Abstract
The pre-meiotic, meiotic and tetrad stages of development in microsporangia of Alsophila setosa were studied with particular emphasis on the early establishment of patterning in the microspore wall and the subsequent development of the sporoderm. The data obtained were compared with corresponding ontogenetic stages of Psilotum nudum. Tapetal behaviour was also examined. During the tetrad period, only one layer, a thin undulating sheet, appeared alongside the plasma membrane of the tetraspores, and this was evidently formed on a pre-patterned structure – a fibrillar layer, corresponding to a kind of primexine matrix. The early free microspores had a wavy plasma membrane with a parallel, sinusoidal, thin initial sporoderm layer. The proximal apertural fold was observed to be an extended outgrowth of this initial spore envelope. Sporoderm ontogeny during the tetrad period in Alsophila and Psilotum show some common points, but also fundamental differences, mainly in the relative timing of events: in Alsophila the end of the tetrad period is the starting point for exospore development, whereas in Psilotum the exospore is already complete at this stage. Considerable differences were also observed in the tapetum of the two species.
Ultrastructural ontogenetic studies have always been a powerful tool for understanding the processes involved in sporoderm development and also for the correct interpretation of mature spore or microspore walls, which at maturity are ‘silent’ or closed structures from which ontogeny cannot be deduced. In spite of the series of a number of important and informative studies on spore wall formation (Lugardon, Citation1966, 1968, Citation1969, 1973, 1979, 1990; Pettitt, Citation1971, 1976, Citation1977; Sheffield & Bell, Citation1979, Bell, Citation1981; Brown & Lemmon, Citation1982a , Citation1982b , 1996, 2001a, 2001b, 2009; Sheffield et al., Citation1983; Parkinson, Citation1987, Citation1995; Uehara & Kurita, Citation1989 a, Citation1989 b, Citation1991; Uehara et al., Citation1991; Parkinson & Pacini, Citation1995), the development of pteridophyte spores has received far less attention than corresponding events in seed plants. There have also been fewer attempts to bring pteridophyte spore development into a phylogenetic context (e.g. Blackmore et al., Citation2000) or to interpret the basic organisation and mechanisms of spore or microspore formation (Wellman, Citation2004; Blackmore, Citation2007; Blackmore et al., Citation2007).
No previous ontogenetic studies have been undertaken on representatives of Cyatheaceae, including Alsophila, the focus of the present study. Gastony (Citation1974) reported some embryological data including the sporangium and spore morphology of Cyatheaceae: he reported the chromosome number n = 69 in Alsophila and observed that there were four spore mother cells (SMCs) per sporangium and, correspondingly, 16 spores in a sporangium. The form of the sporangium is elongate with a rather narrow loculus. Spore morphology of the Cyatheaceae, including Alsophila, was also studied by Gastony and Tryon (Citation1976). Later, mature spores of several genera of the Cyatheaceae (including Alsophila) were described and illustrated with scanning electron microscopy (SEM) and transmission electron microscopy (TEM) by Tryon and Lugardon (Citation1991). The mature sporoderm of an endangered homosporous, leptosporangiate Argentinian species, Alsophila setosa Kaulf., was studied with SEM and TEM by Marquez et al. (Citation2009) as well. The authors demonstrated some differences between the number of perispore layers they recognised and those defined by Tryon and Lugardon (Citation1991).
Our aim in this study was to trace at the ultrastructural level of the developmental events in Alsophila setosa from sporogenous cells through spore mother cell and meiosis stages, and, in particular, to pay special attention to the sporoderm development during the tetrad period. We also wanted to compare the ontogenetic events in the leptosporangiate fern Alsophila setosa (Cyatheaceae) and in Psilotum nudum (L.) P.Beauv. Psilotaceae, with the two genera Psilotum and Tmesipteris, had for many years been regarded as the most primitive living vascular plants (Takhtajan, Citation1978; Parenti, Citation1980; Bremer, Citation1985), although Bierhorst (Citation1977) had argued that Psilotum was, in fact, a morphologically reduced fern. Later, Stevenson and Loconte (Citation1996) pointed out that Psilotum should be placed as second branch within the true ferns (see Blackmore et al., Citation2000, and references cited therein). Analyses based on molecular sequence evidence strongly support the conclusion that Psilotum is a morphologically reduced fern (Qiu & Palmer, Citation1999; Schuettpelz & Pryer, Citation2007). In comparing Alsophila and Psiltoum, our intention is to add to our understanding of early sporogenesis in pteridophytes, and also to find similarities with gymnosperms and angiosperms.
Materials and methods
Sporangia of Alsophila setosa were collected during February 2009 in the Reserva de la Biósfera Yabotí, Misiones, Argentina. The material was fixed in the field using two fixatives: (1) 1% glutaraldehyde (GA) + 0.0025% ruthenium red (RR) + 0.1 M phosphate buffer (pH 7.4; 4 °C; 24 h), washed in phosphate buffer + 0.0025% RR, then post-fixed with 1% osmium tetroxide (OsO4) + 0.0025 % RR + 0.1 M phosphate buffer (pH 7.4; 4 °C; 2 h); (2) the same, but without RR. The material was dehydrated in an acetone series and then embedded in Spurr medium mixture. Ultrathin sections were stained with 1% uranyl acetate for 15 min followed by lead citrate for 5 min. The observations were made with a Hitachi H-600 TEM at the Komarov Botanical Institute, St. Petersburg.
Results
Pre-meiotic stages: sporogenous cells
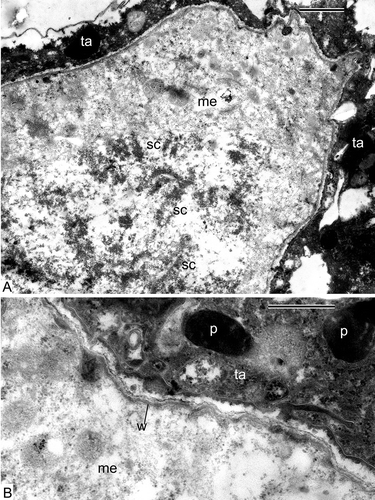
The earliest stage that we managed to obtain in our material of Alsophila represented the stage at which the process of differentiation of the archesporial cells had been completed and sporogenous and tapetal cells were present. The sporogenous cells are tightly packed and have angular outlines and rather dark-contrasted cytoplasm with many ribosomes (, E). The common wall between the tapetum and sporogenous cells is represented by the middle lamella (). The latter is bordered with the plasma membranes of tapetal and sporogenous cells (, D). The plasma membrane of the sporogenous cells has many outgrowths, both small and large (plasmalemmasomes), which are seen in sections as clusters of small or large vesicles (–E). Note the displacement of the middle lamella towards the tapetal cell because of appearance of these plasmalemmasomes of the sporogenous cell (), when compared with the central position of the middle lamella at a slightly earlier stage (). Plasmodesmata between the tapetal cells and sporogenous cells are at first well pronounced (, C), but become partly occluded at a slightly later stage ().
Figure 1. Pre-meiotic stages in Alsophila setosa and in Psilotum nudum: sporogenous cells. A–E. Alsophila setosa: A. Group of sporogenous cells, surrounded by tapetal cells; elongate tapetal cells contain large vacuoles with dark-contrasted precipitations; B. The border between tapetal cell and sporogenous cell; the middle lamella is high contrasted (arrow); all the tapetal organelles are aligned in the parietal layer of the cytoplasm with plasmodesma between tapetal and sporogenous cells (arrowhead); C. Clusters of plasmalemmasomes at the surface of sporogenous cell (asterisks); lipophilic accumulations penetrate from the tapetum to the sporogenous cell through plasmodesma (arrowhead); D. The border between the tapetal cell and the sporogenous cell with distinct middle lamella (arrows), plasma membrane of tapetal cell and plasma membrane of the sporogenous cell; the boundaries of mitochondria and dictyosome in the parietal cytoplasm of the tapetal cell are almost indistinguishable; plasmodesmata between the tapetal cell and the sporogenous cell become occluded (arrowheads); many outgrowths (plasmalemmasomes) of the plasma membrane of the sporogenous cell (asterisks) displaced the middle lamella closer to the tapetal cell; E. Outgrowths of the plasma membrane of the sporogenous cell (asterisk); a lipoid substance penetrating from the tapetal to the sporogenous cell appears in the form of a distinct ordered substructure (arrowhead). F, G. Psilotum nudum (from Gabarayeva, Citation1984a , ; reproduced with permission): F. Clusters of plasmalemmasomes at the surface of a sporogenous cell (asterisk) with ampoule-like dilation of the cisterna of the rough endoplasmic reticulum (arrow); G. The border between sporogenous cells and plasmodesmata; plasmalemmasomes at the surface of the cell (asterisk). Scale bars – 5 μm (A), 500 nm (B, F, G), 200 nm (C–E). Legend to all figures: ce – central element, ch – chromosome, cm – cytoplasm of meiocyte, cp – cup-like plastid, d – dictyosome, er – endoplasmic reticulum, fl – fibrillar layer, ga – Golgi apparatus, is – intercellular space, lg – lipid globule, me – meiocyte, mi – mitochondrion, mvb – multivesicular body, n – nucleus, ne – nucleus envelope, nu – nucleolus, p – plastid, pd – plasmodesma, pe – plastid envelope, pi – invagination of the plastid, pl – plasma membrane of the sporogenous cell, plta – plasma membrane of tapetal cell, rer – rough endoplasmic reticulum, s – young spore, sc – sporogenous cell, sco – synaptonemic complex, smc – spore mother cell, ss – sporangium space, ta – tapetal cell/tapetum, tet – tetrad, ts – tetraspore, v – vacuole, w – spore mother cell wall.
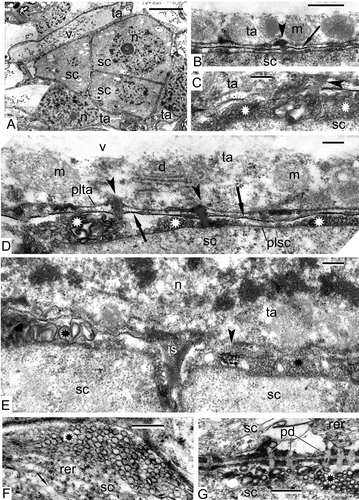
The tapetal cells form a single layer around the sporogenous cells and have an elongate form (). Some of them have large vacuoles with dark contrasted precipitations (). The large vacuole squeezes the cytoplasm of tapetal cells out to the cell periphery, and all the organelles are aligned along the plasma membrane (, D). Nevertheless, the tapetal cells are active, they secrete an osmiophilic substance, probably lipophilic in nature, which accumulates outside the plasma membrane (, D). This osmiophilic substance penetrates into the sporogenous cells (–D) and in some places forms highly ordered substructures ().
At comparable stage in Psilotum, sporogenous cells also show clusters of plasmalemmasomes (, G) and numerous plasmodesmata (). The rough endoplasmic reticulum (RER) forms stacks of cisternae with dilations.
