Abstract
Megaspore ornamentation is one of the few morphological characters used in the taxonomy of the genus Isoëtes. In the present work, we test the application of this character for distinguishing some Isoëtes species occurring in the Mediterranean: Isoëtes sicula, which according to some authors should be included as a variety in I. histrix, and the recently described I. todaroana, whose affinity with other taxa is currently unknown. Two additional species (I. duriei, I. velata) were also included in the analysis. The megaspores were studied using scanning electron microscopy combined with energy-dispersive X-ray spectroscopy and chemical treatment. In all species, the megaspore surface is covered by a siliceous coating; removal of this coating reveals an underlying three-dimensional network of fused rodlets. A unique pattern of this network occurs in I. sicula, supporting its separation from I. histrix. Comparison between the patterns observed in the examined Mediterranean species shows that I. todaroana is most similar to I. histrix.
Phylogenetic analyses based on DNA sequence data reveal that lycophytes (including the extant families Isoëtaceae, Selaginellaceae and Lycopodiaceae) form a distinct clade, branching off from other tracheophytes early in land plant evolution (Pryer et al., Citation2001). The Isoëtaceae are a small, cosmopolitan family of heterosporous plants; the only extant genus (Isoëtes) comprises approximately 200 species (Hoot et al., Citation2004). Based on a number of shared derived characters such as the ligule and heterospory, the family appears to be most closely related to the Selaginellaceae.
Moore et al. (Citation2006) suggested homology of the megaspore sporoderm layers in Isoëtes and Selaginella based on their own investigations and previously published data by Taylor (Citation1993) and Macluf et al. (Citation2003): both share a siliceous outer coating, followed by an (outer and inner) sporopolleninous exospore, an inner separable layer and a cellulosic endospore, an organisation already identified by Tchistiakoff (Citation1873).
Despite their probable Palaeozoic origin, worldwide distribution and diverse ecological adaptations (from seasonal, deciduous terrestrials to obligate, evergreen aquatics), Isoëtes species show very little morphological variation (Hoot et al., Citation2004). Although variation in megaspore ornamentation indicates that surface morphology is liable to convergence, this character remains one of the most significant sources of phylogenetically informative data in morphological analyses, and is one of the few characters that has proven useful in identifying and separating Isoëtes species (Hickey, Citation1986).
The aim of this study is to highlight affinities and differences among some Mediterranean species of Isoëtes using scanning electron microscopy (SEM) observation of the megaspore surface. Since it is known that in several species of this genus an outer silica layer covers the real megaspore surface, it was thought important to ascertain the presence of the silica layer in the examined species by means of energy-dispersive X-ray spectroscopy (EDS), including one species that grows on basic soil, and to observe and compare the megaspore surface after the silica was removed by a chemical treatment.
The species selected for this study were Isoëtes histrix Bory and I. sicula Tod., which according to some authors are synonyms or conspecific taxa, I. duriei Bory, whose megaspores, despite an apparent different morphology at low magnification, are very similar to those of I. histrix at greater magnification (Berthet & Lecocq, Citation1977), the recently described I. todaroana Troia & Raimondo, whose affinity with other taxa is currently unknown, and I. velata A. Braun, whose megaspore surface background is similar to that of I. todaroana (Troia & Raimondo, Citation2010).
Materials and methods
Specimens of the selected Isoëtes species were collected in Sicily (Italy) by the first author and deposited in the Herbarium Mediterraneum (PAL) (). Megaspores from all specimens were studied under SEM, one set clean, without any chemical treatment, and another set after chemical treatment that removed the siliceous outer coating. For the latter, megaspores were washed with distilled water and then soaked into hydrofluoric acid (40% HF) for 20 minutes, caught on a polypropylene mesh, rinsed with distilled water, dried and prepared for SEM. The megaspores were mounted onto SEM stubs using double-stick tape, were then coated with gold/palladium and examined under an Oxford Leo 440 SEM.
Table I. Details of the examined specimens
EDS was carried out for untreated megaspores of all species to document major chemical components of the megaspore surface sculpture. The analytical system used for detecting megaspore surface components was a PentaFET detector connected to Oxford Instruments (ISIS) software.
In the morphological descriptions, we follow the terminology of Hickey (Citation1986) and Taylor (Citation1993). Note that Taylor (Citation1993, p. 169) showed that the use of the term ‘perispore’ to identify the siliceous coating in the genus Isoëtes is not correct.
Results
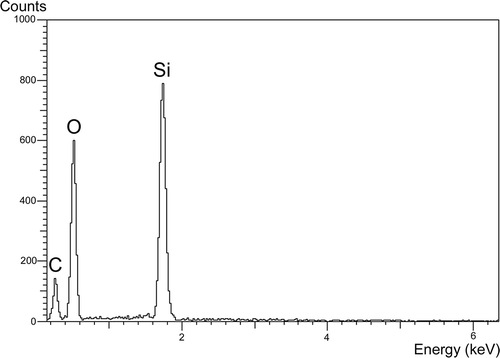
The EDS spectrum of the untreated Isoëtes todaroana megaspore surface shows peaks corresponding to silicon (Si) and oxygen (O) revealing the presence of silica (); similar spectra were obtained for the untreated megaspore surface of the four other species examined here.
Figure 1. Spectrum of the energy-dispersive X-ray spectroscopy (EDS) detecting silica in the megaspore surface of Isoëtes todaroana. A similar pattern was observed in the outer megaspores of all the species examined here.
All five species have trilete megaspores (), which are more or less globose in shape with convex proximal face and hemispherical distal face in equatorial view (Ferrarini et al., Citation1986). The surface ornamentation of the untreated megaspores is retate in Isoëtes duriei () and composed of irregularly anastamosing muri () without prominent ridges; tuberculate in I. histrix (), I. sicula () and I. todaroana (), and pustulate in I. velata (). In all species, the ornamentation of the proximal is similar to that of the distal face.
Figure 2. Scanning electron microscopy (SEM) images of Isoëtes duriei megaspores. A. Proximal view, untreated megaspore. B. Proximal view, after treatment with hydrofluoric acid (HF), which removed the siliceous coating. C. Detail of the surface, untreated megaspore. D, E. Detail of the megaspore surface after HF-treatment. Scale bars – 100 μm (A, B), 10 μm (C, D), 1 μm (E).
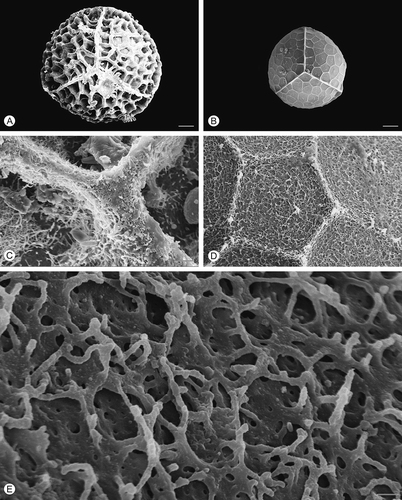
Figure 3. Scanning electron microscopy (SEM) images of Isoëtes histrix megaspores. A. Proximal view, untreated megaspore. B. Proximal view, after treatment with hydrofluoric acid (HF), which removed the siliceous coating. C. Detail of the surface, untreated megaspore. D, E. Detail of the megaspore surface after HF-treatment. Scale bars – 100 μm (A, B), 10 μm (C, D), 1 μm (E).
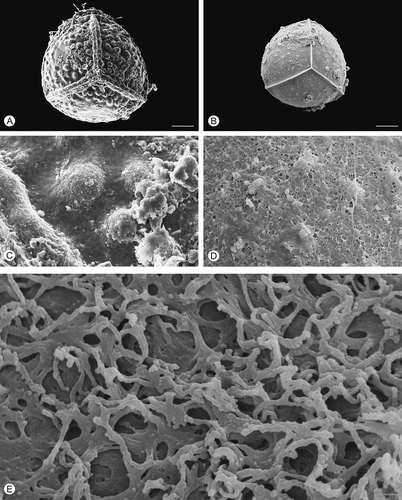
Figure 4. Scanning electron microscopy (SEM) images of Isoëtes sicula megaspores. A. Proximal view, untreated megaspore. B. Proximal view, after treatment with hydrofluoric acid (HF), which removed the siliceous coating. C. Detail of the surface, untreated megaspore. D, E. Detail of the megaspore surface after HF-treatment. Scale bars – 100 μm (A, B), 10 μm (C, D), 1 μm (E).
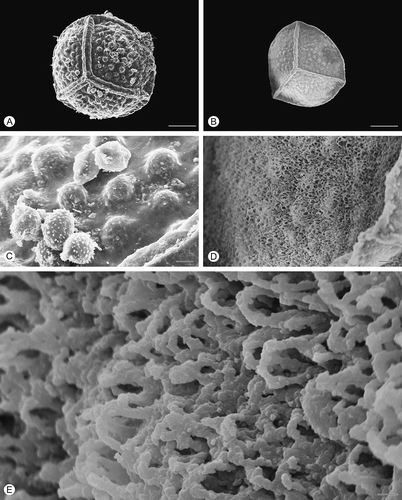
Figure 5. Scanning electron microscopy (SEM) images of Isoëtes todaroana megaspores. A. Proximal view, untreated megaspore. B. Proximal view, after treatment with hydrofluoric acid (HF), which removed the siliceous coating. C. Detail of the surface, untreated megaspore. D, E. Detail of the megaspore surface after HF-treatment. Scale bars – 100 μm (A, B), 10 μm (C, D), 1 μm (E).
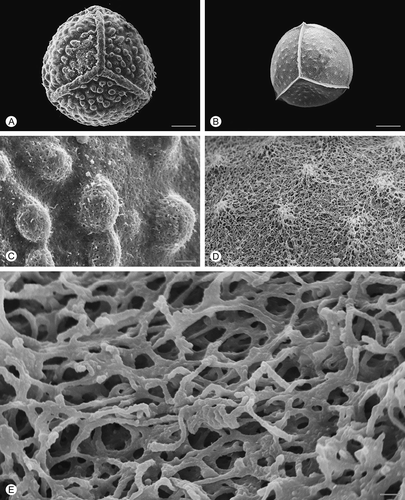
Figure 6. Scanning electron microscopy (SEM) images of Isoëtes velata megaspores. A. Proximal view, untreated megaspore. B. Proximal view, after treatment with hydrofluoric acid (HF), which removed the siliceous coating. C. Detail of the surface, untreated megaspore. D, E. Detail of the megaspore surface after HF-treatment. Scale bars – 100 μm (A, B), 10 μm (C, D), 1 μm (E).
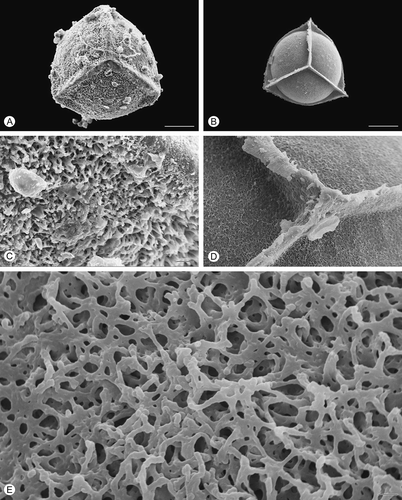
After HF treatment, which removed the siliceous outer coating, the exospore of all species reflects, in a reduced form, the specific ornamentation pattern seen in untreated specimens (, , , , ). In HF-treated megaspores of Isoëtes velata, the expansion of the equatorial ridge at the junctures with the proximal ridges is distinct (). Removal of the siliceous coating causes a significant reduction in the diameter of the megaspores in all species studied.
In all species, the exospore ornamentation is composed of a three-dimensional network of fused rodlets forming heterogeneous spaces in all species (, E, , E, , E, , E, , E); the surface is essentially open (discontinuous; cf. Macluf et al., Citation2003).
The pattern of this network is similar in all species, except for Isoëtes sicula where rodlets form arch-like structures (, E). A slightly different pattern with more densely packed rodlets and smaller spaces is recognisable in I. velata ().
Discussion
Morphological data are often considered of less importance in phylogenetic studies than are DNA sequence data, but are never-the-less of value in phylogenetic reconstructions (Schneider et al., Citation2009), and our results demonstrate that morphological and structural features of the megaspores may be useful in delimitation of the Isoëtes taxa occurring.
Isoëtes duriei is the only polyploid species among the species studied. Polyploidy has proven to affect some morphological traits, such as spore size (cf. Troia, Citation2001), but no particular features that could be ascribed to polyploidy were observed for morphology and structure in the megaspores studied here.
Isoëtes velata is a rare west-Mediterranean species, the only strictly amphibian species studied here (Jermy & Akeroyd, Citation1993). The other species are basically ‘terrestrial’ (i.e. living in seasonally wet or inundated soils, with their leaves emergent in the air).
All species studied grow on acid soils except for Isoëtes todaroana that is reported to grow on basic soils (Troia & Raimondo, Citation2010) in a peculiar geological substrate consisting of calcareous sandstones with a thin layer of clays and quartzitic sands on top.
Our study confirms the presence of a deposit of pure amorphous silica forming most of the ornamentation as observed by previous studies of Isoëtes megaspores including those of I. duriei and I. velata (e.g. Tchistiakoff, Citation1873; Robert et al., Citation1973; Taylor, Citation1993). Of particular interest is the presence of this siliceous layer also in I. todaroana, which grows on basic soils (Troia & Raimondo, Citation2010) and our results are, therefore, significant in asserting that a siliceous coating of the megaspores is present also under these different ecological conditions.
The important role of the silica layer for the ornamentation and for the biology of the megaspore is confirmed by the observed reduction (in all species examined) of the diameter of the megaspores, following removal of the silica by treatment in HF. After HF-treatment, the ornamentation pattern is still visible on the exospore, although in a reduced form. In other words, the surface ornaments of the siliceous coating seem to conform to the sporopolleninous exospore as a deposit.
In all studied species, the outer part of the megaspore wall is composed of a three-dimensional network of fused rodlets separated by heterogeneous spaces. The uniformity of the network indicates that it is highly conservative, and/or that all the examined species are closely related (Taylor, Citation1993). Isoëtes duriei, I. histrix, I. todaroana and I. velata are particularly similar, although I. velata may be distinguished by more densely packed rodlets and smaller spaces between the aggregation of rodlets. Only megaspores of I. sicula show a divergent pattern with rodlets fused to form arch-like structures. This supports the separation of I. sicula from I. histrix at species level as also suggested by molecular studies by Bolin et al. (Citation2008), who referred I. sicula to I. subinermis (Durieu) Cesca & Peruzzi (see later). The unique network pattern may also suggest an isolated position of I. sicula in relation to the other examined taxa.
The status of Isoëtes sicula as a separate species has not been universally accepted. The species was described by Todaro (Citation1866), but immediately (Cesati et al., Citation1868) considered a synonym of I. histrix f. subinermis Durieu, invalidly established in 1861; both names were successively neglected in later descriptions (Pignatti, Citation1982; Jermy & Akeroyd, Citation1993). Recently, Cesca and Peruzzi (Citation2001) raised the subinermis form to species level on the basis of morphological and karyological data. Troia (Citation2005) accepted the species level status, but noted that I. subinermis is a junior synonym of I. sicula. The name I. subinermis was also rejected as nomenclaturally invalid by Arrigoni (Citation2005), who instead suggested the use of the name I. gymnocarpa (Gennari) A. Braun. However, according to the original description of Gennari (Citation1862), I. gymnocarpa is not conspecific with I. subinermis and I. sicula, since I. gymnocarpa lacks a ‘velum’ covering the sporangium that is present in the latter ones. For this reason, the correct name for the species should be I. sicula.
Conclusions
The megaspore surface of Isoëtes species can supply useful data to better define the species and their relationships once the siliceous coating has been removed.
With this approach, the specific separation of Isoëtes sicula from I. histrix can be confirmed and the isolated position of I. sicula ascertained as well as the morphological affinity between I. histrix and I. todaroana. The presence of pure amorphous silica forming most of the spore surface ornamentation in all examined species confirms that the siliceous coating of the megaspores is an important and typical trait of the genus as a whole.
Acknowledgements
The authors acknowledge C. Di Liberto and M. Sajeva (Dipartimento di Biologia ambientale e Biodiversità, Università di Palermo) for technical assistance with scanning electron microscopy and suggestions on hydrofluoric acid-treatment, respectively; F. Parello and S. Calabrese (Dipartimento di Scienze della Terra e del Mare, Università di Palermo) for use of energy-dispersive X-ray spectroscopy; Else Marie Friis, Christian Pott and two anonymous reviewers for their helpful comments and suggestions for the improvement of this manuscript.
References
- Arrigoni , P. V. 2005 . Note floristiche e tassonomiche sulla flora di Sardegna . Parlatorea , 7 : 17 – 21 .
- Berthet , P. and Lecocq , M. 1977 . Morphologie sporale des espèces françaises du genre Isoetes L . Pollen et Spores , 19 : 329 – 359 .
- Bolin , J. F. , Bray , R. D. , Keskin , M. and Musselman , L. J. 2008 . The genus Isoetes L. (Isoetaceae, Lycophyta) in south-western Asia . Turkish Journal of Botany , 32 : 447 – 457 .
- Cesati , V. , Passerini , G. and Gibelli , E. G. 1868 . “ Isoetes L ” . In Compendio della Flora Italiana , Edited by: Cesati , V. , Passerini , G. and Gibelli , E. G. 25 Milan : Francesco Vallardi .
- Cesca , G. and Peruzzi , L. 2001 . Isoëtes (Lycophytina, Isoetaceae) with terrestrial habitat in Calabria (Italy). New karyological and taxonomical data . Flora Mediterranea , 11 : 303 – 309 .
- Ferrarini , E. , Ciampolini , F. , Pichi Sermolli , R. E. G. and Marchetti , D. 1986 . Iconographia Palynologica Pteridophytorum Italiae . Webbia , 40 : 1 – 202 .
- Gennari , P. 1862 . Rivista delle Isoëtee della Flora Italiana (continuazione) . Commentario Società Crittogamologica Italiana , 1 : 111 – 116 .
- Hickey , R. J. 1986 . Isoëtes megaspore surface morphology: nomenclature, variation, and systematic importance . American Fern Journal , 76 : 1 – 16 .
- Hoot , S. B. , Napier , N. S. and Taylor , W. C. 2004 . Revealing unknown or extinct lineages within Isoëtes (Isoëtaceae) using DNA sequences from hybrids . American Journal of Botany , 91 : 899 – 904 .
- Jermy , A. C. and Akeroyd , J. R. 1993 . “ Isoetes L ” . In Flora Europaea 1, Psilotaceae to Platanaceae , Edited by: Tutin , T. G. , Burges , N. A. , Charter , A. O. , Edmondson , J. R. , Heywood , V. H. , Moore , D. M. , Valentine , D. H. , Walters , S. M. and Webb , D. A. 6 – 7 . Cambridge : Cambridge University Press .
- Macluf , C. C. , Morbelli , M. A. and Giudice , G. E. 2003 . Morphology and ultrastructure of megaspores and microspores of Isoetes savatieri Franchet (Lycophyta) . Review of Palaeobotany and Palynology , 126 : 197 – 209 .
- Moore , S. E. M. , Hemsley , A. R. and Borsch , T. 2006 . Micromorphology of outer exospore coatings in Selaginella megaspores . Grana , 45 : 9 – 21 .
- Pignatti , S. 1982 . Flora d'Italia , Bologna : Edagricole .
- Pryer , K. M. , Schneider , H. , Smith , A. R. , Cranfill , R. , Wolf , P. G. , Hunt , J. S. and Sipes , S. D. 2001 . Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants . Nature , 409 : 618 – 622 .
- Robert , M. D. , Roland-Heydacker , F. , Denizot , J. , Laroche , J. , Fougeroux , P. and Davignon , L. 1973 . La paroi mégasporale de l’Isoetes setacea Bosc. ex Delile. Etude en microscopies photonique et électroniques. Localisation et nature de la silice entrant dans sa constitution . Adansonia, Série. 2 , 13 : 313 – 332 .
- Schneider , H. , Smith , A. R. and Pryer , K. M. 2009 . Is morphology really at odds with molecules in estimating fern phylogeny? . Systematic Botany , 34 : 455 – 475 .
- Taylor , W. A. 1993 . Megaspore wall ultrastructure in Isoetes . American Journal of Botany , 80 : 165 – 171 .
- Tchistiakoff , J. 1873 . Notice préliminaire sur l'histoire du développement des sporanges et des spores de l’Isoetes duriei Bory . Nuovo Giornale Botanico Italiano , 5 : 207 – 212 .
- Todaro , A. 1866 . Synopsis plantarum acotyledonearum vascularium in Sicilia insulisque adjacentibus sponte provenientium . Giornale di Scienze Naturali ed Economiche di Palermo , 1 : 208 – 254 .
- Troia , A. 2001 . The genus Isoëtes L. (Lycophyta, Isoëtaceae): synthesis of karyological data . Webbia , 56 : 201 – 218 .
- Troia , A. 2005 . Note corologiche e tassonomiche sul genere Isoëtes L. (Isoëtaceae, Lycophyta) in Sicilia . Informatore Botanico Italiano , 37 : 382 – 383 .
- Troia , A. and Raimondo , F. M. 2010 . Isoëtes todaroana (Isoëtaceae, Lycopodiophyta), a new species from Sicily (Italy) . American Fern Journal , 99 : 238 – 243 .