Abstract
The boll weevil, Anthonomus grandis, entered the United States of America in the early twentieth century and became a major pest in cotton, Gossypium spp. Shortly after the passage of Tropical Storm Erin on 16 August 2007 through the South Texas/Winter Garden boll weevil eradication zone, over 150 boll weevils were captured in the Southern Rolling Plains (SRP) eradication zone that was essentially weevil-free since 2003. Pollen analyses were made of the SRP weevils and weevils collected in two suspected source zones, Cameron (Southern Blacklands eradication zone) and Uvalde (Winter Garden eradication zone). An additional examination of the palynological evidence and examination of additional pollen residue shed new light on this event and strengthens the conclusion that the Uvalde area was the source of the SRP weevils. A total of 192 pollen grains from 39 taxa were found in the SRP weevils: 1904 pollen grains from ten taxa from the Cameron weevils and 148 grains from 28 taxa in the Uvalde weevils. The SRP weevils shared 16 taxa, including Phermeranthus sp. (flameflower) with the Uvalde weevils and only five taxa with the Cameron weevils. Common taxa between SRP and Uvalde weevils and the lack of the dominant ‘low spine’ Asteraceae that occurred in all Cameron samples confirm that the SRP weevils originated from the South Texas/Winter Garden zone. Problems associated with this type of research are similar to those in forensic palynology. These problems include the unknown origin of the weevils, pollen contamination and care and storage of the samples.
Keywords:
The boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae), entered the United States of America (USA) in the early twentieth century and successfully invaded the southern USA leaving economic and social devastation in its wake. Boll weevils damage cotton, Gossypium spp., because the developing larvae feed on the flower buds and fruits causing the flowers to fall off and damaging the fruits. This reduces cotton yield and increases the cost of growing cotton because insecticide applications are necessary. Both the larvae and adults forage on pollen. The larval stage is restricted to foraging within flower buds and fruits of the cotton tribe (Gossypieae) of the Malvaceae (cotton family; Lukefahr & Martin, Citation1962; Burke & Clark, Citation1976; Benedict et al., Citation1991). However, the adults are not restricted and can fly to other flowering plants.
Initially, only taxa in the Malvaceae such as Abutilon, Callirhoë, Cienfuegosia, Hibiscus, Malvaviscus, Sphaeralcea, Sida and Wissadula were known as foraging resources for adult boll weevils (Coad, Citation1914; Gaines, Citation1934; Szumkowski, Citation1953, Citation1954; Stoner, Citation1968; Cross et al., Citation1975). Today, the list of adult foraging resources has expanded to include numerous non-malvaceous taxa (Benedict et al., Citation1991; Jones et al. Citation1993; Jones, Citation1997; Hardee et al., Citation1999; Jones & Coppedge, Citation1999, Citation2001).
The boll weevil eradication programme was developed to eradicate this insect pest from the USA. In many states including North Carolina, South Carolina and Georgia (), the programme has been very successful and the boll weevil is considered to be eradicated. Pheromone traps are placed along cotton fields to monitor for weevil re-introductions, which trigger insecticide applications to prevent re-colonisation of this insect pest. Unfortunately, substantial weevil populations remain in parts of Texas.
Figure 1. Maps of the United States of America and Texas. A. The United States of America showing College Station, Texas (C), North (N) and South (S) Carolina and Georgia (G). B. A map of Texas showing three boll weevil eradication zones: Southern Rolling Plains (SRP) zone, Southern Blacklands (SBL) zone, where Cameron is located, and Southern Texas/Winter Garden (WG) zone, where Uvalde is located.
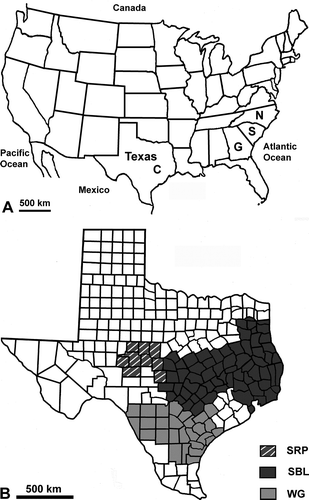
Shortly after the passage of Tropical Storm Erin through the Winter Garden (WG) eradication zone of Texas () in fall of 2007, over 150 boll weevils were unexpectedly captured in the Southern Rolling Plains (SRP) eradication zone of Texas (). This eradication zone had been essentially weevil-free since 2003. In order to mitigate this unexpected re-invasion, expenses of more than one million US dollars (USD) were incurred to increase the number of traps and insecticide applications during the fall of 2007 (Jones et al., Citation2009; Kim et al., Citation2010). The synchrony and broad distribution of captured weevils along the southern perimeter of the SRP eradication zone suggest long-distance dispersal was responsible for the re-invasion.