Pre-meiotic interphase: spore mother cells (SMCs)
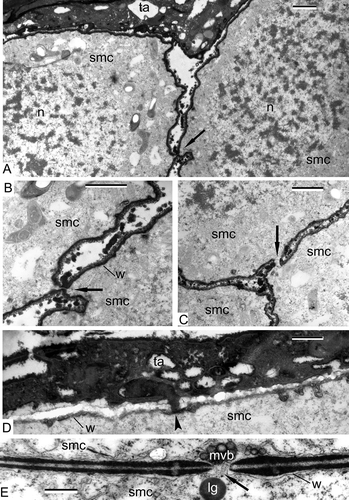
SMCs of Alsophila are separated from each other by narrow spaces, but connected via cytomictic channels (–C). The SMCs develop a dark contrasted envelope, which is slightly wavy; granules, most probably of tapetal origin, are accumulated in the space between the cells (–C). Large nuclei occupy a considerable part of the volume of the SMCs (). Plastids with starch grains are pleomorphic, their form varies from round to amoeboid (). The wall of SMCs has a jagged profile; a lipophilic substance penetrates from tapetum into SMCs ().
In Psilotum, SMCs are also connected by cytomictic channels () and multivesicular bodies; lipid globules and other organelles are in close vicinity, probably penetrating through the cytomictic channels.
Figure 2. Pre-meiotic interphase in Alsophila setosa and Psilotum nudum: spore mother cells (SMCs). A–D. Alsophila setosa: A. Cytomictic channels appear between the SMC (arrow); B. Two SMCs with a cytomictic channel (arrow); the wall of the SMC is well pronounced; roundish particles are present in the space between the SMC; C. Three SMCs with a cytomictic channel (arrow); D. The border between the tapetal cell and the SMC; the wall of the SMC has a jagged profile; penetration of lipoid substance from the tapetal cell into the SMC (arrowhead). E. Psilotum nudum (from Gabarayeva, Citation1984 a, ; reproduced with permission); a cytomictic channel between the SMC (arrow); the multivesicular body is ready to penetrate the channel. Abbreviations: see Scale bars – 1 μm (A–C), 500 nm (D, E).
Meiosis, prophase I: leptotene
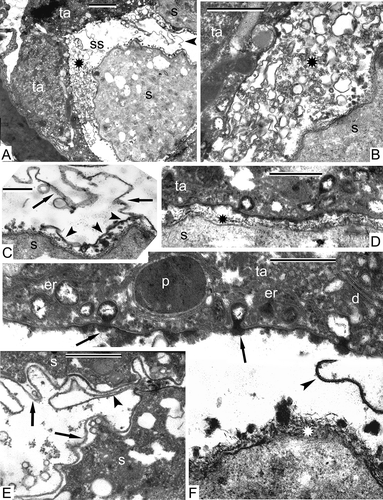
When entering meiosis, the SMCs of Alsophila retain an amoeboid form; the nucleus still occupies most of the cell volume (). The axial elements of chromosomes start to be evident (, B). Many vacuoles appear in the cytoplasm, the remaining organelles are poorly discernible, being poorly differentiated (, B). The tapetal cells have dark-contrasted cytoplasm and contain strongly osmiophilic plastids of ribbon-like or oval form (, B).
Figure 3. Prophase I of meiosis in Alsophila setosa. A. The beginning of the prophase (leptotene stage): The axial elements of the chromosomes start to be visible (arrowheads); the cytoplasm of the meiocytes contains many autolytic vacuoles, elimination of ribosomes and dedifferentiation of plastids take place; the tapetal cells are dark-contrasted and have plastids of ribbon-like and oval form, which are especially osmiophilic. B. Magnified part of (A): Tapetal cells have so-called negative contrast: membranes appear white, but stroma of plastids and the cytoplasm are strongly osmiophilic; the wall of meiocytes is wavy; axial elements of chromosomes visible (arrows). Abbreviations: see Scale bars – 1 μm.
Meiosis, prophase I: zygotene
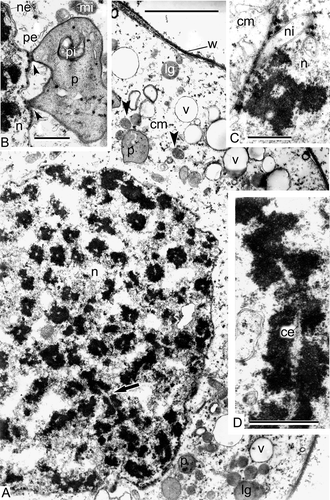
At this stage, the formation of synaptonemic complexes occurs. Pairs of chromosomes are clearly seen and lateral elements of the synaptonemic complexes are parallel to each other (, B, Alsophila); the central element is clearly seen at higher magnification (, Psilotum). The process of meiotic clarification of the cytoplasm is evident in both species: the increase of the number of autolytic vacuoles leads to the reduction of ribosome and organelles number, many multivesicular bodies are seen in the meiocyte cytoplasm of Alsophila, some of them are attached to the microspore wall ().
Figure 4. Prophase I of meiosis in Alsophila setosa and in Psilotum nudum. A–C. Alsophila setosa (zygotene stage): A. The formation of synaptonemic complexes; the process of meiotic clarification of the cytoplasm is in progress and the reduction of ribosome number, increasing of the number of autolytic vacuoles and formation of cup-like plastids are evident in meiocyte cytoplasm; B. Synaptonemic complexes (arrowheads) at higher magnification; lateral elements are visible; C. The cytoplasm of the meiocyte is essentially clarified; clusters of vesicles surrounded with a membrane (arrows) are located in the vicinity of the wall, some of them attached to it: these could be multivesicular bodies. D, E. Psilotum nudum (zygotene and pachytene stage): D. Zygotene sinaptonemic complex of a chromosome dyad with a central element (arrow; from Gabarayeva, Citation1984b , ; reproduced with permission); E. Pachytene synaptonemic comlexes, most are cut longitudinally. Abbreviations: see Scale bars – 2 μm (A), 1 μm (B, E), 500 nm (C, D).
Meiosis, prophase I: early pachytene
Stronger condensation of the chromatin of the bivalents in synaptonemic complexes is typical for this stage in comparison with zygotene in Psilotum () and Alsophila (). In those parts of synaptonemic complexes, which were sectioned longitudinally, it is possible to see coils of chromosome spirals (). The cytoplasm of meiocytes in both species is strongly clarified and contains many vacuoles. The membranes of organelles are either not well preserved or are poorly discernible, the envelope of meiocytes has differential contrast and looks three-layed with a jagged outer surface as in Alsophila ().
Figure 5. Prophase I of meiosis in Alsophila setosa. Early pachytene stage. A. A part of the meiocyte surrounded by tapetal cells; chromosomes of the synaptonemic complexes are more condensed than they were at the zygotene stage; all organelles in the cytoplasm are dedifferentiated, their membranes are not discernible. B. Higher magnification of (A) evidences that the negative contrast of the tapetal cells is still preserved and the wall of the meiocyte becomes thicker and is differentiated into several layers of different contrast; the cytoplasm of meiocytes is clarified. Abbreviations: see Scale bars – 1 μm (A), 500 nm (B).
The condition of tapetal cells in Alsophila shows a sharp difference from that of the meiocytes. Tapetal cells are very active, their cytoplasm contains many organelles and all the membranes of organelles are very well preserved, and the contrast of tapetal cells continues to be negative (all the membranes appear white, but the contents of endoplasmic reticulum cisternae, of plastids and the cytoplasm appear black; ).
In Psilotum, at the late pachytene stage, the compactness of the chromatin in the synaptonemal complexes is very high [, D; cross-sectioned complexes (A), longitudinally-sectioned complex with the central element (D)] and is not less intensive than that observed during metaphase. There are many cup-like plastids and close contact between some of these plastids and the nuclear envelope are a characteristic feature of this stage (). Many finger-like invaginations of the nuclear envelope are also observed ().
Figure 6. Prophase I of meiosis in Psilotum nudum, pachytene stage (from Gabarayeva, 1984b, , 1, 2, 4 and 5, 3; reproduced with permission). A. Part of a meiocyte, general view; most part of the synaptonemic complexes is cross-sectioned with the exception of one or two (arrow); the wall of the meiocyte is fibrillar and dark; many organelles, including mitochondria (arrowheads), are well discernible. B. Close contact between a plastid and the nuclear envelope (arrowheads). C. Finger-like invaginations of the nuclear envelope are characteristic for this stage. D. Magnified image of a pachytene synaptonemic complex with the central element. Abbreviations: see Scale bars – 10 μm (A), 1 μm (B–D).
Meiosis I: telophase
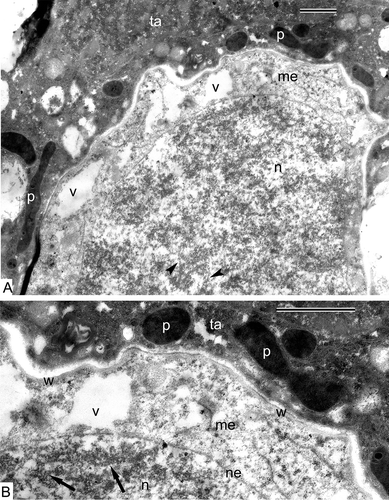
At the late anaphase/early telophase in Alsophila, two groups of sister chromosomes are shared by the equatorial organelle plate, and the nuclear envelopes are indistinct (). This plate is a cluster of many organelles: mitochondria, plastids, lipid globules, small autolytic vacuoles (). Because the membranes of organelles are poorly visible, it is sometimes difficult to distinguish lipid globules from plastoglobules of plastids. No signs of the formation of a new equatorial cell wall is observed at this stage. The wall of Alsophila meiocytes is uneven; it becomes here and there rather thick (, D) and contains an electron transparent matrix with fine fibrillae (). Some tapetal cells, surrounding the four meiocytes in the sporangium, invade inside the loculus, partly embracing each meiocyte from the proximal side; two telophase meiocytes can be seen in this section ().
Figure 7. The end of meiosis I in Alsophila setosa, late anaphase–early telophase. A. Sporangium, where two telophase meiocytes are visible; tapetum spreads outgrowths inside the loculus, embracing each meiocyte from the distal side (arrowheads). B. Overview of the meiocyte with two groups of sister chromosomes (asterisks) and organelle plate at the equatorial plane; nuclear envelopes are not completely restored; dark-contrasted globules could be plastoglobules or free lipid droplets in the cytoplasm (arrows); the tapetum partly embraces the meiocyte and includes large vacuoles; C. Magnified part of (B) with the organelle plate; plastoglobules (plastids are dedifferentiated, restricting membranes are not seen) or free lipid droplets (arrows), many mitochondria (arrowheads), cup-like plastids with remnants of autolysed cytoplasmic inclusions (asterisks) and small vacuoles crowd the equatorial plane of the cell. D. A border between the meiocyte and the tapetum; the meiocyte wall consisting of a network of fine fibrillae becomes thicker, probably as a result of swelling; a chromosome (asterisk) is still attached to a fragment of the nuclear envelope. Abbreviations: see Scale bars – 10 μm (A), 1 μm (B, C), 500 nm (D).
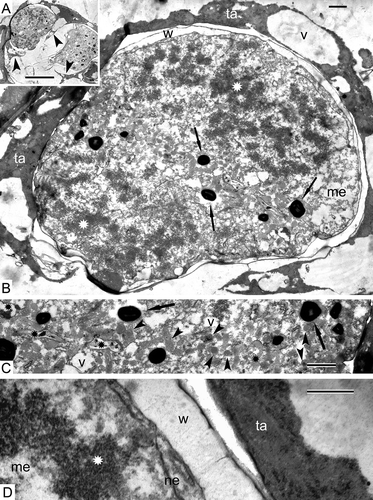
Figure 8. The end of meiosis I in Psilotum nudum, telophase. Nuclear envelopes are completely restored. A. The organelle plate is well pronounced at the equatorial plane and includes cup-like plastids, many mitochondria (arrowheads), vacuoles, lipid globules and dictyosomes; some nuclear invaginations are still present (arrow). B. A fragment of the organelle plate with microtubuli between organelles and attached to them (arrowheads) and ribbon-like double-invaginated plastid (arrow). Abbreviations: see Scale bars – 1 μm.
In Psilotum, the nuclear envelopes are clearly discernible at the advanced telophase stage, surrounding both groups of sister chromosomes and finger-like invaginations of the nuclear envelope are preserved (). The organelle plate consists of readily recognisable plastids (many of them have a cup-like form), mitochondria, vacuoles, lipid globules and Golgi stacks (). Microtubuli penetrate the organelle plate and are attached to organelles (). Some ribbon-like plastids have two cup-like regions at their extremities ().
The tetrad period in Alsophila setosa
Early tetrad stage
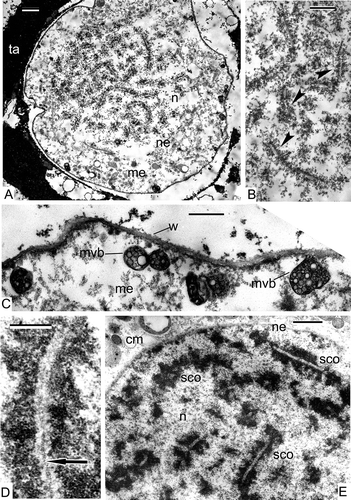
The tetrads are surrounded by elongate, binuclear tapetal cells, which may partly fuse together (, B). The tapetal cells maintain their parietal position and their periclinal walls are poorly discernible. Many osmiophilic spherical units accumulate between the tetraspores (). Long fibrils are discernible on the tetraspore surface (, E). It is difficult to judge whether these fibrillae transformed from the material of the meiocyte envelope (the primary wall of the sporocyte; ), or are newly formed.
Tetrad stage in progress
Sections through tetrahedral tetrads (, B) reveal that each tetraspore is enveloped in a fibrillar layer (). A dark thin unbroken sheet has appeared between the tetraspore plasma membrane and the fibrillar layer (–E). The cytoplasm of the tetraspores, observed at higher magnification, is quite different from that of the meiocytes and contains many ribosomes and polysomes (), although the membranes of organelles are still poorly defined. Some of the tapetal cells have normal contrast and contain large vacuoles with dark contrasted filaments (, D), whereas others retain a negative contrast ().
The end of the tetrad period
Some microspores are retained together in tetrads (), but others start to be separated from each other, and some tapetal cells seem to be going to penetrate between the members of the tetrad (). A dark contrasted sheet, associated with fibrils, becomes more prominent and has a sinusoidal profile (–F). The tapetal cells maintain their parietal position (). Substances, secreted by the tapetum, accumulate in the supporting liquid of the sporangial loculus. As a result, different kinds of osmiophilic accumulations are visible in the loculus of the sporangium including tripartite lamellae, spherical droplets and vesicles of different size and form ().
Very young free spores
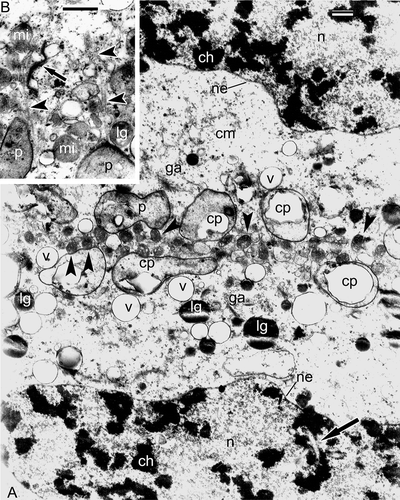
Very young free spores are still surrounded by a parietal tapetum (). An outgrowth of the spore envelope, probably representing the initial proximal fold (), is discernible. Many accumulations of different form and size including vesicles, spherical particles and fragments of membranes fill the loculus of sporangium (): these could either be products of the tapetal synthesis or remnants of disintegrating tapetal cells. A magnified fragment of shows more details of the spore surface, with a membrane on the top of the fibrillar network and with the initial proximal fold visible (). In close vicinity to the tapetum, the distal fibrillar surface of the young spores is rather even and includes osmiophilic roundish particles (, F), but the proximal sides of young spores have a very uneven surface with a sinusoidal initial layer of sporoderm, parallel to the contour of the spore surface and separated from it by a gap (). The tapetum shows high activity and has a negative contrast (, F); its cisternae of endoplasmic reticulum pinch off secretory vesicles; the latter attach to the tapetal plasma membrane and release their contents into the sporangial loculus by exocytosis ().
Discussion
Compared with seed plants, ultrastructural data on pteridophyte spore ontogeny is far less extensive. This is explained by the considerable technical difficulties facing investigators, connected with the poor penetration of fixatives and the corresponding preservation of the study material. The challenges of investigating the extremely thick and dense sporopollenous envelopes of spores, which form as early as the beginning of the post-tetrad period, make the members of this most interesting, polyphyletic group a kind of Cinderella group among researchers. Nevertheless, a small number of determined authors have continued to add to our knowledge of the ultrastructure and development of pteridophyte spore walls. We can therefore compare our new data on meiosis and early sporogenesis in Alsophila with previous data on meiosis (Gabarayeva, 1984a, 1984b, Citation1985) and sporoderm formation (Lugardon, Citation1979) in representatives of other fern groups including Psilotum (Psilotaceae) and with corresponding developmental events in other ferns and higher plants, such as gymnosperms and angiosperms.
Pre-meiotic intercellular connections
At the critical point of the transition from sporophyte to gametophyte, in addition to chromosome reduction, several features of the cells, involved in the transition, deserve special attention.
Clusters of plasmalemmasomes (outgrowths of the plasma membrane of different form) in sporogenous cells of Alsophila (–E) seem to be a typical feature for pre-meiotic cells of different plant groups. At the same point in ontogeny, the plasma membrane in Psilotum nudum sporogenous cells is also very wavy and forms clusters of plasmalemmasomes (). The latter were also observed in Osmunda regalis L., Anemia phyllitidis (L.) Sw. and Lygodium japonicum (Thunb.) Sw., as well as in many gymnosperms and angiosperms, for example, in Stangeria eriopus (Kunze) Baill. and Helleborus foetidus L. (Echlin & Godwin, Citation1968; Lugardon, Citation1968; Surova, Citation1981; Gabarayeva & Grigorjeva, Citation2002). It is likely that such an intensive folding of the plasma membrane is necessary for the rapid growth and rounding-up of the sporogenous cells that follows at the point of transition to SMCs.
Numerous plasmodesmata are characteristic for both sporogenous cells of Alsophila and Psilotum (). In Alsophila, plasmodesmata are observed not only between sporogenous cells, but also between sporogenous and tapetal cells. These intercellular connections in Alsophila (–D) are probably necessary for transition of some lipoid substance from the tapetum to sporogenous cells. Penetration of a lipid or some other hydrophobic surface-active substance is possible also in monomolecular form through the plasma membranes: in this case a hydrophobic substance, being excreted from a tapetal cell, comes into hydrophilic intercellular medium and forms micelle-like ordered structure (). Plasmodesmatal connections in pre-meiosis is a common feature for ferns (Sheffield & Bell, Citation1979) as well as for angiosperms (Dickinson & Heslop-Harrison, Citation1977): the latter authors suggested that up to a certain point in ontogeny, everything was coordinated between cells, because their cytoplasm was interconnected, but later, the closure of cytomictic channels allowed different lines of cells to follow different fates in the course of development.
Before the initiation of meiosis, most of the plasmodesmata become occluded and cytomictic channels appear between SMCs in both Alsophila (–C) and Psilotum (). Lugardon (Citation1968) compared development of cytoplasmic connections in pre-meiotic and meiotic stages in homospore ferns Blechnum spicant (L.) With. and Osmunda regalis: in Blechnum, pre-meiotic plasmodesmata are substituted for cytomictic channels in prophase I of the meiosis; the channels in Osmunda are wider than those of Blechnum and allow passage of such organelles as mitochondria and cisternae of endoplasmic reticulum. In Psilotum, the cytomictic channels are also wide enough to allow passage of multivesicular bodies and lipid globules, although we have not demonstrated the penetration of organelles through them (). The term ‘cytomictic’ itself has appeared to stress their function: to provide the flow of cytoplasm together with its organelles from cell to cell. Cytomictic channels in SMCs are closed earlier in pteridophyte ontogeny than those of angiosperms, and they are less numerous (Lugardon, Citation1968). Blackmore (Citation1990) formulated that ontogeny in angiosperms is an extended process (i.e. has additional steps like the development of a thick primexine) compared to pteridophytes, and it could be that closing the cytomictic channels at an early stage is a characteristic of pteridophytes compared to seed plants. The substitution of plasmodesmata to cytomictic channels, which disappear later, was also shown in the heterosporous fern Marsilea (Bell, Citation1981). The same process is usual at the transition from sporogenous cells to microspore mother cells for angiosperms (Heslop-Harrison, Citation1966; Pacini & Cresti, Citation1978; Gabarayeva et al., Citation1998, Citation2009a ) and is a widespread feature. The time of the appearance of the cytomictic channels varies in different species: sometimes, they appear earlier between sporogenous cells as in Chamaedorea (Gabarayeva & Grigorjeva, Citation2010).
The meaning of these intercellular connections is to create a coenocyte-like organisation and to achieve synchronised meiosis in all the meiocytes of a sporangium. Depending on the number of the cytomictic channels between meiocytes, this synchronism can be complete or limited. In Psilotum, where the number of cytomictic channels is relatively low, incomplete synchronism of meiosis was observed (Gabarayeva, 1984a). Interestingly, the absence of cytomictic channels was shown for gymnosperms (Kozubov et al., Citation1982) and, as a consequence, the asynchronism of meiosis in their microsporocytes.
The walls of SMCs in Alsophila and in Psilotum are clearly discernible, but differ from each other. They are indented and associated with osmiophilic globules in Alsophyla (–D), but smooth in Psilotum (). Many local dilations of stacked RER cisternae (one of them shown in ) were described as connected with the formation of phytolysosomes in Cucurbita pepo L. (Coulomb & Coulon, Citation1972), but in ferns, Pettitt (Citation1979) associated a secretory activity before meiosis with the formation of a fibrillar material of the meiocytes envelopes – the so-called special tetrad envelope. In Hypnum rusciforme Bridel (Bryophyta), a thick fibrillar layer is formed around sporogenous cells and meiocytes; histochemical data confirm its polysaccharide nature, namely callose (Genevès, Citation1971).
Meiosis, meiotic cytoplasmic clarification and re-organisation of organelles
At the transition to meiosis, a general re-organisation of the cytoplasm starts in both species. In the leptotene, when axial elements of chromosomes start to be discernible in Alsophila (), we did not observe the formation of multimembraneous spheres, containing portions of the meiocyte cytoplasm with organelles and ribosomes (as is typical for angiosperms) but rather many autolytic vacuoles of irregular form with electron-transparent contents are the characteristic feature of the cytoplasm. In parallel, the almost total disappearance of starch in Psilotum meiocytes and also of inner and outer membranes of plastids occurs in Alsophila. This was also reported for Polypodiophyta as a whole (Sheffield & Bell, Citation1979). Mitochondria are also poorly differentiated.
These changes of plastids and mitochondria during meiosis are so profound in Alsophila that these organelles are actually unrecognisable from the pachytene until the telophase I (, , C, 5, 7), but they are much better seen in Psilotum (, ). The hyaloplasm of meiocytes looks almost ‘empty’ because of the elimination of most of the ribosomes (, , , ). The stages of meiosis are well recognisable by the nuclear contents: in the leptotene, axial elements of chromosomes start to be discernible (); in the zygotene, pairs of chromosomes form synaptonemic complexes () with a typical central element (); the pachytene is characterised by much stronger condensation of chromatin in bivalents (, , D). After the metaphase and anaphase I stages, when homologous chromosomes have diverged, the organelle plate (band) forms in the telophase (, C, 8).
The main events during meiosis in both species are the same, but details are different. In Psilotum, many plastids invaginate and acquire a cup-like form; some of them are in close contact with the nuclear envelope (). We have not observed invaginations of the nuclear envelope in Alsophila during meiosis, but many finger-like invaginations were found in the pachytene in Psilotum (). The meaning of these invaginations is probably in that the surface of the contact of nucleus and cytoplasm increases considerably (therefore, nuclear-cytoplasmic interaction increases, which is connected with the beginning of another type of growth, in this case: gametophytic). A similar point of view was reported by Bell (Citation1975), who observed another form of nuclear-cytoplasmic interaction: evaginations of the nucleus to the cytoplasm. This is important during the sporophyte-gametophyte transition, most probably in connection with the change of genome functioning and corresponding transfer of the information to the cytoplasm. The cytoplasm of the gametophytes is purged of sporophyte information in the process of cytoplasmic clarification (Heslop-Harrison, Citation1971). A similar intensification of the nucleo-cytoplasmic interaction is a common feature for all land plant groups.
The comparison of all the observed organelle changes with those in other, closely and remotely related plant groups reveals the same changes or variations of them, but in different combinations or confined to slightly different points in ontogeny. They were found in the homosporous ferns Pteridium aquilinum (L.) Kuhn and Anemia phyllitidis (Sheffield & Bell, Citation1979; Surova, Citation1981), in the heterosporous fern Marsilea (Bell, Citation1981), in the apogamous fern Dryopteris borreri Newman (Sheffield et al., Citation1983), in mosses at sporogenesis (Brown & Lemmon, Citation1982 a) and gametogenesis (Manohar Lal & Bell, Citation1977), in gymnosperms at microsporogenesis (Aldrich & Vasil, Citation1970; Dickinson & Bell, Citation1970; Kozubov et al., Citation1982) and in angiosperms at mega- and microsporogenesis (Bogdanov, Citation1983).
In particular, the contact of the plastid with the nuclear envelope in Selaginella during megasporogenesis and in young microspores was reported (Pettitt, Citation1971, 1977); invaginated plastids were described in Isoetes megasporogenesis (Pettitt, Citation1976). The monoplastidic type of meiosis (MTOC, in which the plastid is the site of microtubule organising centre) in eusporangiate ferns (Brown & Lemmon, Citation2001a ) is considered to be more primitive than the polyplastidic type (Brown & Lemmon, Citation2001b ), characteristic of the fern and seed plant clade. Novel meiotic spindle ontogeny in quadrilobed sporocytes of leafy liverworts, unique for bryophytes, was also reported (Brown & Lemmon, Citation2009). In Isoetes sinensis Palmer, a rare and endangered quillwort in China, irregular meiotic behaviour was reported (Wang et al., Citation2007).
Simultaneous meiosis in Alsophila and in Psilotum is accompanied by formation of an organelle plate (=organelle band), associated with microtubules, in the equatorial plane at the telophase I (, ). Distinct polarisation of the meiotic cell is observed at the previous stage, at the metaphase with its polar distribution of organelles, but this kind of polarisation changes to the equatorial in the telophase. The organelle plate represents a special mechanism for the equal distribution of organelles between the sister meiotic cells. The same behaviour of organelles was described in Polypodium (Marengo & Marengo, Citation1972) and Onoclea (Marengo, Citation1977), in Pteridium (Sheffield & Bell, Citation1979) and in the apogamous fern Dryopteris borreri (Sheffield et al., Citation1983). An organelle plate forms in the moss Rhynchostegium serrulatum (Hedwig) A. Jaeger (Brown & Lemmon, Citation1982 b); in the horsetails Equisetum palustre L. (Lewitsky, Citation1926) and E. fluviatile L. (Lehmann et al., Citation1984), in four other species of Equisetum (Bednara et al., Citation1986); in Ginkgo biloba L. (Wolniak, Citation1976); in the angiosperms Helleborus foetidus (Echlin & Godwin, Citation1968) and Cypripedium californicum A. Gray (Brown & Lemmon, Citation1996). Interestingly, the F-actin cytoskeleton plays an important role in meiosis: after experimental destruction of microfilaments in the Psilotum nudum meiocyte, the daughter nuclei were located too close to each other and an organelle plate did not form, causing abnormal meiosis (Tchórzewska & Bednara, Citation2011). During simultaneous meiosis in Lavatera (Malvaceae), an unusual type of microtubular cytoskeleton was described (Tchórzewska et al., Citation2008), in which plastids and mitochondria group around the nucleus forming a kind of envelope, surrounded by radially oriented microtubules at the end of the meiotic prophase I and maintain this position up to the late stages of sporoderm development. No organelle plate and fragmoplast were found in the simutaneous meiosis of the apomictic Asteraceae Chondrilla juncea L. (Koscinska-Pajak & Bednara, Citation2003).
Sporoderm development in the tetrad period in Alsophila setosa
Our main interest in the tetrad period is the sporoderm development. Summarising the main events in the tetraspore wall, we emphasise the appearance of a fibrillar layer and of dark thin sheet around each tetraspore (–E). A layer of clearly discernible fibrilles surrounding the tetraspores is most probably a primexine matrix, which might be polysaccharidic or glycoproteinous. If the latter is true, it could be called a glycocalyx, but only histochemical data could show its chemical composition. Until new data will be gathered, we use the more neutral term ‘primexine matrix’. No histochemical data exist so far on chemical composition of the surface coat (a coat of sporocyte plus a coat of tetraspore) of homosporous leptosporangiate ferns in the tetrad stage, whereas for all other groups of plants (beginning with some Bryophytes) such data exist (even if only fragmentary) that show the presence of glycoproteins comprising a glycocalyx (Gabarayeva & Hemsley, Citation2006). The latter plays a key role in exine development of seed plants (Rowley & Dahl, Citation1977; Rowley, Citation1990), and it is very important to clarify the role of the glycocalyx in sporoderm development of Pteridophyta. In particular, the presence of acid mucopolysaccharides (=glycoproteins) was confirmed histochemically for eusporangiate ferns (Pettitt & Jermy, Citation1974; Pettitt, Citation1979), but for leptosporangiate ferns, the presence of the glycocalyx is still in question.
The new tetraspore envelope, developing between the plasma membrane and the fibrillar layer, is very thin and sinusoidal (, ) and, importantly, its development is evidently connected with the primexine matrix. Lugardon (Citation1990), describing the development of the sporoderm of Oreopteris limbosperma (All.) Holub., another homosporous leptosporangiate fern, has found a very similar sinusoidal thin sheet around each tetraspore – the initial exospore, but unlike Alsophila, the exospore layer in Oreopteris becomes fully developed during the tetrad period. The ‘feuillets’ (leaf-like sheets) of the exospore were considered by Lugardon (Citation1990) to be comparable to corresponding elements in the endexine of gymnosperms and angiosperms. Tryon and Lugardon (Citation1991) have pointed out that in more derived ferns the exospore is reduced to a single sheet. Lugardon (Citation1966) was very careful about naming initial layers in sporoderm development, preferring to call them by numbers in ontogeny and defining them only about maturity.
At present, we have observed only the first step of sporoderm development during the tetrad period and, following Lugardon, call it ‘layer 1’ or initial layer. As in Alsophila, the beginning of sporoderm development in Equisetum fluviatile starts very late (Lehmann et al., Citation1984), even later than in Alsophila in its free spore period. The authors concluded that the development of the whole sporoderm is under tapetal influence. In E. arvense L., the appearance of the thin inner exospore (from roundish plate-like structures) starts at the late tetrad stage and from the proximal side only (Uehara & Kurita, Citation1989 a). On the other side, in the eusporangiate fern Ophioglossum thermale Komarov and in the quillwort Isoetes japonica A. Braun and the liverwort Lycopodium clavatum L., all exospore development proceeds during the tetrad period (Uehara & Kurita, Citation1989 b, 1991; Uehara et al., Citation1991).
We have not observed a well discernible proximal fold in the end of the tetrad period, just a long outgrowth of a thin initial spore envelope (, E). Blackmore and Crane (Citation1998) mentioned that simultaneous cytokinesis, without a dyad stage, generally results in a tetrahedral tetrad, in which all four meiotic products are in direct contact and appear to be reflected in a distinct trilete aperture. Lugardon (Citation1966, 1969), describing the formation of a proximal fold in the leptosporangiate homospore fern Blechnum spicant, calls the forming layers by numbers. Only the first layer is laid down during the tetrad period. It has initially a tripartite structure and later acquires a fibrillar-granular cover.
The sporoderm development was studied by Psilotum triquetrum. According to Lugardon (Citation1979), the whole exospore in Psilotum nudum (≡P. triquetrum) develops during the tetrad period. The tetrads are covered by a special envelope (the envelope of the SMC) and an individual envelope covers every tetraspore, which is very thin from the distal side and thick from the proximal side. Both envelopes consist of slimy polysaccharides and have a fibrillar texture; both gradually disappear in the process of exospore formation. The first elements of the exospore are tripartite membranes. Later, they anastomose each other and join to a sheet. At the same time, a very thin proximal fold occurs. A continuous inner exospore layer is formed gradually from partly independent superficial sheets. Lugardon (Citation1979) concluded that the sporoderm ontogenetic process in Psilotum is very similar to that of Filices, which is in accordance with recent genetic data (Qiu & Palmer, Citation1999), whereas it obviously differs from that of other Pteridophytes.
As is clear from the data obtained, the sporoderm ontogeny during the tetrad period in Alsophila and Psilotum has some common points, but also essential differences. The main difference concerns the temporal aspect of unfolding events: whereas in Alsophila the end of the tetrad period is a starting point of exospore development, in Psilotum, the exospore is completed by this time. In its late starting point for exospore development, Alsophila is similar to Equisetum. Significant differences between the two species concern the tapetum. In Alsophila, we observed a layer of elongated tapetal cells enveloping first a compact complex of the four sporogenous cells () and then every meiocyte (, , , ) and tetrad (, B, 10A, 11A). In Psilotum, the complex of sporogenous cells is highly branched and immersed into ceonocytic nutritive tissue, whereas the outer parietal layer lines the loculus of the sporangium. Later, penetration of the nutritive tissue between the fans of the branched complex of the tapetal cells in Psilotum leads to the isolation of several groups from the complex and, as a consequence, to the slightly asynchronous entry of meiocytes into the meiosis (Gabarayeva, 1984a). In contrast, Alsophila meiocytes develop simultaneously. Parkinson (Citation1987, 1995) showed that in Psilotum (and also in the fern Schizeae), the tapetum is of combination type: the outer layer consists of typical parietal secretory cells, which persist in their position until the end of the sporogenesis and form a sporopolleninous tapetal membrane, but the inner layer turns into a periplasmodium.
It should be stressed that the type of the tapetum in Alsophila is difficult to determine precisely: the inner and outer plasma membranes of the tapetal cells are preserved (at least during the tetrad period), but the periclinal walls of the tapetal cells are poorly visible. The latter can be explained by high osmiophilia and so-called negative contrast, when membranes of organelles appear white in TEM sections, but interiors of the organelles and the cytoplasm are intensively contrasted (, D, 3, 4A, 5, 6A, C, 7A, B, 10D). Considerable amounts of carotenoids cause also negative contrast as shown for sporocytes of Lycopodium saururus Lam. (Sersic, Citation1983). The negative contrast is also known as a kind of fixation artefact, resulting in disruption of the vacuole tonoplast and the exit of some phenolic substances from vacuoles (Mueller & Greenwood, Citation1978). This may be explained by the fact that phenolics are one of the building blocks of sporopollenin (Gubatz & Wiermann, Citation1992; Hemsley et al., Citation1996a; Niester-Nyveld et al., Citation1997).
Blackmore et al. (Citation2000) have analysed studies on spore wall formation in their preliminary phylogenetic review of sporogenesis in pteridophytes and provided very useful data (Blackmore et al., Citation2000, appendix 1) on ontogenetic character states (total 25 characters). Ultrastructural information was available for 18 genera, representing most of the major extant plant lineages. The authors have extended the number of morphological characters available and emphasised that in sporogenesis, a limited number of ontogenetic processes generate a wide variety of sporoderm patterns because of interplay between processes in the haploid microspores and diploid tapetum. According to those character states (Blackmore et al., Citation2000), we summarise here selected characters of Alsophila, which we observed during meiosis, cytokinesis and the tetrad period and which would be useful for future phylogenetic analyses.
Figure 9. Early tetrad stage in Alsophila setosa. A. Part of a tetrad (one of the tetraspores sectioned tangentially) surrounded by a binuclear tapetal cell. B. Section through two tetraspores of a tetrad; tapetal cells keep parietal position. C. Border between two tetraspores (proximal side); many spherical osmiophilic accumulations are seen in the slot (asterisk). D, E. Border between tetraspores and tapetum; long fibrills (asterisks) are visible on the tetraspore surface; initial dark contrasted sheet appears here and there (arrows). Abbreviations: see Scale bars – 2 μm (A, B), 300 nm (C–E).
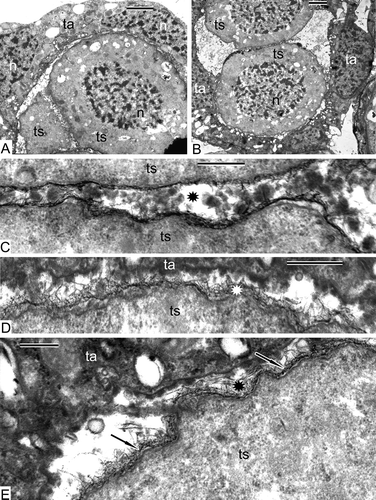
Figure 10. Ongoing tetrad period in Alsophila setosa. A. Three members of a tetrad (lateral section) surrounded by tapetal cells. B. Tetrad (central section) and adjacent tapetal cell. C. Border between two tetraspores of a tetrad and an adjacent tapetal cell; the newcomer is a thin dark sheet (arrow) adjacent to the plasma membrane (arrowhead); a fibrillar layer extends around the tetraspores. D. Tetraspore of a tetrad and adjacent tapetal cell; the vacuole of the tapetal cell is filled with long-fibrillar contents; a sinusoidal dark contrasted layer (arrows) and a fibrillar layer are visible. E. Border between a tetraspore and tapetum with a sinusoidal thin dark sheet at the inner side of the fibrillar layer (arrowheads); the number of ribosomes is restored (asterisk). Abbreviations: see Scale bars – 2 μm (A, B), 500 nm (C–E).
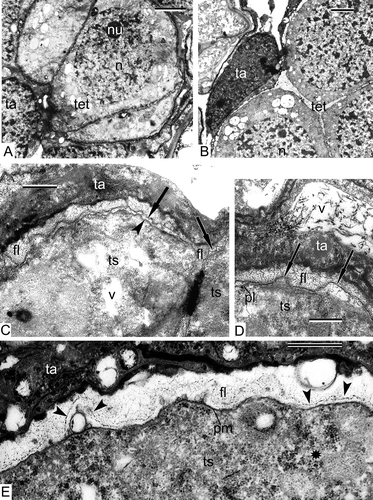
Figure 11. The end of the tetrad period in Alsophila setosa. A. Overview of a tetrad. B. Fragment of a tetrad at the disintegration point, proximal zone. C–F. Distal borders of tetraspores; a thin sinusoidal envelope (arrows), associated with the fibrillar layer (asterisk), is the initial sporoderm layer. G. Sporangia-supporting liquid between the tetraspores is full of accumulations of different forms: tripartite lamellae (arrowhead), spherical droplets (star) and vesicles (black and white asterisks). Abbreviations: see Scale bars – 2 μm (A), 1 μm (B, G), 500 nm (C, E, F), 300 nm (D).
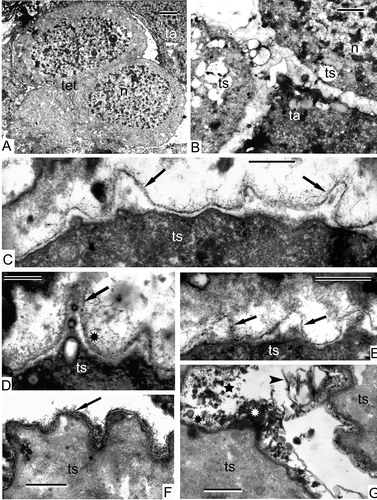
Figure 12. Very young free spores in Alsophila setosa. A. Neighbouring spores are separated from each other; tapetal cells maintain their parietal position; different kinds of accumulations (asterisk) are present in the sporangium space; an outgrowth of a spore envelope, probably the initial proximal fold (arrowhead). B. Vesicles of different size and form and spherical units (asterisk) in sporangia-supporting liquid between the tapetum and a young spore. C. Proximal side of a young spore with initial proximal fold (arrows) and tripartite membrane (arrowheads). D. Fibrillar envelope of a young spore in close vicinity to the tapetum. E. Two neighbouring young spores with wavy outlines and sinusoidal initial layer of the sporoderm (arrows); a loop of the proximal fold (arrowhead). F. A young spore, adjacent to tapetum; the tapetal cell has a negative contrast (white membranes and dark stroma of organelles); in the tapetum, plastids with large inclusion, wrapped in endoplasmic reticulum cisternae, dictyosomes, cisternae of endoplasmic reticulum and their large vesicles are visible, some vesicles open to the tapetal cell surface and release their content into the sporangium loculus (arrows); the surface of the spore is covered by a fibrillar envelope with addition of osmiophilic spherical units (asterisk); a fragment of tripartite membrane in sporangia-supporting liquid (arrowhead) is probably detached from the spore surface. Abbreviations: see Scale bars – 2 μm (A), 1 μm (B, E), 500 nm (C, D, F).
| 1. | Sporangium ontogeny: leptosporangiate (1). | ||||
| 2. | Tetrad wall: not formed (0). | ||||
| 3. | Monoplastidic meiosis: not monoplastidic (1). | ||||
| 4. | Mode of cytokinesis in meiosis (centripetal furrowing or centrifugal cell plate): ? (not evident) | ||||
| 5. | Timing of cytokinesis: simultaneous, with organelle plate – no cell wall after meiosis I (0). | ||||
| 6. | Aperture definition: by folding of plasma membrane (0). | ||||
| 7. | Aperture type: proximal and trilete (3). | ||||
| 8. | Exine (exospore) deposition: white-line centred lamellae (WLCL) not involved in the initial deposition, primexine (matrix) present (2). | ||||
| 9. | Exine patterning: plasma membrane strongly undulating in sectional view, giving rise to parallel undulating first thin layer of the exospore (1). | ||||
| 10. | Tapetal contribution to exine: exine formation definitely involves a tapetum-derived component, incorporated into tetraspore surface (1). | ||||
| 11. | Spore output: four sporocytes give rise to 64 spores (1); (see Gastony (Citation1974). | ||||
| 12. | Spores: homosporous (0). | ||||
| 13. | Tapetal thickness: tapetum of one layer of cells (0). | ||||
| 14. | Tapetal activity: ambiguous. Some tapetal cells remain parietal through the tetrad period, but others invade the loculus (0/2). The type of tapetum differs from the combination type described by Parkinson (Citation1987, 1995) in that we did not observe both a true plasmodial tapetum, co-existing with a parietal one. It might be that later in Alsophila sporogenesis, in the post-tetrad period, plasmodial tapetum appears, but this is unclear at present. | ||||
(Information on characters 11–16, 19–20 and 23–25 is not available yet. We look forward to provide data after the study of the post-tetrad period of the sporoderm development in Alsophila.)
To compare characters of the sporogenesis in the species studied here with those of other ferns, we have put the data we have so far obtained on 14 characters of Alsophila alongside the corresponding data for Psilotum, based on the work of Lugardon (Citation1973, 1979), Gabarayeva (1984a, 1984b, 1985, this study) and Parkinson (Citation1987), and include them to the adapted data matrix of Blackmore et al. (Citation2000, ), from which we took only 14 characters and excluded lycophytes and heterosporous ferns. In result, we receive the following sequences (). The comparison of the first two developmental sequences (Alsophila and Psilotum) shows that such characters as the timing of meiotic cytokinesis, aperture type and mode of its definition, and involvement of tapetal-derived material onto spore surfaces are present in both, but such important features as the presence of a well-defined tetrad wall, the involvement of WLCL and the configuration of the plasma membrane in young free microspores are not. These comparisons are inevitably preliminary; we need additional data on further stages of sporogenesis in Alsophila for a more complete comparison between the developmental features of these species. Interestingly, the initial spore wall layer is formed on WLCL in all pteridophytes taxa sampled by Blackmore et al. (Citation2000); however, assuming that very thin glycocalyx is present in other pteridophytes, whereas this character only changes in taxa that either have a distinct primexine or lack a sporopollenin wall (Blackmore & Barnes, Citation1987). It appears that Alsophila with its distinct primexine matrix has moved into this exceptional group, and then the initial formation of ectexine is a new developmental process representing a non-terminal addition to sporoderm ontogeny, as was pointed out by Blackmore and Barnes (Citation1987).
Table I. Data matrix of selected stages of sporogenesis (pre-tetrad and tetrad stage, some post-tetrad data) in Alsophila setosa and Psilotum nudum, based on character states of Blackmore et al. (Citation2000, appendix 1), with some additions.
By comparing the two species under study with other ferns in the Blackmore et al. (Citation2000) data matrix (), one receives the next sequences: Alsophila has nine characters in common with Osmunda, eight with Anemia, seven with Schizaea and Pteridium, and six with remote genera such as Ophioglossum and Athyrium to Microgramma (in the latter all characters coincide; ), and four characters with Equisetum. Psilotum shares 11 common features with Ophioglossum, ten with Anemia, nine with Schizaea, Equisetum and Pteridium, and eight with the remaining genera from Athyrium to Microgramma.
A lot in these sequences appears surprising, but we should remember that this is pure numerical comparison; every character has its own ‘specific ontogenetic gravity’. We also added data on Oreopteris (Lugardon, Citation1990) to the data matrix in , although incomplete (data on meiosis stages are absent).
A thoughtful review on the origin, function and development of the spore wall in early land plants (Wellman, Citation2004) embraces many aspects, including the substructural organisation of the sporoderm and the role of molecular genetics in sporoderm development. Here, we touch on only some of these points. Our data on Alsophila has shown that a mode of sporoderm formation based upon a pre-patterned cell surface primexine matrix (glycocalyx?) may be not confined to the pollen of seed plants, but could also be characteristic of at least some pteridophytes. Two recent models of spore/pollen wall substructure, the model of universal tufts-units proposed by Rowley (e.g. 1990, 1995, 1996) and the ‘micellar’ (or self-assembly) model (Hemsley et al., Citation1992, Citation1994, Citation2000; Gabarayeva, Citation1993; Scott, Citation1994), discussed in the review of Wellman (Citation2004), can now be regarded as united (Gabarayeva & Hemsley, Citation2006; Hemsley & Gabarayeva, Citation2007; Gabarayeva et al., Citation2009 a, 2009b). This became possible after the conclusion that the tuft-units of Rowley are most probably the second mesophase in self-assembling micellar sequence. Cylindrical micelles and WLCL are so-called ‘neat’ micellae, bi-layers with a gap between the layers. Comparing pteridophytes with gymnosperms and angiosperms on their substructure, Rowley (Citation1996) has found radially oriented units in some pteridophytes; Tryon and Lugardon (Citation1991) described rodlet-like units (cylindrical micelles?) about 0.1 μm in diameter during the early perispore development. As to typical pteridophyte white-lined lamellae (=neat micelles), Rowley (Citation1995) considered them to be similar in exospore of pteridophytes and in endexine of gymnosperms and angiosperms.
Information is gradually accumulating that the processes of self-assembly (which are initially under genetic control) interfere in some stages of (micro)sporogenesis, a hypothesis initiated by Wodehouse (Citation1935) and continued by Heslop-Harrison (Citation1972), van Uffelen (Citation1991), Gabarayeva (Citation1993) and worked out by Hemsley et al. (Citation1992, 1994), including mimic experiments (Hemsley et al., Citation1996b, 1998; Griffiths & Hemsley, Citation2001; Moore et al., Citation2009) and regarding sporoderm development as unfolding system of micellar phases (Collinson et al., Citation1993; Gabarayeva & Hemsley, Citation2006; Hemsley & Gabarayeva, Citation2007). The latter idea received confirmation in ontogenetic studies of many angiosperms and some cycads (Blackmore et al., Citation2010; Gabarayeva & Grigorjeva, Citation2011; Gabarayeva et al., Citation2011, and references cited therein).
During the tetrad period in Alsophila, self-assembly processes participate in the development of high-ordered micelle-like connections between tapetal cells and sporogenous cells (), spherical lipoid units, vesicles and tripartite membranes of tapetal origin (, , , F) and a fibrillar layer (primexine matrix) around tetraspores (, –E, ). All of these are typical self-assembling structures. We were so far not able to observe cylindrical micelles in Alsophila: this fact seems to be meaningful (cylindrical micelles are indeed the framework for angiosperm columellae, which are not typical for pteridophytes). However, we intend to undertake the further investigations needed to trace development in the post-tetrad period.
Conclusions
Clusters of plasmalemmasomes in sporogenous cells as seen in Alsophila and Psilotum, are a typical feature for pre-meiotic cells of other pteridophytes, gymnosperms and angiosperms as well. They are probably necessary for the subsequent rapid growth and rounding-up of the sporogenous cells at the point of transition to SMCs.
Numerous plasmodesmata are characteristic for both sporogenous cells of Alsophila and Psilotum. In Alsophila, they are also present between sporogenous and tapetal cells suggesting that they are necessary for the transfer of some lipoid substance from the tapetum. Plasmodesmatal connections in pre-meiosis are a common feature for both ferns and angiosperms.
Before the intiation of the meiosis, most plasmodesmata become occluded and cytomictic channels appear between SMCs in both Alsophila and Psilotum to provide the flow of cytoplasm, together with its organelles, from cell to cell. The same process is usually observed at the transition from sporogenous cells to microspore mother cells in angiosperms. The meaning of these intercellular connections is to secure coenocyte-like structure and, correspondingly, synchronisation of meiosis in all meiocyte of a sporangium/anther, which can be complete (Alsophila) or incomplete (Psilotum).
At the transition to meiosis, a general re-organisation of the cytoplasm starts in both species: meiotic cytoplasmic clarification and re-organisation of organelles occur. Many autolytic vacuoles are present and the membranes of plastids and mitochondria almost completely disappear in Alsophila, which is less pronounced in Psilotum; elimination of ribosomes, important in connection with the change of genome functioning, were also reported for Polypodiophyta in the whole. An organelle plate (band) forms in the telophase I of simultaneous cytokinesis in Alsophila and Psilotum. The main events during meiosis in both species are the same, but the details are different.
At the beginning of the tetrad period in Alsophila, a fibrillar layer, the primexine matrix, and a dark, thin, sinusoidal sheet associated with these fibrils, appear at the vicinity of the tetraspore plasma membrane. This initial sheet is not tripartite. Only this initial step of the sporoderm development is observed during the tetrad period in Alsophila, similar to Equisetum fluviatile, where sporoderm development starts also very late at the end of the tetrad period.
No clearly discernible proximal fold has been observed at the end of the tetrad period, just a long thin outgrowth of the initial spore envelope. This is similar to the formation of proximal fold in the leptosporangiate homosporous fern Blechnum spicant, where only the first layer is laid down during the tetrad period, but in Blechnum this layer has tripartite structure.
Sporoderm ontogeny during the tetrad period in Alsophila and Psilotum has some common points, but also essential differences. The main difference concerns the relative timing of the events: in Alsophila the end of the tetrad period is the starting point for the exospore development, while in Psilotum the exospore is completed by this point in ontogeny. Considerable differences between the two species concern the tapetum.
During the tetrad period in Alsophila, self-assembly processes participate in the development, judging on the presence of many spherical lipoid units, vesicles and tripartite membranes of tapetal origin in the sporangium loculus and the fibrillar primexine matrix around the tetraspores. These units correspond to spherical, laminate and filament micelles, but cylindrical micelles, typical for gymnosperms and angiosperms, were not observed.
Acknowledgements
This work was supported by grants PICT 0661/08 (ANPCyT and CONICET) for Gonzalo Marquez and by grant RFBR No. 11-04-00462 for Nina Gabarayeva. The authors thank Marta Morbelli for providing inspiration and financial support to this work. The authors also thank the engineer Peter Tzinman for assistance with the Hitachi H-600 TEM. Special thanks go to Steve Blackmore for linguistic improvement.
Notes
This article is dedicated to the memory of John Rowley, my dear teacher and friend (N.G.).
References
- Aldrich , H. C. and Vasil , I. K. 1970 . Ultrastructure of the post meiotic nuclear envelope in microspores of Podocarpus macrophyllus . Journal of Ultrastructure Research , 32 : 307 – 315 .
- Bednara , J. , Giełwanowska , I. and Rodkiewicz , B. 1986 . Regular arrangements of mitochondria and plastids during sporogenesis . Equisetum. Protoplasma , 130 : 145 – 152 .
- Bell , P. R. 1975 . Physical interactions of nucleus and cytoplasm in plant cells . Endeavour , 34 : 19 – 22 .
- Bell , P. R. 1981 . Megasporogenesis in a heterosporous fern: Features of the organelles in meiotic cells and young megaspores . Journal of Cell Science , 51 : 109 – 119 .
- Bierhorst , D. W. 1977 . The systematic position of Psilotum and Tmesipyeris . Brittonia , 29 : 3 – 13 .
- Blackmore , S. 1990 . Sporoderm homologies and morphogenesis in land plants, with a discussion of Echinops sphaerocephala (Compositae) . Plant Systematics and Evolution , 5 : 1 – 12 .
- Blackmore , S. 2007 . Pollen and spores: Microscopic keys to understanding the earth's biodiversity . Plant Systematics and Evolution , 263 : 3 – 12 .
- Blackmore , S. and Barnes , S. H. 1987 . Embryophyte spore walls: Origin, development and gomologies . Cladistics , 3 : 185 – 195 .
