . Directory of important world honey sources , London : International Bee Research Association .
- de Santiago , G. J. R. 2000 . Miconia teotepecensis (Melastomataceae), una nueva especie de la Sierra Madre del Sur de Guerrero y Oaxaca, México . Acta Botánica Mexicana , 50 : 21 – 25 .
- Erdtman , G. 1960 . The acetolysis method; a revised description . Svensk Botanisk Tidskrift , 54 : 561 – 564 .
- Espina , P. D. and Ordetx , S. G. 1983 . Flora apícola tropical Costa Rica , Costa Rica : Tecnológica de Costa Rica .
- García-Mendoza , A. J. , Ordóñez , M. J. and Briones-Salas , M. 2004 . Biodiversidad de Oaxaca , Mexico : Instituto de Biología, Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la conservación de la naturaleza and World Wildlife Fund .
- Girón-Vanderhuck , M. 1995 . Análisis palinológico de la miel y la carga de polen colectada por Apis mellifera en el suroeste de Antoquia, Colombia . Boletín del Museo de Entomología de la Universidad del Valle , 3 : 35 – 54 .
- Girón-Vanderhuck , M. 1996 . Melitopalinología. Recolección de polen y néctar por Apis mellifera en algunas especies de plantas silvestres y cultivadas del municipio de Salgar Antioquia , Colombia : Universidad del Quindío, Colciencas .
- Gómez-Ferreras , C. and Sáenz de Rivas , C. 1980 . Análisis polínico de Cáceres España . Anales del Jardín Botánico de Madrid , 36 : 191 – 201 .
- Grether , R. , Martínez-Bernal , A. , Luckow , M. and Zárate , S. 2006 . Flora del valle de Tehuacán-Cuicatlán. Mimosaceae, Tribu Mimoseae , México : Instituto de Biología, Universidad Nacional Autónoma de México .
- Lewis , G. P. and Elias , T. S. 1981 . “ Tribu Mimoseae ” . In Advances in legume systematics , Edited by: Polhill , R. M. and Raven , P. H. 55 – 168 . Kew : Royal Botanic Gardens Kew .
- Lima , L. L. C. , Silva , F. H. M. , Santos , S. A. and dos Santos , F. A. 2006 . “ Morfología polínica de species de Mimosa L. (Leguminosae) apícolas do Semi-árido ” . In Apium Plantae , Edited by: Ribeiro dos Santos , F. A. 87 – 102 . Recife : Instiuto do Milênio do Semi-árido and Mistério de Ciência e Tecnologia .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1978 . Methods of melissopalynology . Bee World , 59 : 139 – 157 .
- Luis-Villota , P. and Gómez-Fereas , C. 1989 . Contribución al análisis polínico de mieles de Astuias Occidental (España) . Botanica Complutensis , 1S : 163 – 173 .
- Maher , L. J. Jr . 1981 . Statistics for microfossil concentration measurements employing samples spiked with marker grains . Review of Palaeobotany and Palynology , 32 : 153 – 191 .
- Martínez-Bernal , A. , Grether , R. and González-Amaro , R. M. 2008 . Flora de Veracruz. Leguminosae I, Mimosoideae: Mimosa , Xalapa : Instituto de Ecología, A. C .
- Martínez-Hernández , E. , Cuadriello , A. J. I. , Téllez , V. O. , Ramírez-Arriaga , E. , Sosa , N. M. S. , Melchor , S. J. E. , Medina , C. M. and Lozano , G. M. S. 1993 . Atlas de las plantas y el polen utilizados por las cinco especies principales de las abejas productoras de miel en la región del Tacaná, Chiapas, México , Mexico : Universidad Nacional Autónoma de México .
- Maurizio , A. 1939 . Untersuchungen zur quantitativen Pollenanalyse des Honigs . Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene , 30 : 27 – 69 .
- Michener , C. D. 1979 . Biogeography of the bees . Annals of the Missouri Botanical Garden , 66 : 277 – 347 .
- Miranda , F. 1976 . La vegetación de Chiapas , Mexico : Gobierno del Estado de Chiapas .
- Oliveira , P. P. , Van der Berg , C. and dos Santos , F. A. R. 2010 . Pollen analysis of honeys from Caatinga vegetation of the state of Bahia, Brazil . Grana , 49 : 66 – 75 .
- Palacios-Chávez , R. , Ludlow-Wiechers , B. and Villanueva , G. R. 1991 . Flora palinológica de la reserva de la biosfera de Sian Ka´an Quintana Roo , México : CIORO .
- Pennington , T. D. and Sarukhán , J. 1998 . Árboles tropicales de México. Manual para la identificación de las principales especies , Mexico : Universidad Nacional Autónoma de México and Fondo de Cultura Económica .
- Piedras , G. B. and Quiroz , G. D. L. 2007 . Estudio melisopalinológico de dos mieles de la porción sur del Valle de México . Polibotánica , 23 : 57 – 75 .
- Pielou , E. C. 1977 . Mathematical ecology , New York : Wiley .
- Quiroz-García , D. L. and Palacios-Chávez , R. 1999 . Determinación de los recursos florales utilizados por Centris inermis Friese (Hymenoptera: Apidae) en Chamela, Jalisco, México . Polibotánica , 10 : 59 – 72 .
- Ramírez-Arriaga , E. 1989 . Explotación de los recursos florales por Plebeia sp. (Apidae) en dos zonas con diferente altitud y vegetación en el Soconusco, Chiapas , Mexico : Facultad de Ciencias, Universidad Nacional Autónoma de México, PhD Diss .