The initial reports on this event used DNA, meteorological and palynological techniques to determine the possible origin of the SRP weevils (Jones et al., Citation2009; Kim et al., Citation2010). The purpose of this research was to examine additional pollen residue from the collected weevils, identify as many of the unknown taxa as possible, and examine the palynological challenges of this type of research.
Material and methods
Processing
Boll weevils were captured in boll weevil pheromone traps in the SRP eradication zone () between 27 August and 10 September 2007 and shipped to the US Department of Agriculture, Agricultural Research Service (USDA-ARS), College Station, TX, for analyses. Nineteen boll weevils were used for pollen analyses. Some of the weevils were not whole but rather body parts (legs, head, etc.).
To increase the pollen diversity and the number of recovered pollen grains, the whole insect was processed using light microscopy (Jones, Citation2012). Each boll weevil or all boll weevil parts were put into a uniquely marked 1.5 ml micro-centrifuge tube. Samples were acetolysed by pipetting 0.5 ml of a 9:1 ratio of acetic acid to sulphuric acid into each tube (Erdtman, Citation1960, Citation1963; Jones & Coppedge, Citation1999; Jones, 2011). Samples were heated in a hot block at 100 °C for 15 min and stirred every 3–4 min. To stop the acetolysation process, 0.5 ml of glacial acetic acid was added to the samples. The samples were centrifuged for 3 min at 1060 g, decanted and mixed on a vortex stirrer for 15 s. Distilled water was added and the samples were centrifuged, decanted and mixed. The samples were rinsed twice more with distilled water, centrifuging, decanting and mixing each time. To stain the pollen so that it could be easily seen, a clean wooden stick was put into Safranin-O stain, then placed into the sample and used to stir the sample. A new, clean wooden stick was used for each sample. Each micro-centrifuge tube was filled with 95% ethanol. Once again the samples were centrifuged, decanted and stirred. Three drops of glycerine were stirred into each sample and they were placed into a hot block at 25 °C and left over night to remove the ethanol and leave the pollen residue.
To determine where the boll weevils originated, a pollen-print was developed from the recovered pollen residue. A pollen-print is similar to a local pollen spectrum or pollen rain of an area. The main difference is that a pollen-print is the pollen recovered from an object (in this case boll weevils), a person or items usually of forensic interest. Once a pollen-print from the boll weevils was developed, it was compared to pollen-prints of boll weevils collected at the most likely originating locations. The pollen-print of boll weevils from known locations acts as controls so that similarities and differences can be ascertained among the pollen-prints from known locations and that of the boll weevils from an unknown origin.
To determine the pollen-print of the boll weevils from the two most likely origin locations, weevils were collected from eight sites near Cameron (Southern Blacklands eradication zone; ) and eight sites near Uvalde, TX (Southern Texas/WG eradication zone; ). Five boll weevils from each site were pooled to enhance the pollen-print of the suspected possible origin. The Cameron and Uvalde boll weevils were processed in a similar manner except they were put into individual 15 ml glass centrifuge tubes and more chemicals were used (Jones, 2011); 7 ml of acetolysation mixture was added to the Cameron and Uvalde samples instead of one. After acetolysation, 5 ml of glacial acetic acid was added to the Cameron and Uvalde samples, and five drops of Safranin-O stain was added. For a more detailed processing technique of the light microscopy technique LM6, see Jones (2011).
The next morning, glass slides were made from each sample using one drop of the pollen residue. The glycerine/stain drop was covered with a glass cover slip and the edges were sealed by painting a coat of fingernail polish along the edges. After the initial report on these weevils (Jones et al., Citation2009; Kim et al., Citation2010), additional slides of the pollen residue were made and examined of the SRP weevils to enhance their pollen-print. Micrographs of the Cameron and Uvalde boll weevils were re-examined and unknown taxa identified.
Pollen analysis
An Olympus AX-70 compound light microscope was used for examination. Pollen grains were identified to the lowest taxonomic level possible (family, genus or species ranks). The Areawide Pest Management Research Unit Pollen Reference Collections were used for comparisons and identifications. Light micrographs were made of all pollen types. Total number of pollen grains and taxa were summed. Because of their morphological similarity, some pollen groups are lumped together. Chenopodiaceae (goosefoot family) and Amaranthus (pigweed) pollen are traditionally put into a single category, ‘Cheno-Am’ (Martin, Citation1963). Asteraceae pollen grains initially were divided into ‘low spine’ and ‘high spine’. ‘Low spine’ Asteraceae were Asteraceae pollen grains that had spines or processes that were less than 3 mm long. ‘High spine’ Asteraceae were Asteraceae grains with spines greater than 3 mm long. If there was a difference in the number of processes, the process length, width or shape, the pore size or shape or the number of small pores surrounding the processes, they were considered different taxa within the ‘low and high spine’ categories (Skvarla & Larson, Citation1965; Payne & Skvarla, Citation1970; Tomb et al., Citation1974; Feuer & Tomb, Citation1977; Robbins et al., Citation1979). The pollen-prints developed from the SRP weevils were compared with the pollen-prints of the Cameron and Uvalde weevils and other known pollen-printed weevil areas (Jones & Coppedge, Citation1999).
The percentage of pollen grains was calculated by totalling all pollen grains in a taxon and dividing by the total number of pollen grains. Frequency of occurrence was calculated by dividing the number of samples in which a taxon occurred by the total number of samples then multiplying by 100 (Hardee et al., Citation1999; Jones & Coppedge, Citation1999). Multiplying by 100 puts the results in percentages, which make them easier to read and to see any differences.
Results
Seventeen (89%) of the SRP weevils contained pollen (). A total of 192 pollen grains from 39 taxa were found in the pollen residue of the SRP weevils (). Pollen grain numbers per SRP weevil ranged from two to 36, and the number of taxa ranged from one to eight ().
Table I. General pollen data found in the boll weevils from the three eradication zones
All samples (100%) from Cameron and Uvalde contained pollen (). A total of 1904 pollen grains from ten taxa were found in the Cameron boll weevils. In the Uvalde boll weevils, 148 grains from 28 taxa were encountered (). The number of pollen grains in the Cameron boll weevils ranged from 105 to 524 per sample, and from five to 30 in the Uvalde samples.
The SRP weevils contained the greatest diversity of pollen taxa (39), those from Cameron contained the least ten (, ). More Asteraceae types were found than any other plant family (). Based on differences in ‘spine’ length, width, shape and the pore arrangement at the base of the spines, Asteraceae pollen grains were represented by 12 taxa in the SRP samples, ten taxa in Uvalde samples and three taxa in Cameron samples (). Pollen recovered from Cameron was dominated by a single ‘low spine’ Asteraceae (94%; ).
Table II. Each taxon's percentage of the total number of pollen grains per location
Some taxa were found at all locations, while others were found at only one. No taxon was found in all the SRP or all the Uvalde boll weevils (). However, Asteraceae ‘low spine’ #13, Iva sp. #2 and Cheno-Am pollen were found in all Cameron boll weevils ().
Cheno-Am (,), cotton () and Poaceae (grass; ) pollen were found in the samples from all three locations (). Sagittaria sp. (arrowhead), Artemisia sp. (sagebrush), and Celtis laevigata Willd. var. reticulata (Torr.) L. Benson (net-leaf hackberry, ≡ C. reticulata Torr.) were only found in the SRP weevils (). Asteraceae ‘low spine’ #13 (poison ivy; ) was found only in the Cameron samples and Toxicodendron radicans (L.) Kuntze (poison ivy; ) only in the Uvalde samples ().
Figure 2. Pollen found in boll weevils collected in the Southern Rolling Plains, Southern Blacklands (Cameron) and Winter Garden (Uvalde) eradication zones. A. Cheno-Am, Amaranthus palmerii. B. Malvaceae, cotton, Gossypium hirsutum. C. Poaceae, grass. D. Asteraceae ‘low spine’ #13. E. Anacardiaceae, poison ivy, Toxicodendron radicans. F. Portulacaceae, flameflower, Phermeranthus sp. Scale bars – 50 μm (B), 20 μm (A, C–F).
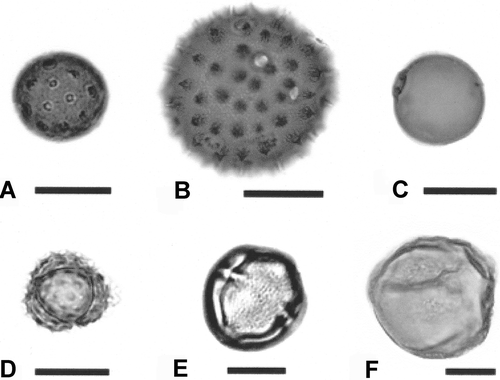
The SRP weevils shared five taxa with the Cameron samples and 16 taxa with the Uvalde samples (). Only two of the taxa in common between the SRP and Cameron weevils were not found in the Uvalde samples (Cyperaceae and cedar elm, Ulmus crassifolia Nutt.; , ). Taxa in common between the SRP and Uvalde weevils included all five of the Fabaceae taxa encountered, Phermeranthus sp. (flameflower; ) and Juniperus sp. (juniper). Of the total pollen encountered in the SRP samples, 33% were from taxa found in Cameron and 58% were from taxa found at Uvalde (calculated from ).
Table III. Frequency of occurrence of the pollen taxa found in the boll weevil samples
Asteraceae pollen had the highest frequency of occurrence in samples from all three eradication zones (calculated from ). In the SRP samples, Iva sp. #1 occurred in more than 50% of the samples while any of the Fabaceae occurred in only 5% of the samples (). Iva sp. #2, ‘low spine’ #13 and Cheno-Am pollen occurred in every Cameron sample (). In the Uvalde samples, 62% contained Solidago sp. pollen ().
Discussion
The origin of the SRP weevils was unknown. Since they were trapped in an eradication zone that had been weevil-free for four years, it was presumed that they originated from elsewhere. The two most likely zones were the Southern Blacklands (Cameron) or the Southern Texas/WG (Uvalde) zones because boll weevils had not been eradicated from these zones.
Boll weevil samples are not often collected or maintained in a sterile and contaminant-free environment. Weevils can remain in traps for days or weeks before being collected. It is not known how much pollen is lost during this time or how much the pollen is degraded by fungi, sun, rain, wind, etc. Some of the SRP weevils were deteriorated or partially eaten because only body parts were available for analyses. Those SRP weevils that were whole were very dehydrated. It is logical that the more dehydrated and deteriorated the sample, the fewer pollen grains can be recovered. Collecting boll weevils every 24 hours and freezing them is ideal for pollen analysis. Freezing prevents pollen loss due to digestion, while the boll weevils are dying and also prevents fungal attack.
As with any sample, pollen contamination can occur. Possible pollen contamination of the boll weevils can come from the person collecting the weevils, unclean containers, samples left unsealed, etc. Jones and Greenberg (Citation2009 a) found that boll weevil traps collect and retain little or no pollen, so any pollen contamination most likely comes during collection or leaving the samples unsealed and left open. Weevils usually land on the outside of the trap skirt, walk downwards, over the skirt edge and then climb up the inside of the trap skirt to the mesh cone (Jones & Greenberg, Citation2009 a). The mesh cone where the weevils remain before being collected seldom contained any pollen. Thus, it is doubtful that weevils become contaminated with pollen while sitting in a trap.
The accuracy of the pollen-print from any insect depends on the palynologist examining and identifying the pollen, the available pollen reference collections and pollen-prints from known locations. It is important to compare the pollen-prints of the same species of insects rather than look at the pollen-prints of different insects. Different insects have different food ‘preferences’ and feeding habits. Some insects, such as honeybees, actively search for pollen, while other insects, such as butterflies, become dusted with pollen when they feed on nectar. Although the pollen-prints will be similar, they will also be quite different because of the food preferences of the insect species. Furthermore, pollen retention in and on different insects varies. For example, boll weevils retained pigweed (Amaranthus sp.) pollen for 48 h (Jones et al., Citation2007); however, Lygus lineolaris Palisot (tarnished plant bug) retained pigweed pollen for 96 h (Jones & Allen, Citation2012).
In honey samples, Jones and Bryant (Citation1998, Citation2001) found that increasing a pollen count by 100 grains increased the number of new taxa. They also found that in a 2500 grain count only 60% of the taxa were encountered. Increasing the amount of the SRP residue examined increased the number of pollen grains and taxa encountered. The number of grains increased from 158 to 192 and the number of taxa from 33 to 39 (Jones et al., Citation2009; Kim et al., Citation2010).
The amount of pollen recovered from most boll weevil samples is usually low. Making a 200+ grain count is virtually impossible. This was not the case with the Cameron boll weevils where over 1900 pollen grains were counted and the number of pollen grains per sample ranged from 105 to 525 grains. A 200 or even 1000 grain count can easily be made from the Cameron samples. However, making a 200 grain count per sample from the SRP or Uvalde weevils might be difficult, because pollen grain numbers were low per sample. Even with the Uvalde samples containing five weevils per sample, only 148 pollen grains were found and the range was 5–30 per sample.
The number of pollen grains encountered per individual weevil is usually relatively low, although a high number of pollen grains per individual weevil have been reported. The high number of pollen grains per weevil captured in Cameron may have been due to several factors. The number of pollen grains seems to depend on the season or time of year, the taxa of pollen encountered and the length of time that has passed after feeding. In controlled laboratory studies, the number of pollen grains per boll weevil varied according to the taxa provided as a food source and the length of time after digestion (Jones et al., Citation2007). In the control weevils fed Prunus dulcis (Mill.) D.A. Webb (almond) pollen at 0 h, one boll weevil contained 1053 pollen grains. After 1 h, the highest number of pollen grains per boll weevil was 58 (Jones et al., Citation2007). How much variation in pollen grain numbers is actually due to the size and the ornamentation of the pollen grains themselves is unknown.
Similarly, Jones and Greenberg (Citation2009 b) found that the number of cotton pollen grains was highest at 0 h after removal from prior feeding and decreased with time. They found over 1270 cotton pollen grains in a single weevil. After 48 h, the highest number of cotton pollen grains was four in one individual. Thus, the longer the boll weevils remain in the traps, the fewer the pollen grains are going to be recovered.
Although the ranges are similar, weevils captured in pheromone traps contained fewer pollen grains per individual. The mean number of pollen grains found in weevils captured in Mississippi ranged from 80 during the fall to 18 during the winter (Hardee et al., Citation1999). In Uvalde, the number of pollen grains per individual varied according to the month from 10 to 354 during April and from three to 73 during July (Jones & Coppedge, Citation1999).
In honey samples, when fewer than 500 pollen grains are counted per sample, the percentage of the pollen grains is reported in frequency classes (Louveaux et al., Citation1970, Citation1978; Lieux, Citation1972, Citation1975, Citation1981; Jones & Bryant, Citation1998, Citation2004; Bryant & Jones, Citation2001). Dominant pollen has a percentage of 45% or more. Secondary types are those that occurred between 45–16%; important minor pollen types occur between 15–3% and minor pollen types occurred below 3% (Louveaux et al., Citation1970).
Examining the frequency classes in an entomopalynological study along with its pollen-print can help establish a more accurate assessment of the habitat because frequency classes classify a pollen type according to its rank among the total number of pollen grains. This makes trends in species more easily seen. In other words, frequency classes make the dominant, secondary and minor types more obvious.
Following the use of frequency classes for melissopalynology, similar frequency categories could be used in entomopalynological studies to classify pollen according to its rank among the total number of pollen grains. Jones and Bryant (Citation1998, Citation2001) found that the frequency classes hardly varied regardless of the number of pollen grains that were counted. Accordingly, Asteraceae ‘low spine’ #13 was the only dominant pollen type from the three eradication zones and dominated the pollen-print from Cameron. Uvalde weevils contained no dominant pollen types, but three secondary pollen types (all Asteraceae). The SRP weevils also contained no dominant types, but two secondary types (Asteraceae and Cheno-Am).
It was expected that some taxa would be common in all three locations and some would be found in only one. Asteraceae, Cheno-Am, cotton and Poaceae pollen occurred in the samples from all three locations. Asteraceae, Cheno-Am and Poaceae pollen have been reported as alternative food sources. Pollen from 14 non-malvaceous plant families including Asteraceae, Cheno-Am and Poaceae was found in boll weevils captured in southern Texas and in north-eastern Mexico (Benedict et al., Citation1991). Asteraceae, Poaceae and Cheno-Am pollen have been found in weevils captured in Tamaulipas, Mexico, Mississippi and Texas (Jones et al., Citation1993; Hardee et al., Citation1999; Jones & Coppedge, Citation1999). One of the first non-malvaceous taxa found as an alternative feeding source was an Asteraceae, Hymenopappus flavescens A. Gray (yellow woolywhite; Rummel et al., Citation1978).
Asteraceae, Cheno-Am and Poaceae occur in all vegetational zones of Texas, flower almost year-round and usually produce an abundance of pollen. Asteraceae and Poaceae are the two largest plant families in Texas (Jones et al., Citation1997). Furthermore, pollen from these three families is distinctive and easily put into the family ranking.
In the samples from all three locations, Asteraceae had the highest occurrence of pollen and thus was encountered more frequently than other types. Asteraceae is the largest plant family in Texas, containing over 196 genera and over 680 species (Jones et al., Citation1997). These samples were collected in August and September. During this time, the summer flowering taxa have gone to fruit and the fall flowering taxa are just beginning to flower. Taxa in the Asteraceae make up a large portion of the fall blooming plants in Texas.
Upon re-examination of the micrographs, several unknowns from each location were identified and some were placed into already identified taxa. Increasing the taxa list makes the pollen-print a more closely accurate reflection of the habitat. Unfortunately none of the newly identified unknowns were habitat specific.
It is likely that the SRP weevils obtained some pollen from SRP plants before being captured. One SRP weevil contained 16 cotton pollen grains indicating that it had foraged on cotton 24 h before being trapped and killed (Jones & Greenberg, Citation2009b ). Thus, this individual likely foraged locally after it reached the SRP. Foraging on plants in the SRP prior to being trapped would explain why the pollen-prints were slightly different between the weevils captured in the SRP and WG eradication zones.
Both the SRP and Uvalde weevils contained flameflower. Flameflower is an entomophilous taxon that does not grow throughout Texas. There are nine taxa of flameflower in Texas (Jones et al., Citation1997). Although flameflower is not restricted to the Uvalde area, it is more common in south and west Texas (Gould, Citation1975; Correll & Johnston, Citation1979).
Since there were no real habitat specific taxa in the samples, determination of the origin of the SRP weevils is therefore based on the taxa the SRP have in common with the Cameron and Uvalde samples and the diversity of the taxa. The SRP pollen-print only shared five taxa with the Cameron pollen-print including Cheno-Ams, Poaceae and cotton, which were common throughout Texas and also found in the Uvalde boll weevil. The pollen-print of weevils captured near Cameron was dominated by Asteraceae ‘low spine’ #13 that was present in high numbers in all the Cameron samples. The complete absence of Astereaceae ‘low spine’ #13 and the lack of common taxa rules out Cameron as the origin of the SRP weevils.
Although the Uvalde pollen-print does not perfectly match the SRP pollen-print, over 50% of the pollen counted were from taxa common to both locations. In addition, the SRP and Uvalde weevils had 16 of the 39 taxa in common. Of the 28 taxa found in Uvalde, 57% were found in the SRP samples. These taxa included state-wide taxa such as Asteraceae, Cheno-Am and Poaceae but also included entomophilous taxa that are not common (Leucaena sp., Mimosa sp. and Mimosa strigillosa Torr. & A. Gray). The presence of flameflower in the SRP and Uvalde samples also contributes to Uvalde being the likely source of the SRP weevils. Finally, boll weevil collected in 1995 in the Southern Texas/WG zone contained a high diversity of pollen taxa and a large number of Asteraceae and Fabaceae pollen types (Jones & Coppedge, Citation1999, 2001), which is similar to the pollen-print of the weevils captured in the SRP eradication zone.
Conclusion
Pollen-prints reflect a picture of the blooming plants in a habitat at a particular time. The pollen-print will change throughout the year due to biotic and abiotic factors. Boll weevils within a habitat obtain pollen either by eating the flowers and buds or by walking around on the plants in search of food, shelter, etc. The SRP boll weevils should contain a blend of pollen types from their originating habitat and from the habitat prior to being trapped. The lack of pollen diversity, the few taxa in common and the absence of Asteraceae ‘low spine’ #13 pollen from the SRP pollen-print discount Cameron as a possible source region. Conversely, the presence of taxa in common, the high diversity of pollen taxa and the number of Asteraceae and Fabaceae taxa indicated that the SRP weevils most likely came from the Uvalde area.
In forensic palynology, soil samples are collected and used to compare the sample in question. Whether or not examining the pollen assemblage from the soil around the traps would add more information is not known. It would be interesting to examine the correlation of the pollen-print of boll weevils trapped in various eradication zones to that of the soil around the boll weevil traps.
Acknowledgements
The author would like to thank Ester F. Wilson, USDA-ARS, APMRU, for her dedication and hard work on the research projects. The author also appreciates Vaughn M. Bryant, Jr (TAMU) and Cynthia L. Sheffield (USDA-ARS, FFSRU) for their review of this manuscript. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- Benedict , J. H. , Wolfenbarger , D. A. , Bryant , V. M. Jr. and George , D. M. 1991 . Pollens ingested by boll weevils (Coleoptera: Curculionidae) in southern Texas and northeastern México . Journal of Economic Entomology , 84 : 126 – 131 .
- Bryant , V. M. Jr and Jones , G. D. 2001 . The R-values of honey: Pollen coefficients . Palynology , 25 : 11 – 28 .
- Burke , H. R. and Clark , W. E. 1976 . “ Cienfuegosia drummondii as a host of the boll weevil, Anthonomus grandis, in south Texas ” . In Boll weevil suppression, management, and elimination technology , Edited by: Davich , T. B. 12 – 21 . Lincoln , NE : Agricultural Research Service, USDA, ARS-S-71 .
- Coad , B. R. 1914 . Feeding habits of the boll weevil on plants other than cotton . Journal of Agricultural Research , 2 : 235 – 245 .
- Correll , D. S. and Johnston , M. C. 1979 . Manual of the vascular plants of Texas , Richardson , TX : The University of Texas at Dallas Press .
- Cross , W. H. , Lukefahr , M. J. , Fryxell , P. A. and Burke , H. R. 1975 . Host plants of the boll weevil . Environmental Entomology , 4 : 19 – 26 .
- Erdtman , G. 1960 . The acetolysis method . Svensk Botanisk Tidskrift , 54 : 561 – 564 .
- Erdtman , G. 1963 . Palynology . Advances in Botanical Research , 1 : 149 – 208 .
- Feuer , S. and Tomb , A. S. 1977 . Pollen morphology and detailed structure of family Compositae, tribe Cichorieae. II. Subtribe Microseridinae . American Journal of Botany , 64 : 230 – 245 .
- Gaines , R. D. 1934 . The development of the boll weevil on plants other than cotton . Journal of Economic Entomology , 27 : 745 – 748 .
- Gould , F. W. 1975 . Texas plants – a checklist and ecological summary. Texas Agricultural Experiment Station Bulletin, MP-585 , College Station , TX : Texas A&M University .
- Hardee , D. D. , Jones , G. D. and Adams , L. C. 1999 . Emergence, movement, and host plants of boll weevils (Coleoptera: Curculionidae) in the delta of Mississippi . Journal of Economic Entomology , 92 : 130 – 139 .
- Jones , G. D. 2012 . Pollen extraction from insects . Palynology , 36 : 86 – 109 .
- Jones , G. D. and Allen , K. C. 2012 . Using Amaranthus palmeri pollen to mark captured tarnished plant bugs . Palynology , doi: 10.1080/01916122.2012.662178
- Jones , G. D. and Bryant , V. M. Jr . 23–28 June 1996 1998 . “ Pollen recovery from honey ” . In Proceedings of the IX International Palynological Congress 23–28 June 1996 , 483 – 487 . Houston , Texas
- Jones , G. D. and Bryant , V. M. Jr . 2001 . “ Is one drop enough? ” . In Palynology: Principles and applications , Edited by: Jansonius , J. and McGregor , D. C. 933 – 938 . Dallas , TX : American Association of Stratigraphic Palynologists Foundation .
- Jones , G. D. and Bryant , V. M. Jr . 2004 . The use of ethanol for the dilution of honey . Grana , 43 : 174 – 182 .
- Jones , G. D. and Coppedge , J. R. 1999 . Foraging resources of boll weevils (Coleoptera: Curculionidae) . Journal of Economic Entomology , 92 : 860 – 869 .
- Jones , G. D. and Coppedge , J. R. 23–28 June 1996 2001 . “ Uvalde, Texas, boll weevils are hungry critters ” . In Proceedings of the IX International Palynological Congress 23–28 June 1996 , 501 – 503 . Houston , Texas
- Jones , G. D. and Greenberg , J. R. 2009a . Pollen contamination of boll weevil traps . Grana , 48 : 297 – 309 .
- Jones , G. D. and Greenberg , J. R. 2009b . Cotton pollen retention in boll weevils: A laboratory experiment . Palynology , 33 : 157 – 165 .
- Jones , G. D. , Greenberg , S. M. and Eischen , F. A. 2007 . Almond, melon, and pigweed pollen retention in the boll weevils (Coleoptera: Curculionidae) . Palynology , 31 : 81 – 93 .
- Jones , G. D. , Westbrook , J. K. , Sappington , T. W. and Kim , K. S. 3–6 January 2006 2009 . “ A multidisciplinary approach to reconstruction of a boll weevil re-invasion ” . In Proceedings of the Beltwide Cotton Production Research Conferences 3–6 January 2006 , 786 San Antonio , Texas
- Jones , R. W. 1997 . Pollen feeding by the boll weevil (Coleoptera: Curculionidae) following cotton harvest in East Central Texas . Southwestern Entomology , 22 : 419 – 429 .
- Jones , R. W. , Cate , J. R. , Hernandez , E. M. and Sosa , E. S. 1993 . Pollen feeding and survival of the boll weevil (Coleoptera: Curculionidae) on selected plants species in northeastern México . Environmental Entomology , 22 : 99 – 108 .
- Jones , S. D. , Wipff , J. K. and Montgomery , P. M. 1997 . Vascular plants of Texas , Austin , TX : University of Texas Press .
- Kim , K. S. , Jones , G. D. , Westbrook , J. K. and Sappington , T. W. 2010 . Multidisciplinary fingerprints: Forensic reconstruction of an insect reinvasion . Journal of the Royal Society Interface , 7 : 677 – 686 .
- Lieux , M. H. 1972 . A melissopalynological study of 54 Louisiana (U.S.A.) honeys . Review of Palaeobotany and Palynology , 13 : 95 – 124 .
- Lieux , M. H. 1975 . Dominant pollen types recovered from commercial Louisiana honeys . Economic Botany , 29 : 78 – 96 .
- Lieux , M. H. 1981 . An analysis of Mississippi USA honey: Pollen, color and moisture . Apidologie , 12 : 137 – 158 .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1970 . Methods of melissopalynology . Bee World , 51 : 125 – 131 .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1978 . Methods of melissopalynology . Bee World , 59 : 139 – 157 .
- Lukefahr , M. J. and Martin , D. F. 1962 . A native host plant of the boll weevil and other cotton insects . Journal of Economic Entomology , 55 : 150 – 151 .
- Martin , P. S. 1963 . The last 10 000 years: A fossil pollen record of the American Southwest , Tucson , AZ : University of Arizona Press .
- Payne , W. W. and Skvarla , J. J. 1970 . Electron microscopy study of Ambrosia pollen (Compositae: Ambrosieae) . Grana , 10 : 89 – 100 .
- Robbins , R. R. , Dickinson , D. B. and Rhodes , A. M. 1979 . Morphometric analysis of pollen from four species of Ambrosia (Compositae) . American Journal of Botany , 66 : 538 – 545 .
- Rummel , D. R. , While , J. R. and Pruitt , G. R. 1978 . A wild feeding host of the boll weevil in west Texas . Southwestern Entomologist , 3 : 171 – 175 .
- Skvarla , J. J. and Larson , D. A. 1965 . An electron microscopic study of pollen morphology in the Compositae, with special reference to the Ambrosiinae . Grana , 6 : 210 – 269 .
- Stoner , A. 1968 . Sphaeralcea spp. as hosts of the boll weevil in Arizona . Journal of Economic Entomology , 61 : 1100 – 1102 .
- Szumkowski , W. 1953 . Nota preliminar sobre Cienfuegosia heterophylla Garcke, plantas hospedera de Alabama argillacea (Hbn.) y Anthonomus grandis Boh. en Venezuela . Agronomia Tropical (Maracay, Venezuela) , 1 : 121 in Spanish
- Szumkowski , W. 1954 . Lista de plantas hospedera de Anthonomus grandis Boh. en Venezuela . Agronomia Tropical (Maracay, Venezuela) , 4 : 29 – 42 . in Spanish
- Tomb , A. S. , Larson , D. A. and Skvarla , J. J. 1974 . Pollen morphology and detailed structure of family Compositae, tribe Cichorieae. I. Subtribe Stephanomeriinae . American Journal of Botany , 61 : 486 – 498 .