- Blackmore , S. and Crane , P. R. 1998 . “ The evolution of apertures in the spores and pollen grains of embryophytes ” . In Reproductive biology , Edited by: Owens , S. J. and Rudall , P. J. 159 – 182 . Kew : Royal Botanic Gardens .
- Blackmore , S. , Takahashi , M. and Uehara , K. 2000 . “ Preliminary phylogenetic analysis of sporogenesis in pteridophytes ” . In Pollen and spores: Morphology and biology , Edited by: Harley , M. M. , Morton , C. M. and Blackmore , S. 109 – 124 . Kew : Royal Botanic Gardens .
- Blackmore , S. , Wortley , A. H. , Skvarla , J. J. and Rowley , J. R. 2007 . Pollen wall development in flowering plants . New Phytologist , 174 : 483 – 498 .
- Blackmore , S. , Wortley , A. H. , Skvarla , J. J. , Gabarayeva , N. I. and Rowley , J. R. 2010 . Developmental origins of structural diversity in pollen walls of Compositae . Plant Systematics and Evolution , 284 : 17 – 32 .
- Bremer , K. 1985 . Summary of green plant phylogeny and classification . Cladistics , 1 : 369 – 385 .
- Bogdanov , Y. F. 1983 . Ultrastructure of chromosomes and sinaptonemic complexes in meiotic prophase in lily . Tsitologiya , 25 : 17 – 23 . in Russian
- Brown , R. C. and Lemmon , B. E. 1982a . Ultrastructure sporogenesis in the moss Amblystegium riparium. I. Meiosis and cytokinesis . American Journal of Botany , 69 : 1096 – 1107 .
- Brown , R. C. and Lemmon , B. E. 1982b . Ultrastructural aspect of moss meiosis: Cytokinesis and organelle apportionment in Rhynchostegium serrulatum . Journal of the Hattori Club , 53 : 41 – 50 .
- Brown , R. C. and Lemmon , B. E. 1996 . Nuclear cytoplasmic domains, microtubules and organelles in microsporocytes of the slipper orchid Cypripedium californicum A. Gray dividing by simultaneous cytokinesis . Sexual Plant Reproduction , 9 : 145 – 152 .
- Brown , R. C. and Lemmon , B. E. 2001a . Sporogenesis in eusporangiate ferns: I. Monoplastidic meiosis in Angiopteris (Marattiales) . Journal of Plant Research , 114 : 223 – 235 .
- Brown , R. C. and Lemmon , B. E. 2001b . Sporogenesis in eusporangiate ferns: I. Polyplastidic meiosis in Ophioglossum (Ophioglossaceae) . Journal of Plant Research , 114 : 237 – 246 .
- Brown , R. C. and Lemmon , B. E. 2009 . Premeiotic bands and novel meiotic spindle ontogeny in quadrilobed sporocytes of leafly liverworts (Jungermannidae, Bryophyta) . Protoplasma , 237 : 41 – 49 .
- Collinson , M. E. , Hemsley , A. R. and Taylor , W. A. 1993 . Sporopollenin exhibiting colloidal organization in spore walls . Grana, Supplement , 1 : 31 – 39 .
- Coulomb , P. and Coulon , J. 1972 . Origine et fonctions des phytolysosomes dans le meristeme radiculaire de la courge (Cucurbita pepo L. cucurbitacée). I. Origine de phytolysosomes. Relations reticulum endoplasmique – dictiosomes – phytolysosomes . Journal de Microscopie , 13 : 263 – 280 .
- Dickinson , H. G. and Bell , P. R. 1970 . Nucleocytoplasmic interaction at the nuclear envelope in post-meiotic microspores of Pinus banksiana . Journal of Ultrastructure Research , 33 : 356 – 359 .
- Dickinson , H. G. and Heslop-Harrison , J. 1977 . Ribosomes, membranes and organelles during meiosis in angiosperms . Philosophical Transactions of the Royal Society of London B , 277 : 327 – 342 .
- Echlin , P. and Godwin , H. 1968 . The ultrastructure and ontogeny of pollen in Helleborus foetidus L. II. Pollen grain development through the callose special wall stage . Journal of Cell Science , 3 : 175 – 186 .
- Gabarayeva , N. I. 1984a . The development of spores in Psilotum nudum (Psilotaceae). The changes of cytoplasm and organelles of spore mother cells from the premeiotic interphase to leptothene of proface I of meiosis . Botanicheski Zhurnal , 69 : 1441 – 1450 . in Russian
- Gabarayeva , N. I. 1984b . The development of spores in Psilotum nudum (Psilotaceae): The changes of cytoplasm and organelles in spore mother cells from zygothene to pachytene . Botanicheski Zhurnal , 69 : 1612 – 1622 . in Russian
- Gabarayeva , N. I. 1985 . The development of spores in Psilotum nudum (Psilotaceae): The changes of cytoplasm and organelles in spore mother cells in metaphase and telophase I of meiosis . Botanicheski Zhurnal , 70 : 441 – 450 . in Russian
- Gabarayeva , N. I. 1993 . Hypothetical ways of exine pattern determination . Grana, 33 Supplement , 2 : 54 – 59 .
- Gabarayeva , N. I. and Grigorjeva , V. V. 2002 . Exine development in Stangeria eriopus (Stangeriaceae): Ultrastructure and substructure, sporopollenin accumulation, the equivocal character of the aperture, and stereology of microspore organelles . Review of Palaeobotany and Palynology , 122 : 185 – 218 .
- Gabarayeva , N. I. and Grigorjeva , V. V. 2010 . Sporoderm ontogeny in Chamaedorea microspadix (Arecaceae). Self-assembly as the underlying cause of development . Grana , 49 : 91 – 114 .
- Gabarayeva , N. I. and Grigorjeva , V. V. 2011 . Sporoderm development in Swida alba (Cornaceae), interpreted as a self-assembling colloidal system . Grana , 50 : 81 – 101 .
- Gabarayeva , N. , Grigorjeva , V. and Polevova , S. 2011 . Exine and tapetum development in Symphytum officinale (Boraginaceae). Exine substructure and its interpretation . Plant Systematics and Evolution , 296 : 101 – 120 .
- Gabarayeva , N. I. , Grigorjeva , V. V. , Rowley , J. R. and Hemsley , A. R. 2009a . Sporoderm development in Trevesia burckii (Araliaceae). I. Tetrad period: Further evidence for the participation of self-assembly processes . Review of Paleobotany and Palynology , 156 : 211 – 232 .
- Gabarayeva , N. I. , Grigorjeva , V. V. , Rowley , J. R. and Hemsley , A. R. 2009b . Sporoderm development in Trevesia burckii (Araliaceae). II. Post-tetrad period: Further evidence for participation of self-assembly processes . Review of Palaeobotany and Palynology , 156 : 233 – 247 .
- Gabarayeva , N. I and Hemsley , A. R. 2006 . Merging concepts: The role of self-assembly in the development of pollen wall structure . Review of Palaeobotany and Palynology , 138 : 121 – 139 .
- Gabarayeva , N. I. , Rowley , J. R. and Skvarla , J. J. 1998 . Exine development in Borago (Boraginaceae). 1. Microspore tetrad period . Taiwania , 43 : 203 – 214 .
- Gastony , G. J. 1974 . Spore morphology in the Cyatheaceae. I. The perine and sporangial capasity: General considerations . American Journal of Botany , 61 : 672 – 680 .
- Gastony , G. J. and Tryon , R. 1976 . Spore morphology in the Cyatheaceae. 2. The genera Lophosoria, Metaxia, Sphaeropteris, Alsophila and Nephelea . American Journal of Botany , 63 : 738 – 758 .
- Genevès , M. L. 1971 . Mise en place de polysaccharides membranaires dans le tissue sporogène de l’Hypnum rusciforme (Hypnacées) au cours la phase de prolifération, et au début de la différenciation . Comptes Rendus de l'Académie des Sciences Paris , 273 : 723 – 726 .
- Griffiths , P. C. and Hemsley , A. R. 2001 . Rasberries and muffins – Mimicking biological pattern formation . Colloids and Surfaces B: Biointerfaces , 25 : 163 – 170 .
- Gubatz , S. and Wiermann , R. 1992 . Studies on sporopollenin biosynthesis in Tulipa anthers. 3. Incorporation of specifically labeled C-14 phenylalanine in comparison to other precursors . Botanica Acta , 105 : 407 – 413 .
- Hemsley , A. R. , Barrie , P. J. and Chaloner , W. G. 1996a . Studies of fossil and modern spore wall biomacromolecules using 13C solid state NMR . Annals of Botany , 78 : 83 – 94 .
- Hemsley , A. R. , Collinson , M. E. and Brain , A. P. R. 1992 . Colloidal crystal-like structure of sporopollenin in the megaspore walls of recent Selaginella and similar fossil spores . Botanical Journal of the Linnean Society , 108 : 307 – 320 .
- Hemsley , A. R. , Collinson , M. E. , Kovach , W. L. , Vincent , B. and Williams , T. 1994 . The role of self-assembly in biological systems: Evidence from iridescent colloidal sporopollenin in Selaginella megaspore walls . Philosophical Transactions of the Royal Society of London B , 345 : 163 – 173 .
- Hemsley , A. R. , Collinson , M. E. , Vicent , B. , Griffiths , P.C. and Jenkins , P. D. 2000 . “ Self-assembly of colloidal units in exine development ” . In Pollen and spores: Morphology and biology , Edited by: Harley , M. M. , Morton , C. M. and Blackmore , S. 31 – 44 . Whitstable : Whitstable Printers Ltd .
- Hemsley , A. R. and Gabarayeva , N. I. 2007 . Exine development: The importance of looking through a colloid chemistry “window . Plant Systematics and Evolution , 263 : 25 – 49 .
- Hemsley , A. R. , Jenkins , P. D. , Collinson , M. E. and Vincent , B. 1996b . Experimental modelling of exine self-assembly . Botanical Journal of the Linnean Society , 121 : 177 – 187 .
- Hemsley , A. R. , Vincent , B. , Collinson , M. E. and Griffiths , P. C. 1998 . Simulated self-assembly of spore exines . Annals of Botany , 82 : 105 – 109 .
- Heslop-Harrison , J. 1966 . Cytoplasmic continuities during spore formation in flowering plants . Endeavour , 25 : 65 – 72 .
- Heslop-Harrison , J. 1971 . “ The cytoplasm and its organelles during meiosis ” . In Pollen: Development and physiology , Edited by: Heslop-Harrison , J. 16 – 31 . London : Butterworths .
- Heslop-Harrison , J. 1972 . Pattern in plant cell walls: Morphogenesis in miniature . Proceedings of the Royal Institute of Great Britain , 45 : 335 – 351 .
- Koscinska-Pajak , M. and Bednara , J. 2003 . Microtubule patterns and organelles during microsporogenesis in apomictic Chondrilla juncea L . Acta Biologica Cracoviensia, Botanica , 45 : 169 – 176 .
- Kozubov , G. M. , Trenin , V. V. , Tichova , M. A. and Kondratjeva , V. P. 1982 . Reproductive structures of gymnosperms (comparative description) , Leningrad : Nauka . in Russian
- Lehmann , H. , Neidhart , H. V. and Schlenkermann , G. 1984 . Ultrastructural investigations on sporogenesis . Equisetum fluviatile. Protoplasma , 123 : 38 – 47 .
- Lewitsky , M. 1926 . Die Chondriosomen in der Gonogenese bei Equisetum palustre L . Planta , 1 : 301 – 317 .
- Lugardon , B. 1966 . Cytology végétale. Formation de l'exospore chez Blechnum spicant (L.) Roth . Comptes Rendus de l'Académie des Sciences Paris, Série D , 262 : 2029 – 2031 .
- Lugardon , B. 1968 . Sur l'existence de liaisons protoplasmiques entre les cellules-mères des microspores de Ptéridophytes au cours de la prohase hétérotypique . Comptes Rendus de l'Académie des Sciences Paris , 267 : 593 – 596 .
- Lugardon , B. 1969 . Sur le processus de la formation du “pli proximal” au cours de la sporogenèse chez Blechnum spicant (L.) Roth . Comptes Rendus de l'Académie des Sciences Paris, Série D , 268 : 286 – 289 .
- Lugardon , B. 1973 . Sur les parois sporales de Psilotum triquetrum Sw. et leur structure fine . Comptes Rendus de l'Académie des Sciences Paris , 276 : 1277 – 1280 .
- Lugardon , B. 1979 . Sur la formation du sporoderme chez Psilotum triquetrum Sw. (Psilotaceae) . Grana , 18 : 145 – 165 .
- Lugardon , B. 1990 . “ Pteridophyte sporogenesis: A survey of spore wall ontogeny and fine structure in a polyphyletic plant group ” . In Microspores: Evolution and ontogeny , Edited by: Blackmore , S. and Knox , R. B. 95 – 120 . London : Academic Press .
- Lal , Manohar and Bell , P. R. 1977 . Aspects of the differentiation of the egg of the moss Phyomitrium coorgense Broth . Annals of Botany , 41 : 127 – 131 .
- Marengo , N. P. 1977 . Ultrastructural features of the dividing meiocyte of Onoclea sensibilis . American Journal of Botany , 64 : 600 – 601 .
- Marengo , N. P. and Marengo , M. M. 1972 . The ultrastructure of the mitochondrial plate of the meiocyte of Polypodium aureum . Bulletin of the Torrey Botanical Club , 99 : 21 – 23 .
- Marquez , G. J. , Morbelli , M. A. and Giudice , G. E. 2009 . Comparative analysis of spores of Alsophila (Cyatheaceae) species from southern South America . Review of Palaeobotany and Palynology , 156 : 165 – 176 .
- Moore , S. E. M. , Gabarayeva , N. and Hemsley , A. R. 2009 . Morphological, developmental and ultrastructural comparison of Osmunda regalis L. spores with spore mimics . Review of Palaeobotany and Palynology , 156 : 177 – 184 .
- Mueller , W. C. and Greenwood , A. W. 1978 . The ultrastructure of phenolic-storing cells fixed with caffeine . Journal of Experimental Botany , 29 : 757 – 764 .
- Niester-Nyveld , C. , Haubrich , A. , Kampendonk , H. , Gubatz , S. , Tenberge , K. B. , Rittscher , M. , Willmesmeier , S. and Wiermann , R. 1997 . Immunocytochemical localization of phenolic compounds in pollen walls using antibodies against p-coumaric acid coupled to bovine serum albumin . Protoplasma , 197 : 148 – 159 .
- Pacini , E. and Cresti , M. 1978 . Ultrastructural characteristics of the tapetum and microspore mother cells in Lycopersicum peruvianum during meiotic prophase . Bulletin de la Société Botanique de France , 125 : 121 – 128 .
- Parenti , L. K. 1980 . A phylogenetic analysis of the land plants . Biological Journal of the Linnean Society , 13 : 225 – 242 .
- Parkinson , B. M. 1987 . Tapetal organization during sporogenesis . Psilotum nudum. Annals of Botany , 60 : 353 – 360 .
- Parkinson , B. M. 1995 . The tapetum in Schizeae pectinata (Schizeaceae) and a comparison with the tapetum in Psilotum nudum (Psilotaceae) . Plant Systematics and Evolution , 196 : 161 – 172 .
- Parkinson , B. M. and Pacini , E. 1995 . A comparison of tapetal structure and function in pteridophytes and angiosperms . Plant Systematics and Evolution , 198 : 55 – 88 .
- Pettitt , J. M. 1971 . Developmental mechanisms in heterospory. I. Megasporocyte degeneration . Selaginella. Botanical Journal of the Linnean Society , 64 : 237 – 246 .
- Pettitt , J. M. 1976 . Developmental mechanisms in heterospory. III. The plastid cycle during megasporogenesis in Isoetes . Journal of Cell Science , 20 : 671 – 685 .
- Pettitt , J. M. 1977 . Developmental mechanisms in heterospory: Features of post-meiotic regression . Selaginella. Annals of Botany , 41 : 117 – 125 .
- Pettitt , J. M. 1979 . “ Ultrastructure and cytochemistry of spore wall morphogenesis ” . In The experimental biology of ferns , Edited by: Dyer , A. F. 211 – 252 . London : Academic Press .
- Pettitt , J. M. and Jermy , A. C. 1974 . The surface coats on spores . Biological Journal of the Linnean Society , 6 : 245 – 257 .
- Qiu , Y.-L. and Palmer , J. 1999 . Phylogeny of early land plants: Insights from genes and genomes . Trends in Plant Science , 4 : 26 – 30 .
- Rowley , J. R. 1990 . The fundamental structure of the pollen exine . Plant Systematics and Evolution , 5 : 13 – 29 .
- Rowley , J. R. 1995 . Are the endexines of pteridophytes, gymnosperms and angiosperms structurally equivalent? . Review of Palaeobotany and Palynology , 85 : 13 – 34 .
- Rowley , J. R. 1996 . “ Exine origin, development and structure in pteridophytes, gymnosperms, and angiosperms ” . In Palynology: Principles and applications , Edited by: Jansonius , J. and McGregor , D. C. 445 – 462 . Salt Lake City : American Association of Stratigraphic Palynologists Publishers Press .
- Rowley , J. R. and Dahl , A. O. 1977 . Pollen development in Artemisia vulgaris with special reference to glycocalyx material . Pollen et Spores , 19 : 169 – 284 .
- Schuettpelz , E. and Pryer , K. M. 2007 . Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes . Taxon , 56 : 1037 – 1050 .
- Scott , R. J. 1994 . “ Pollen exine – the sporopollenin enigma and the physics of pattern ” . In Molecular and cellular aspects of plant reproduction , Edited by: Scott , R. J. and Stead , M. A. 49 – 81 . Cambridge : Cambridge University Press .
- Sersic , A. N. 1983 . Carotenoides y esporodermis de Lycopodium saururus (Lycopodiaceae) . Kurtziana , 16 : 161 – 163 .
- Sheffield , E. and Bell , P. R. 1979 . Ultrastructural aspects of sporogenesis in a fern, Pteridium aquilinum (L.) Kuhn . Annals of Botany , 44 : 393 – 405 .
- Sheffield , E. , Laird , S. and Bell , P. R. 1983 . Ultrastructural aspects of sporogenesis in the apogamous fern Dryopteris borreri . Journal of Cell Science , 63 : 125 – 134 .
- Stevenson , D. W. and Loconte , H. 1996 . “ Ordinal and familial relationships of pteridophytes ” . In Pteridology in perspective , Edited by: Camus , J. M. , Gibby , M. and Johns , R. J. 435 – 467 . Kew : Royal Botanic Gardens .
- Surova , T. D. 1981 . On the formation of lipid complexes in sporogenesis of the fern Anemia phyllitidis (Schisaeaceae) . Botanicheski Zhurnal , 66 : 1276 – 1280 . in Russian
- Takhtajan , A. L. 1978 . “ Psilotophyta ” . In The life of plants , Edited by: Fedorov , A. A. 125 – 130 . Moscow : Prosveschenie . in Russian
- Tchórzewska , D. and Bednara , J. 2011 . The dynamics of the actin cytoskeleton during sporogenesis in Psilotum nudum L . Protoplasma , 248 : 289 – 299 .
- Tchórzewska , D. , Winiarczyk , K. , Pietrusiewicz , J. and Bednara , J. 2008 . A new type of microtubular cytoskeleton in microsporogenesis of Lavatera thuringiaca L . Ptotoplasma , 232 : 223 – 231 .
- Tryon , R. and Lugardon , B. 1991 . Spores of Pteridophyta. Surface, wall structure and diversity based on electron microscope studies , New York : Springer .
- Uehara , K. and Kurita , S. 1989a . An ultrastructural study of spore wall morphogenesis in Equisetum arvense . American Journal of Botany , 76 : 939 – 951 .
- Uehara , K. and Kurita , S. 1989b . Ultrastructural study on spore wall morphogenesis in Ophioglossum thermale Kom. var. nipponicum (Miyabe et Kudo) Nishida . The Botanical Magazine Tokyo , 102 : 413 – 427 .
- Uehara , K. and Kurita , S. 1991 . Ultrastructural study on spore wall morphogenesis in Lycopodium clavatum (Lycopodiaceae) . American Journal of Botany, 78 , : 24 – 36 .
- Uehara , K. , Kurita , S. , Sanashi , N. and Ohmoto , T. 1991 . Ultrastructural study on microspore wall morphogenesis in Isoetes japonica (Isoetaceae) . American Journal of Botany , 78 : 1182 – 1190 .
- Van Uffelen , G. A. 1991 . “ The control of spore wall formation ” . In Pollen and spores: Patterns of diversification , Edited by: Blackmore , S. and Barnes , S. H. 89 – 102 . Oxford : Clarendon Press .
- Wang , H.-C. , Li , J.-Q. and He , Z.-C. 2007 . Irregular meiotic behaviour in Isoetes sinensis (Isoetaceae), a rare and endangered fern in China . Caryologia , 60 : 358 – 363 .
- Wellman , C. H. 2004 . “ Origin, function and development of the spore wall in early land plants ” . In The evolution of plant physiology , Edited by: Hemsley , A. R. and Poole , I. 43 – 63 . Kew : Royal Botanic Gardens .
- Wodehouse , R. P. 1935 . Pollen grains: Their structure, identification and significance in science and medicine , New York : McGraw-Hill .
- Wolniak , S. M. 1976 . Organelle distribution and apportionment during meiosis in the microsporocyte of Ginkgo biloba L . American Journal of Botany , 63 : 251 – 258 .