- Ramírez-Arriaga , E. and Martínez-Hernández , E. 2007 . Melissopalynological characterization of Scaptotrigona mexicana Gúerin (Apidae: Meliponini) and Apis mellifera L. (Apidae: Apini) honey samples in northern Puebla state, Mexico . Journal of Kansas Entomologycal Society , 80 : 377 – 391 .
- Ramírez , R. and Montenegro , G. 2004 . Certificación de origen botánico de miel y polen corbicular pertenecientes a la comuna de Litueche, VI Región de Chile, Departamento de Ciencias vegetales. Casilla 306-22, Santiago, Chile . Ciencia e Investigación Agraria , 31 : 197 – 211 .
- Ribeiro dos Santos , F. A. , Moura , O. J. , Pereira , O. P. , Batista , L. K. R. and Carneiro , C. E. 2006 . “ Plantas do semi-árido importantes para as abelhas ” . In Apium Plantae , Edited by: Giulietti , A. M. , Paganucci de Queiroz , L. and dos Santos , F. A. Ribeiro . 61 – 86 . Recife : Instituto do Milênio do Semi-árido and Ministério da Ciência e Tecnologia .
- Roubik , D. W. and Moreno , P. J. E. 1991 . Pollen and spores of Barrio Colorado Island. St , Louis, MO : Missouri Botanical Gardens .
- Ruoff , K. , Luginbühl , W. , Künzli , R. , Iglesias , M. T. , Bogdanov , S. , Olivier , B. J. , von der Ohe , K. and Amadò , R. 2006 . Authentication of the botanical and geographical origin of honey by mid-infrared spectroscopy . Journal of Agricultural and Food Chemistry , 54 : 6873 – 6880 .
- Salas-Morales , S. H. , Saynes-Vásquez , A. and Schibli , L. 2003 . Flora de la Costa de Oaxaca, México: Lista florística de la región de Zimatán . Boletín de la Sociead Botánica de México , 72 : 21 – 58 .
- Salas-Morales , S. H. , Schibli , L. , Naa-Zafra , A. and Saynes-Vásquez , A. 2007 . Flora de la Costa de Oaxaca, México (2): Lista florística comentada del Parque Nacional Huatulco . Boletín de la Sociedad Botánica de México , 81 : 101 – 130 .
- Souza-Novelo , N. 1940 . Plantas melíferas y políniferas de Yucatán , Mérida : Taller de Litografía El Porvenir .
- Shannon , C. E. and Weaver , W. 1949 . The mathematical theory of communication , Urbana, IL : University of Illinois Press .
- Stockmarr , J. 1971 . Tablets with spores used in absolute pollen analysis . Pollen et Spores , 13 : 615 – 621 .
- Tejero-Díez , J. D. , Ledesma-Corral , J. C. and Torres-Díaz , A. N. 2008 . El palmar de Orbignya guacayule al sur de Nayarit, México . Polibotánica , 26 : 67 – 100 .
- Terrab , A. , Pontes , A. , Heredia , F. J. and Díez , M. J. 2004 . Palynological and geographical characterization of avocado honeys in Spain . Grana , 43 : 116 – 121 .
- Tseng-Chieng , H. 1972 . Pollen flora de Taiwan , Taipeh : Botany Department Press, National Taiwan University .
- Valle , A. , Andrada , E. , Aramayo , L. and Gallez , S. L. 2001 . Mieles de la región periserrana de Ventania, Argentina . Invstigación Argraria: Producción y Protección Vegetales , 16 : 343 – 354. .
- Verduzco , C. and Rodríguez , C. L. 1995 . “ El rincón de la Vía ” . In Estudios florísticos de Guerrero , Edited by: Diego-Pérez , N. and Fonseca , M. R. Mexico : Facultad de Ciencias, Universidad Nacional Autónoma de México .
- Villanueva , G. R. 1984 . Plantas de importancia apícola en el Ejido de Plan del Río, Veracruz, México . Biotica , 9 : 279 – 340 .
- Villanueva , G. R. 2002 . Polliniferous plants and foraging strategies of Apis mellifera (Hymenoptera: Apidae) in the Yucatán Peninsula, Mexico . Revista de Biología Tropical , 50 : 1035 – 1043 .
- Villanueva , G. R. , Moguel-Ordóñez , Y. B. , Echazarreta , G. C. M. and Arana , L. G. 2009 . Monofloral honeys in the Yucatán Peninsula, Mexico . Grana , 48 : 214 – 223 .
- Villegas-Durán , G. , Bolaños-Medina , A. , Miranda-Sánchez , J. A. , Quintana-Rocha , I. L. , Guzmán-Quintana , O. E. and Zavala-Ruiz , M. J. 1999 . Flora nectarífera y polinífera en el Estado de Michoacán , Mexico : Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación .
- Villegas-Durán , G. , Bolaños-Medina , A. , Miranda-Sánchez , J. A. and Zenón-Abarca , A. J. 2002 . Flora nectarífera y polinífera en el Estado de Chiapas , Mexico : Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación .
- Villegas-Durán , G. , Bolaños-Medina , A. , Miranda-Sánchez , J. A. , Sandoval-Hernández , R. and Lizama-Manrique , J. M. 2003 . Flora nectarífera y polinífera en la península de Veracruz , Mexico : Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación .
- Villegas-Durán , G. , Cajero , A. , Bolaños , A. , Miranda , S. , Pérez , M. , Guzmán , Q. , Tah , B. , Osorno , L. and Sánchez , R. 1998 . Flora nectarífera y polinífera en la península de Yucatán , Mexico : Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación .
- Villegas-Durán , G. , Rodríguez-Rodríguez , A. M. , Miranda-Sánchez , J. A. and Córdoba-Wade , H. 2004 . Flora nectarífera y polinífera en el estado de Tabasco , Mexico : Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación .